Maternal Plasma Glycerophospholipids LC-PUFA Levels Have a Sex-Specific Association with the Offspring’s Cord Plasma Glycerophospholipids-Fatty Acid Desaturation Indices at Birth
Abstract
:1. Introduction
2. Methods and Materials
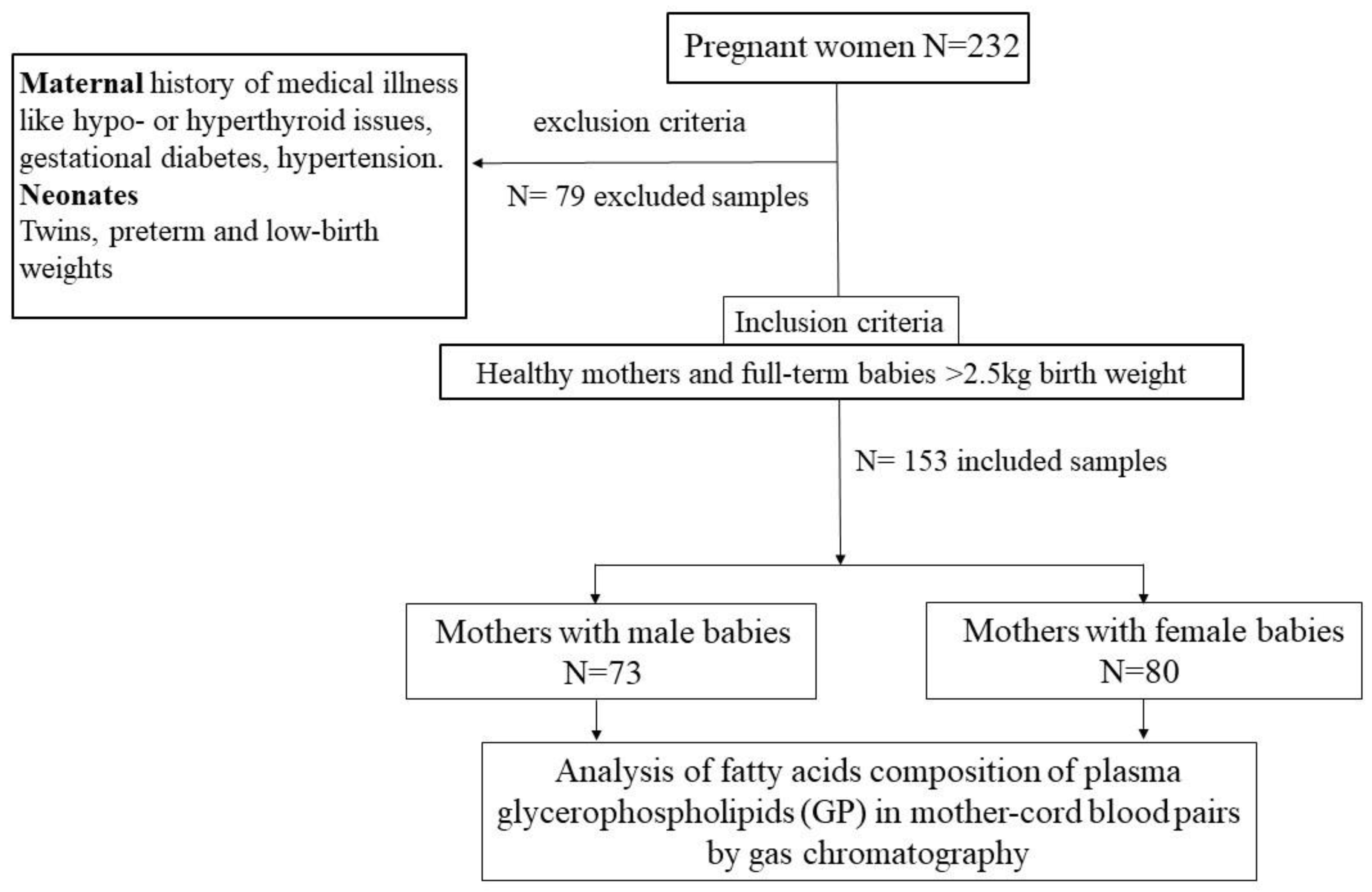
2.1. Human Observation Study Design
2.2. Quantification of Fatty Acids in Plasma Glycerophospholipids of the Mother and Cord Blood Pairs by Gas Chromatography
2.3. Quality Controls of Gas Chromatography Analysis
2.4. Desaturation Indices Calculation
2.5. Statistical Analysis
3. Results
3.1. Study Subjects
3.2. Comparisons of Maternal and Sex-Specific Newborn’s Cord Blood Fatty Acid Composition at Birth
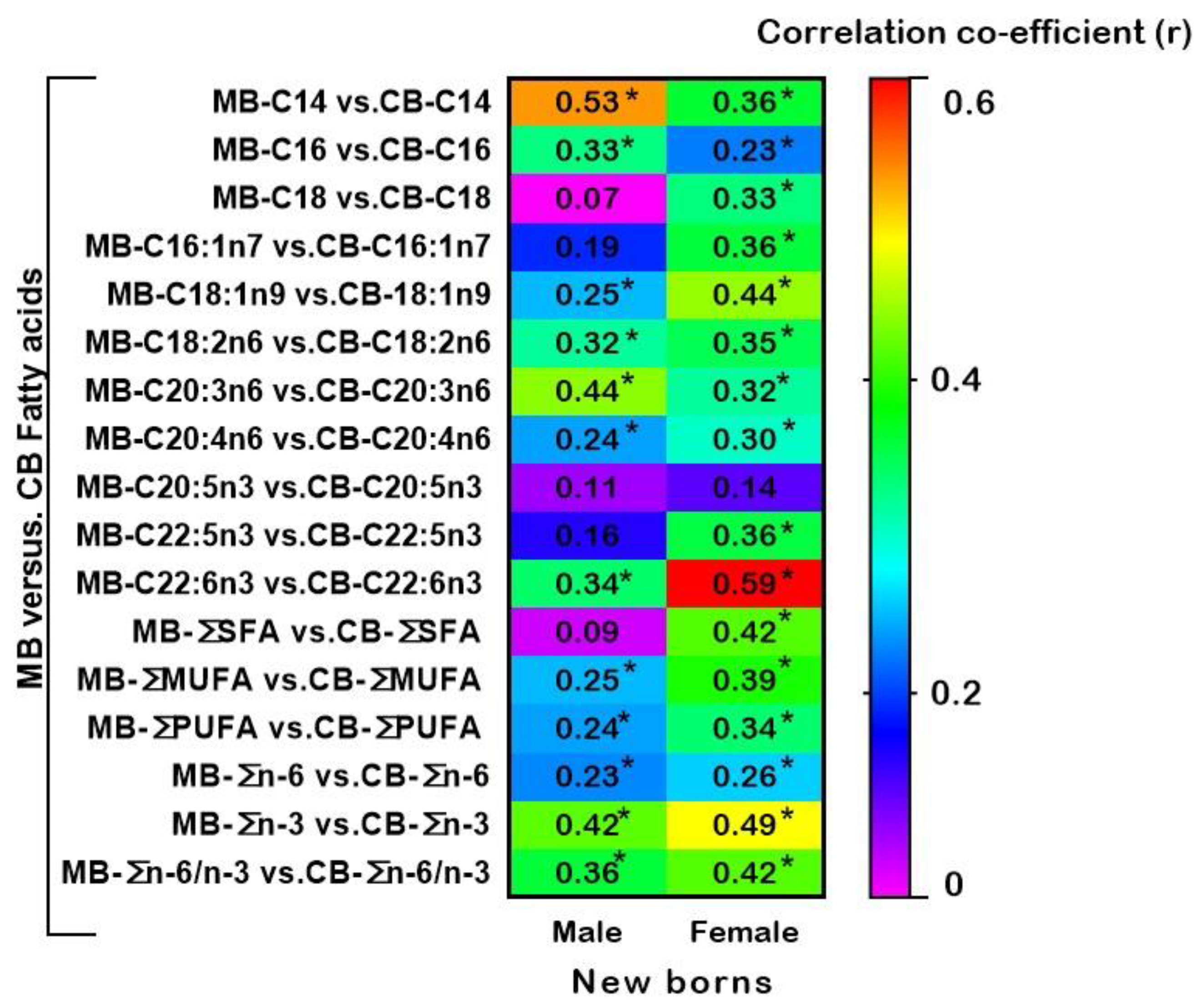
3.3. Relationships between Fatty Acids of Maternal and Cord Blood Plasma Glycerophospholipids in Male and Female New-Borns
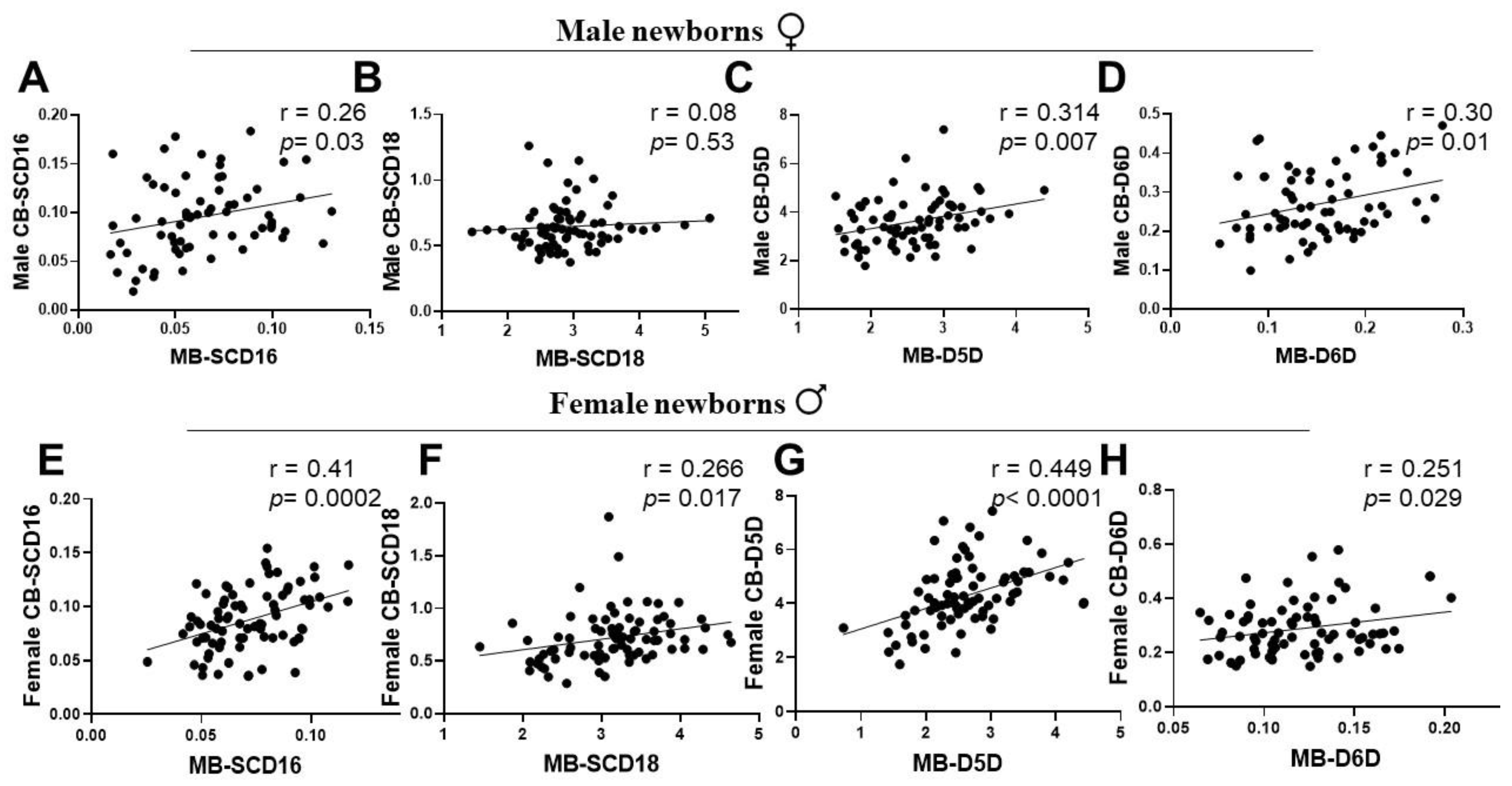
3.4. Associations of Maternal and New-Born’s Sex-Specific Cord Blood Desaturation Indices at Birth
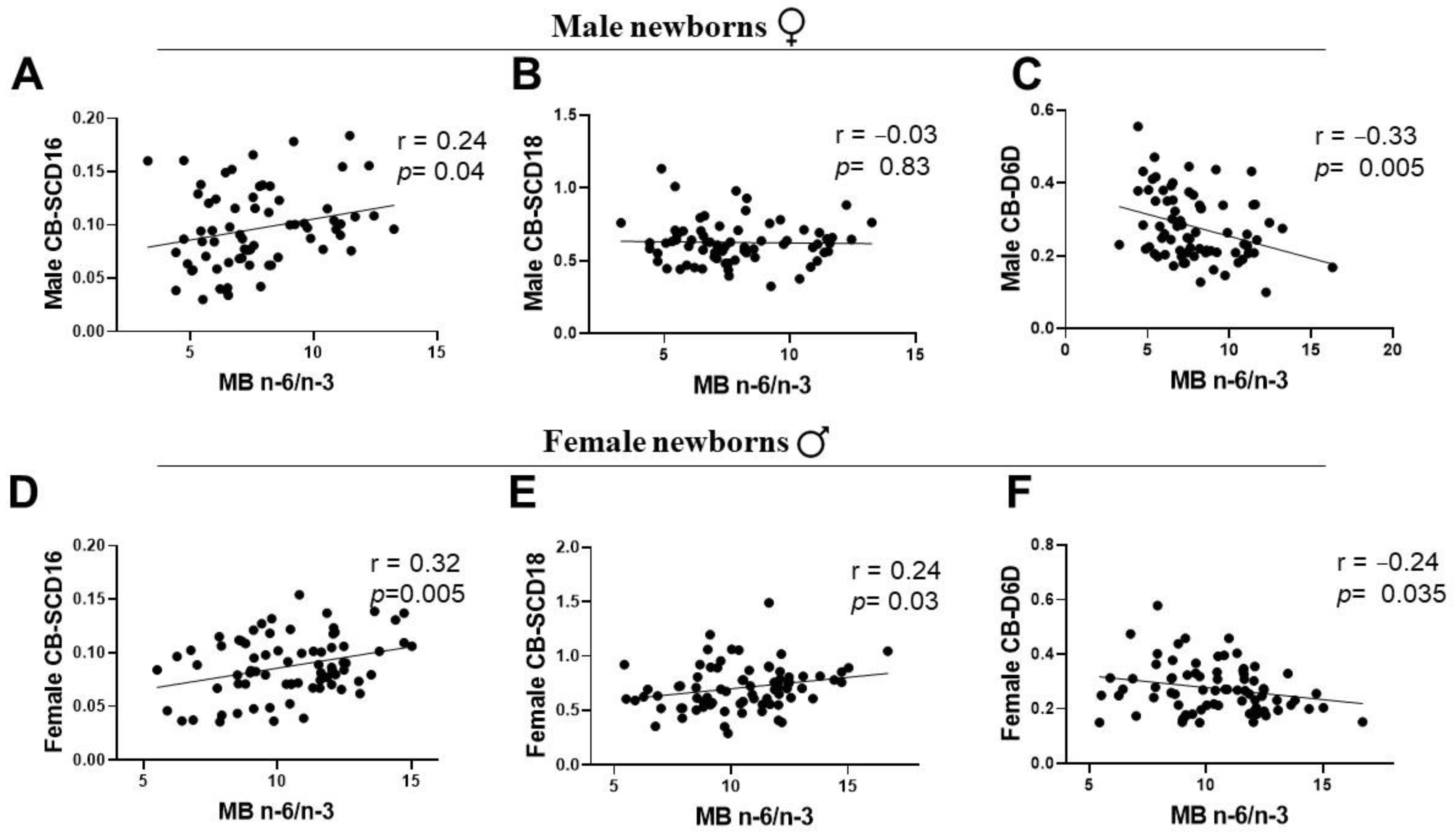
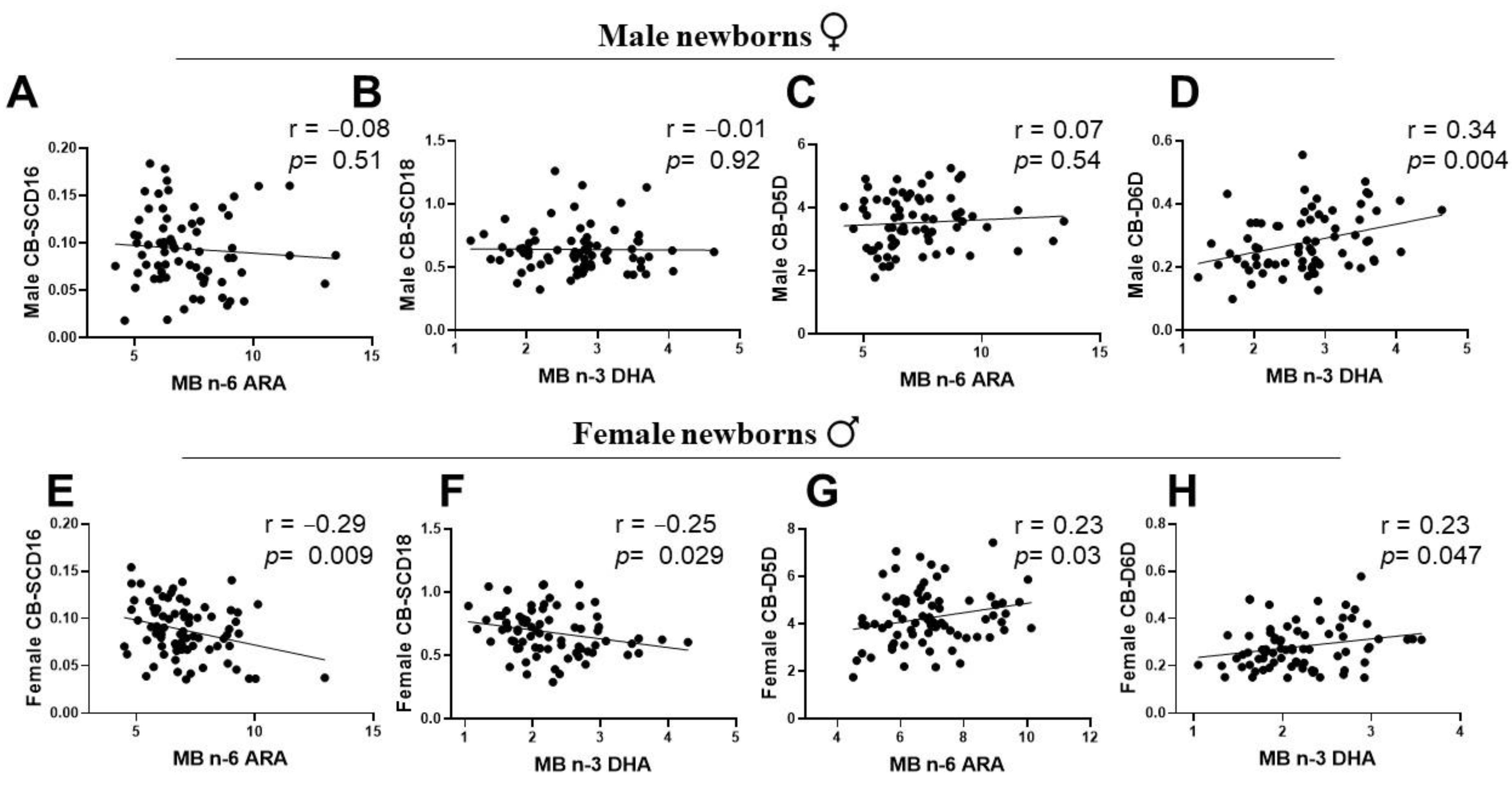
3.5. Associations of Maternal LC-PUFA with the Newborn Gender-Related Cord Blood Desaturation Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Godfrey, K.M. The Role of the Placenta in Fetal Programming—A Review. Placenta 2002, 23, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, N.; Patil, V.; Joshi, S. Maternal long chain polyunsaturated fatty acid status and pregnancy complications. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehra, D.; Le, H.D.; Fallon, E.M.; Carlson, S.J.; Woods, D.; White, Y.A.; Pan, A.H.; Guo, L.; Rodig, S.J.; Tilly, J.L.; et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell 2012, 11, 1046–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akerele, O.A.; Cheema, S.K. A balance of omega-3 and omega-6 polyunsaturated fatty acids is important in pregnancy. J. Nutr. Intermed. Metab. 2016, 5, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Butruille, L.; Marousez, L.; Pourpe, C.; Oger, F.; Lecoutre, S.; Catheline, D.; Görs, S.; Metges, C.C.; Guinez, C.; Laborie, C.; et al. Maternal high-fat diet during suckling programs visceral adiposity and epigenetic regulation of adipose tissue stearoyl-CoA desaturase-1 in offspring. Int. J. Obes. 2019, 43, 2381–2393. [Google Scholar] [CrossRef]
- Robertson, R.C.; Kaliannan, K.; Strain, C.R.; Ross, R.P.; Stanton, C.; Kang, J.X. Maternal omega-3 fatty acids regulate offspring obesity through persistent modulation of gut microbiota. Microbiome 2018, 6, 95. [Google Scholar] [CrossRef]
- Coletta, J.M.; Bell, S.J.; Roman, A.S. Omega-3 Fatty acids and pregnancy. Rev. Obstet. Gynecol. 2010, 3, 163–171. [Google Scholar]
- Meyer, B.J.; Onyiaodike, C.C.; Brown, E.A.; Jordan, F.; Murray, H.; Nibbs, R.J.; Sattar, N.; Lyall, H.; Nelson, S.M.; Freeman, D.J. Maternal Plasma DHA Levels Increase Prior to 29 Days Post-LH Surge in Women Undergoing Frozen Embryo Transfer: A Prospective, Observational Study of Human Pregnancy. J. Clin. Endocrinol. Metab. 2016, 101, 1745–1753. [Google Scholar] [CrossRef]
- Crawford, M.A.; Costeloe, K.; Ghebremeskel, K.; Phylactos, A.; Skirvin, L.; Stacey, F. Are deficits of arachidonic and docosahexaenoic acids responsible for the neural and vascular complications of preterm babies? Am. J. Clin. Nutr. 1997, 66, 1032S–1041S. [Google Scholar] [CrossRef] [Green Version]
- Haggarty, P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, A.; Larqué, E.; Demmelmair, H.; Acien, M.I.; Faber, F.L.; Parrilla, J.J.; Koletzko, B. Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am. J. Clin. Nutr. 2010, 92, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Yee, J.K.; Mao, C.S.; Ross, M.G.; Lee, W.N.P.; Desai, M.; Toda, A.; Kjos, S.L.; Hicks, R.A.; Patterson, M.E. High oleic/stearic fatty-acid desaturation index in cord plasma from infants of mothers with gestational diabetes. J. Perinatol. 2014, 34, 357–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brass, E.; Hanson, E.; O’Tierney-Ginn, P. Placental oleic acid uptake is lower in male offspring of obese women. Placenta 2013, 34, 503–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kröger, J.; Schulze, M.B. Recent insights into the relation of Δ5 desaturase and Δ6 desaturase activity to the development of type 2 diabetes. Curr. Opin. Lipidol. 2012, 23, 4–10. [Google Scholar] [CrossRef]
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. Annu. Rev. Nutr. 2019, 39, 21–44. [Google Scholar] [CrossRef]
- Popeijus, H.E.; Saris, W.H.M.; Mensink, R.P. Role of stearoyl-CoA desaturases in obesity and the metabolic syndrome. Int. J. Obes. 2008, 32, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of Δ6, Δ5, and Δ9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men. Br. J. Nutr. 2007, 88, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C.; Wootton, S.A. Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Lohner, S.; Fekete, K.; Marosvölgyi, T.; Decsi, T. Gender Differences in the Long-Chain Polyunsaturated Fatty Acid Status: Systematic Review of 51 Publications. Ann. Nutr. Metab. 2013, 62, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.; Slater-Jefferies, J.; Grant, R.; Chung, W.-S.; West, A.; Lillycrop, K.; Hanson, M.; Calder, P. Sex, but not maternal protein or folic acid intake, determines the fatty acid composition of hepatic phospholipids, but not of triacylglycerol, in adult rats. Prostaglandins Leukot. Essent. Fat. Acids 2008, 78, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Extier, A.; Langelier, B.; Perruchot, M.-H.; Guesnet, P.; Van Veldhoven, P.P.; Lavialle, M.; Alessandri, J.-M. Gender affects liver desaturase expression in a rat model of n−3 fatty acid repletion. J. Nutr. Biochem. 2010, 21, 180–187. [Google Scholar] [CrossRef]
- Childs, C.E.; Romeu-Nadal, M.; Burdge, G.C.; Calder, P.C. Gender differences in the n-3 fatty acid content of tissues. Proc. Nutr. Soc. 2008, 67, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Decsi, T.; Kennedy, K. Sex-specific differences in essential fatty acid metabolism. Am. J. Clin. Nutr. 2011, 94, 1914S–1919S. [Google Scholar] [CrossRef] [Green Version]
- Marangoni, F.; Colombo, C.; Martiello, A.; Negri, E.; Galli, C. The fatty acid profiles in a drop of blood from a fingertip correlate with physiological, dietary and lifestyle parameters in volunteers. Prostaglandins Leukot. Essent. Fat. Acids. 2007, 76, 87–92. [Google Scholar] [CrossRef]
- Glaser, C.; Demmelmair, H.; Koletzko, B. High-throughput analysis of fatty acid composition of plasma glycerophospholipids. J. Lipid Res. 2010, 51, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Glaser, C.; Demmelmair, H.; Sausenthaler, S.; Herbarth, O.; Heinrich, J.; Koletzko, B. Fatty Acid Composition of Serum Glycerophospholipids in Children. J. Pediatr. 2010, 157, 826–831.e1. [Google Scholar] [CrossRef]
- Glaser, C.; Rzehak, P.; Demmelmair, H.; Klopp, N.; Heinrich, J.; Koletzko, B. Influence of FADS Polymorphisms on Tracking of Serum Glycerophospholipid Fatty Acid Concentrations and Percentage Composition in Children. PLoS ONE 2011, 6, e21933. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Kawabata, T.; Kagawa, Y.; Shoji, K.; Kimura, F.; Miyazawa, T.; Tatsuta, N.; Arima, T.; Yaegashi, N.; Nakai, K. Associations of umbilical cord fatty acid profiles and desaturase enzyme indices with birth weight for gestational age in Japanese infants. Prostaglandins Leukot. Essent. Fat. Acids 2021, 165, 102233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.W.; Zhu, Q.; Zhang, H.Y. Mineral Nutrition and the Risk of Chronic Diseases: A Mendelian Randomization Study. Nutrients 2019, 11, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellur, G.; Sukhdeo, S.V.; Khan, M.T.; Sharan, K. Maternal high protein-diet programs impairment of offspring’s bone mass through miR-24-1-5p mediated targeting of SMAD5 in osteoblasts. Cell Mol. Life Sci. 2021, 78, 1729–1744. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, T.; Clausen, T. The fetal origins hypothesis: Placental insufficiency and inheritance versus maternal malnutrition in well-nourished populations. Acta Obstet. Gynecol. Scand. 2002, 81, 112–114. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Sex-Specific Placental Responses in Fetal Development. Endocrinology 2015, 156, 3422–3434. [Google Scholar] [CrossRef] [Green Version]
- Alur, P. Sex Differences in Nutrition, Growth, and Metabolism in Preterm Infants. Front. Pediatr. 2019, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Lecoutre, S.; Deracinois, B.; Laborie, C.; Eberlé, D.; Guinez, C.; Panchenko, P.; Lesage, J.; Vieau, D.; Junien, C.; Gabory, A.; et al. Depot- and sex-specific effects of maternal obesity in offspring’s adipose tissue. J. Endocrinol. 2016, 230, 39–53. [Google Scholar] [CrossRef]
- Kabaran, S.; Besler, H.T. Do fatty acids affect fetal programming? J. Health Popul. Nutr. 2015, 33, 14. [Google Scholar] [CrossRef] [Green Version]
- Sedlmeier, E.-M.; Brunner, S.; Much, D.; Pagel, P.; Ulbrich, S.E.; Meyer, H.H.; Amann-Gassner, U.; Hauner, H.; Bader, B.L. Human placental transcriptome shows sexually dimorphic gene expression and responsiveness to maternal dietary n-3 long-chain polyunsaturated fatty acid intervention during pregnancy. BMC Genom. 2014, 15, 941. [Google Scholar] [CrossRef] [Green Version]
- Herrera, E.; Ortega-Senovilla, H. Maternal lipid metabolism during normal pregnancy and its implications to fetal development. J. Clin. Lipidol. 2010, 5, 899–911. [Google Scholar] [CrossRef] [Green Version]
- Kuijper, E.A.; Ket Jc Fau-Caanen, M.R.; Caanen Mr Fau-Lambalk, C.B.; Lambalk, C.B. Reproductive hormone concentrations in pregnancy and neonates: A systematic review. Reprod. Biomed. Online 2013, 27, 33–63. [Google Scholar] [CrossRef] [PubMed]
- Hanebutt, F.L.; Demmelmair, H.; Schiessl, B.; Larque, E.; Koletzko, B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin. Nutr. 2008, 27, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Al, M.D.; van Houwelingen, A.C.; Hornstra, G. Long-chain polyunsaturated fatty acids, pregnancy, and pregnancy outcome. Am. J. Clin. Nutr. 2000, 71, 285s–291s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, C.; Heinrich, J.; Koletzko, B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism 2010, 59, 993–999. [Google Scholar] [CrossRef]
- Conway, M.C.; McSorley, E.M.; Mulhern, M.S.; Strain, J.J.; van Wijngaarden, E.; Yeates, A.J. Influence of fatty acid desaturase (FADS) genotype on maternal and child polyunsaturated fatty acids (PUFA) status and child health outcomes: A systematic review. Nutr. Rev. 2020, 78, 627–646. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005, 25, 317–340. [Google Scholar] [CrossRef]
- Symonds, M.E.; Pope, M.; Sharkey, D.; Budge, H. Adipose tissue and fetal programming. Diabetologia 2012, 55, 1597–1606. [Google Scholar] [CrossRef] [Green Version]
- Pardo, I.M.; Geloneze, B.; Tambascia, M.A.; Pereira, J.L.; Barros Filho, A.A. Leptin as a marker of sexual dimorphism in newborn infants. J. Pediatr. 2004, 80, 305–308. (In Portuguese) [Google Scholar] [CrossRef]
- Yee, J.K.; Mao, C.S.; Hummel, H.S.; Lim, S.; Sugano, S.; Rehan, V.K.; Xiao, G.; Lee, W.-N.P. Compartmentalization of stearoyl-coenzyme A desaturase 1 activity in HepG2 cells. J. Lipid Res. 2008, 49, 2124–2134. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef] [Green Version]
- Moon, R.J.; Harvey, N.C.; Robinson, S.M.; Ntani, G.; Davies, J.H.; Inskip, H.M.; Godfrey, K.M.; Dennison, E.M.; Calder, P.C.; Cooper, C.; et al. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J. Clin. Endocrinol. Metab. 2013, 98, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Enninga, E.A.L.; Nevala, W.K.; Creedon, D.J.; Markovic, S.N.; Holtan, S.G. Fetal Sex-Based Differences in Maternal Hormones, Angiogenic Factors, and Immune Mediators during Pregnancy and the Postpartum Period. Am. J. Reprod. Immunol. 2015, 73, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Qaraghouli, M.; Fang, Y.M.V. Effect of Fetal Sex on Maternal and Obstetric Outcomes. Front. Pediatr. 2017, 5, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, C.; Hobel, C. Fetal sex differences in maternal corticotrophin releasing hormone levels during pregnancy for term and preterm deliveries. Am. J. Obstet. Gynecol. 2006, 195, S157. [Google Scholar] [CrossRef]
- Mani, I.; Dwarkanath, P.; Thomas, T.; Thomas, A.; Kurpad, A.V. Maternal fat and fatty acid intake and birth outcomes in a South Indian population. Int. J. Epidemiol. 2016, 45, 523–531. [Google Scholar] [CrossRef]
- Mani, I.; Kurpad, A.V. Fats & fatty acids in Indian diets: Time for serious introspection. Indian J. Med. Res. 2016, 144, 507–514. [Google Scholar] [CrossRef]

| Characteristics | Values | |
|---|---|---|
| Male Newborns | Female Newborns | |
| Maternal Gestational age (weeks) | 39 ± 0.1 | 39 ± 0.3 |
| Maternal age (years) | 26 ± 4.2 | 26 ± 4.6 |
| Number of new-borns, n = 153 | n = 73 | n = 80 |
| Birth weight (kg) | 3.04 ± 0.38 | 3.05 ± 0.36 |
| Delivery method, n | ||
| Spontaneous labor | 48 | 55 |
| Elective caesarean | 25 | 25 |
| Vegetarians | 54 | 59 |
| Non-habitual consumer of n-3 PUFA | 19 | 21 |
| Newborn Sex | Males | Females | ||
|---|---|---|---|---|
| FA (%) | MB | CB | MB | CB |
| SFA | ||||
| C14(MA) | 2.53 ± 1.54 | 3.39 ± 0.32 * | 1.16 ± 0.49 | 0.75 ± 0.33 # |
| C16(PA) | 32.10 ± 2.17 | 29.1 ± 3.7 * | 33.39 ± 2.1 | 31.84 ±2.23 # |
| C18(SA) | 7.797 ± 1.4 | 12.39 ±1.99 * | 7.49 ± 1.19 | 12.93 ± 1.98 # |
| MUFA | ||||
| C16:1n7(POA) | 2.17 ± 1.01 | 2.82 ± 1.39 * | 2.41 ± 0.71 | 2.78 ± 0.9 # |
| C18:1n9(OA) | 22.3 ± 3.1 | 7.81 ± 1.82 * | 22.96 ± 3.4 | 8.92 ± 1.81 # |
| PUFA n-6 family | ||||
| C18:2n6(LA) | 18.44 ± 3.99 | 15.32 ± 3.1 * | 19.59 ± 2.56 | 15.57 ± 2.61 # |
| C20:3n6(DHGLA) | 2.91 ± 0.81 | 4.05 ± 0.83 * | 2.86 ± 0.97 | 4.25 ± 1.2 # |
| C20:4n6(ARA) | 7.21 ± 1.85 | 14.23± 3.17 *$ | 6.99 ± 1.54 | 16.96 ± 2.54 #$ |
| PUFA n-3 family | ||||
| C20:5n3(EPA) | 0.26 ± 0.18 | 1.296 ± 1.11 * | 0.01 ± 0.005 | 0.23 ± 0.06 # |
| C22:5n3(DPA) | 0.88 ± 0.34 | 1.36 ± 0.78 * | 0.69 ± 0.39 | 0.84 ± 0.29 # |
| C22:6n3(DHA) | 2.75 ± 0.785 | 4.75 ± 1.40 * | 2.25 ± 0.65 | 4.43 ± 1.25 # |
| ∑SFA | 42.43 ± 2.69 | 46.83 ± 4.14 * | 42.03 ± 2.56 | 45.53 ± 3.54 # |
| ∑MUFA | 24.47 ± 2.78 | 10.63 ± 2.39 * | 25.37 ± 3.07 | 11.71 ± 1.99 # |
| ∑PUFA | 32.46 ± 2.96 | 41.02 ± 4.14 * | 32.4 ± 2.75 | 42.26 ± 3.21 # |
| ∑n-6 | 28.56 ± 2.83 | 33.60 ± 2.86 * | 29.44 ± 2.34 | 36.77 ± 2.8 # |
| ∑n-3 | 3.90 ± 1.1 | 7.41 ± 2.27 * | 2.95 ± 0.84 | 5.49 ± 1.41 # |
| ∑n-6/n-3 | 7.96 ± 2.5 | 4.94 ± 1.49 * | 10.61 ± 2.46 | 7.17 ± 2.03 # |
| Desaturation Indices = Product/Precursor | ||||
| SCD16 index | 0.07 ± 0.03 | 0.098 ± 0.05 * | 0.072 ± 0.02 | 0.09 ± 0.03 # |
| SCD18 index | 2.93 ± 0.61 | 0.64 ± 0.17 * | 3.14 ± 0.68 | 0.72 ± 0.24 # |
| D5D index | 2.58 ± 0.61 | 3.62 ± 0.99 *$ | 2.59 ± 0.71 | 4.27 ± 1.19 #$ |
| D6D index | 0.18 ± 0.1 | 0.28 ± 0.09 * | 0.12 ± 0.04 | 0.28 ± 0.09 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamadeva, S.G.; Bhattacharyya, N.; Sharan, K. Maternal Plasma Glycerophospholipids LC-PUFA Levels Have a Sex-Specific Association with the Offspring’s Cord Plasma Glycerophospholipids-Fatty Acid Desaturation Indices at Birth. Int. J. Environ. Res. Public Health 2022, 19, 14850. https://doi.org/10.3390/ijerph192214850
Vamadeva SG, Bhattacharyya N, Sharan K. Maternal Plasma Glycerophospholipids LC-PUFA Levels Have a Sex-Specific Association with the Offspring’s Cord Plasma Glycerophospholipids-Fatty Acid Desaturation Indices at Birth. International Journal of Environmental Research and Public Health. 2022; 19(22):14850. https://doi.org/10.3390/ijerph192214850
Chicago/Turabian StyleVamadeva, Sowmya Giriyapura, Nagalakshmi Bhattacharyya, and Kunal Sharan. 2022. "Maternal Plasma Glycerophospholipids LC-PUFA Levels Have a Sex-Specific Association with the Offspring’s Cord Plasma Glycerophospholipids-Fatty Acid Desaturation Indices at Birth" International Journal of Environmental Research and Public Health 19, no. 22: 14850. https://doi.org/10.3390/ijerph192214850
APA StyleVamadeva, S. G., Bhattacharyya, N., & Sharan, K. (2022). Maternal Plasma Glycerophospholipids LC-PUFA Levels Have a Sex-Specific Association with the Offspring’s Cord Plasma Glycerophospholipids-Fatty Acid Desaturation Indices at Birth. International Journal of Environmental Research and Public Health, 19(22), 14850. https://doi.org/10.3390/ijerph192214850








