Prognostic Potential of the Body Composition Indices in Predicting Positive Changes in Resting Blood Pressure after High-Intensity Interval Training in Adolescents
Abstract
1. Introduction
2. Materials and Methods
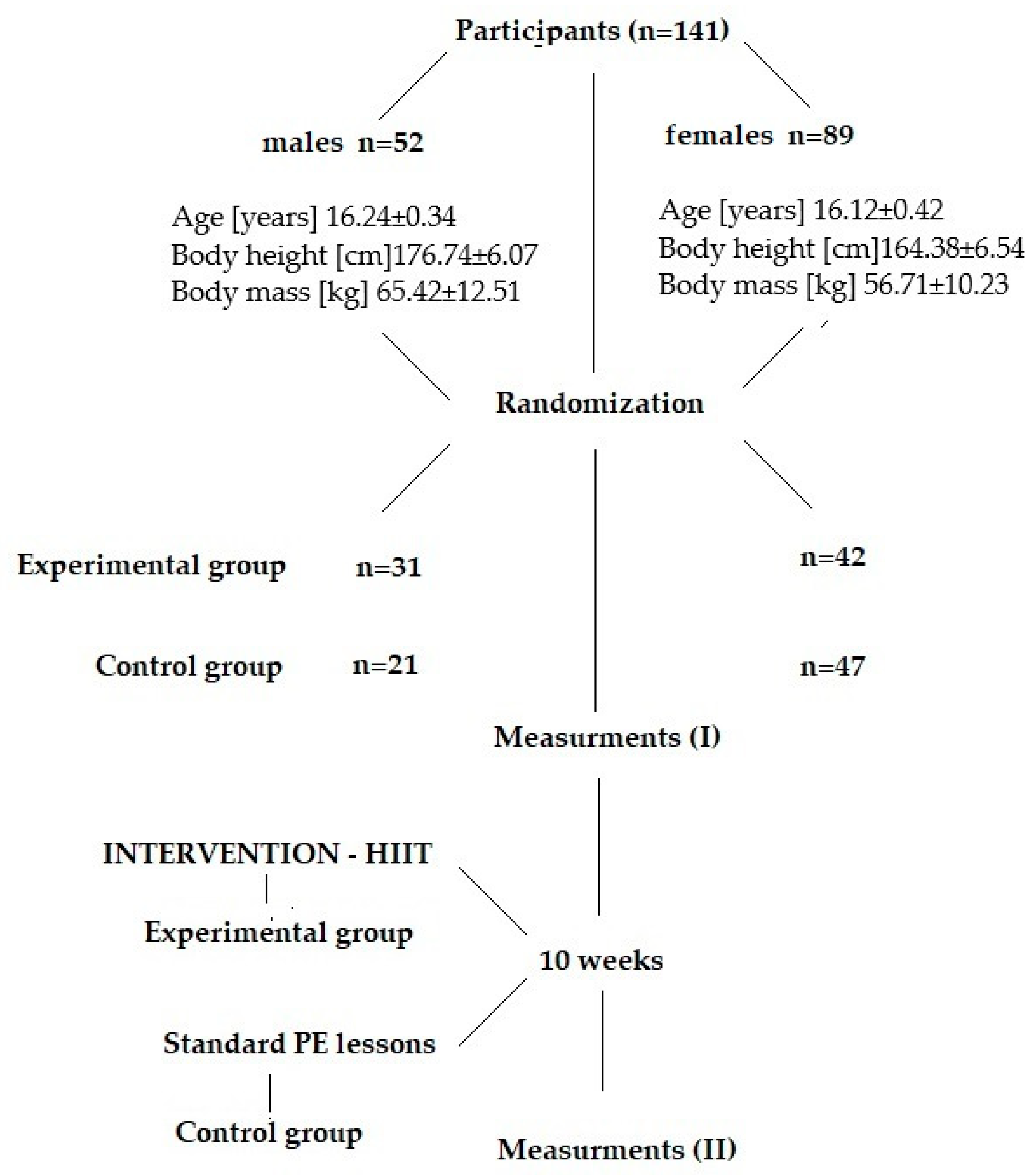
2.1. Participants and Study Design
2.2. Intervention
2.3. Procedures
2.4. Anthropometric and Body Composition Measurements
2.5. Resting Blood Pressure
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Bleyer, A. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Zhou, B.; Bentham, J.; Di Cesare, M.; Bixby, H.; Danaei, G.; Cowan, M.J.; Paciorek, C.J.; Singh, G.; Hajifathalian, K.; Bennett, J.E.; et al. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19· 1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef]
- Kokubo, Y.; Matsumoto, C. Hypertension is a risk factor for several types of heart disease: Review of prospective studies. Adv. Exp. Med. Biol. 2017, 956, 419–426. [Google Scholar] [PubMed]
- Salem, M.M. Pathophysiology of hypertension in renal failure. Semin. Nephrol. 2002, 22, 17–26. [Google Scholar] [CrossRef]
- Cohen, L.; Curhan, G.C.; Forman, J.P. Influence of age on the association between lifestyle factors and risk of hypertension. J. Am. Soc. Hypertens. 2012, 6, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global prevalence of hypertension in children: A systematic review and meta-analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Falkner, B. Hypertension in children and adolescents: Epidemiology and natural history. Pediatr. Nephrol. 2010, 25, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. S5), S213–S256. [Google Scholar] [CrossRef]
- Halbert, J.A.; Silagy, C.A.; Finucane, P.; Withers, R.T.; Hamdorf, P.A.; Andrews, G.R. The effectiveness of exercise training in lowering blood pressure: A meta-analysis of randomised controlled trials of 4 weeks or longer. J. Hum. Hypertens. 1997, 11, 641–649. [Google Scholar] [CrossRef]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U.; Lancet Physical Activity Series Working Group. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2010; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Domaradzki, J.; Koźlenia, D.; Popowczak, M. The Mediation Role of Fatness in Associations between Cardiorespiratory Fitness and Blood Pressure after High-Intensity Interval Training in Adolescents. Int. J. Environ. Res. Public Health 2022, 19, 1698. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Ruiz, J.R.; Castillo, M.J.; Sjöström, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 2008, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dale, C.E.; Fatemifar, G.; Palmer, T.M.; White, J.; Prieto-Merino, D.; Zabaneh, D.; Casas, J.P. Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes, and type 2 diabetes mellitus: A Mendelian randomization analysis. Circulation 2017, 135, 2373–2388. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.A.; Joo, H.J.; Cho, J.Y.; Lee, S.H.; Park, J.H.; Hong, S.J.; Lim, D.S. Visceral fat area and serum adiponectin level predict the development of metabolic syndrome in a community-based asymptomatic population. PLoS ONE 2017, 12, e0169289. [Google Scholar] [CrossRef]
- Shah, R.V.; Murthy, V.L.; Abbasi, S.A.; Blankstein, R.; Kwong, R.Y.; Goldfine, A.B.; Allison, M.A. Visceral adiposity and the risk of metabolic syndrome across body mass index: The MESA Study. JACC Cardiovasc. Imaging 2014, 7, 1221–1235. [Google Scholar] [CrossRef]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Garcia-Hermoso, A.; Prieto-Benavides, D.H.; Correa-Bautista, J.E.; Quino-Ávila, A.C.; Rubio-Barreto, C.M.; Rio-Valle, J.S. Muscle mass to visceral fat ratio is an important predictor of the metabolic syndrome in college students. Br. J. Nutr. 2019, 121, 330–339. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Carrillo, H.A.; González-Ruíz, K.; Vivas, A.; Triana-Reina, H.R.; Martínez-Torres, J.; Prieto-Benavidez, D.H.; Correa-Bautista, J.E.; Ramos-Sepúlveda, J.A.; Villa-González, E.; et al. Fatness mediates the influence of muscular fitness on metabolic syndrome in Colombian collegiate students. PLoS ONE 2017, 12, e0173932. [Google Scholar] [CrossRef]
- Okosun, I.S.; Liao, Y.; Rotimi, C.N.; Choi, S.; Cooper, R.S. Predictive values of waist circumference for dyslipidemia, type 2 diabetes and hypertension in overweight White, Black, and Hispanic American adults. J. Clin. Epidemiol. 2000, 53, 401–408. [Google Scholar] [CrossRef]
- Jackson, A.S.; Stanforth, P.R.; Gagnon, J.; Rankinen, T.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Bouchard, C.; Wilmore, J.H. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int. J. Obes. 2002, 26, 789–796. [Google Scholar] [CrossRef]
- Racil, G.; Ben Ounis, O.; Hammouda, O.; Kallel, A.; Zouhal, H.; Chamari, K.; Amri, M. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur. J. Appl. Physiol. 2013, 113, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; De Schutter, A.; Patel, D.; Artham, S.M.; Milani, R.V. Body composition and coronary heart disease mortality—An obesity or a lean paradox? In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2011; Volume 86, pp. 857–864. [Google Scholar]
- Havenetidis, K.; Paxinos, T.; Kardaris, D.; Bissas, A. Prognostic potential of body composition indices in detecting risk of musculoskeletal injury in army officer cadet profiles. Physician Sportsmed. 2017, 45, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Domaradzki, J.; Rokita, A.; Koźlenia, D.; Popowczak, M. Optimal values of body composition for the lowest risk of failure in tabata training’s effects in adolescents: A pilot study. Biomed Res. Int. 2021, 2021, 6675416. [Google Scholar] [CrossRef]
- Domaradzki, J.; Koźlenia, D. The performance of body mass component indices in detecting risk of musculoskeletal injuries in physically active young men and women. PeerJ 2022, 10, e12745. [Google Scholar] [CrossRef] [PubMed]
- Skrzek, A.; Kozieł, S.; Ignasiak, Z. The optimal value of BMI for the lowest risk of osteoporosis in postmenopausal women aged 40–88 years. Homo Int. Z. Fur Die Vgl. Forsch. Am Menschen 2014, 65, 232–239. [Google Scholar] [CrossRef]
- Koźlenia, D.; Domaradzki, J. Prediction and injury risk based on movement patterns and flexibility in a 6-month prospective study among physically active adults. PeerJ 2021, 9, e11399. [Google Scholar] [CrossRef] [PubMed]
- Koźlenia, D.; Struzik, A.; Domaradzki, J. Force, Power, and Morphology Asymmetries as Injury Risk Factors in Physically Active Men and Women. Symmetry 2022, 14, 787. [Google Scholar] [CrossRef]
- Chase, N.L.; Sui, X.; Lee, D.C.; Blair, S.N. The association of cardiorespiratory fitness and physical activity with incidence of hypertension in men. Am. J. Hypertens. 2009, 22, 417–424. [Google Scholar] [CrossRef]
- Gopinath, B.; Hardy, L.L.; Teber, E.; Mitchell, P. Association between physical activity and blood pressure in prepubertal children. Hypertens. Res. 2011, 34, 851–855. [Google Scholar] [CrossRef]
- Muntaner-Mas, A.; Palou, P. Effects of high intensity interval training (HIIT) intervention amongst school adolescents. J. Phys. Educ. Health-Soc. Perspect. 2017, 6, 19–25. [Google Scholar]
- Fairclough, S.J.; Stratton, G. Effects of a physical education intervention to improve student activity levels. Phys. Educ. Sport Pedagog. 2006, 11, 29–44. [Google Scholar] [CrossRef]
- Bond, B.; Weston, K.L.; Williams, C.A.; Barker, A.R. Perspectives on high-intensity interval exercise for health promotion in children and adolescents. Open Access J. Sport. Med. 2017, 8, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Buchan, D.S.; Young, J.D.; Simpson, A.D.; Thomas, N.E.; Cooper, S.M.; Baker, J.S. The effects of a novel high intensity exercise intervention on established markers of cardiovascular disease and health in Scottish adolescent youth. J. Public Health Res. 2012, 1, 155–157. [Google Scholar] [CrossRef]
- Morales-Palomo, F.; Ramirez-Jimenez, M.; Ortega, J.F.; Pallarés, J.G.; Mora-Rodriguez, R. Acute Hypotension after High-Intensity Interval Exercise in Metabolic Syndrome Patients. Int. J. Sport. Med. 2017, 38, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Costigan, S.A.; Eather, N.; Plotnikoff, R.C.; Taaffe, D.R.; Lubans, D.R. High-intensity interval training for improving health-related fitness in adolescents: A systematic review and meta-analysis. Br. J. Sport. Med. 2015, 49, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Popowczak, M.; Rokita, A.; Koźlenia, D.; Domaradzki, J. The high-intensity interval training introduced in physical education lessons decrease systole in high blood pressure adolescents. Sci. Rep. 2022, 12, 1–7. [Google Scholar]
- Martin-Smith, R.; Cox, A.; Buchan, D.S.; Baker, J.S.; Grace, F.; Sculthorpe, N. High Intensity Interval Training (HIIT) Improves Cardiorespiratory Fitness (CRF) in Healthy, Overweight and Obese Adolescents: A Systematic Review and Meta-Analysis of Controlled Studies. Int. J. Environ. Res. Public Health 2020, 17, 2955. [Google Scholar] [CrossRef]
- Batacan, R.B.; Duncan, M.J.; Dalbo, V.J.; Tucker, P.S.; Fenning, A.S. Effects of high-intensity interval training on cardiometabolic health: A systematic review and meta-analysis of intervention studies. Br. J. Sport. Med. 2017, 51, 494–503. [Google Scholar] [CrossRef]
- Campbell, W.W.; Kraus, W.E.; Powell, K.E.; Haskell, W.L.; Janz, K.F.; Jakicic, J.M.; Troiano, R.P.; Sprow, K.; Torres, A.; Piercy, K.L.; et al. High-Intensity Interval Training for Cardiometabolic Disease Prevention. Med. Sci. Sport. Exerc. 2019, 51, 1220–1226. [Google Scholar] [CrossRef]
- Delgado-Floody, P.; Espinoza-Silva, M.; García-Pinillos, F.; Latorre-Román, P. Effects of 28 weeks of high-intensity interval training during physical education classes on cardiometabolic risk factors in Chilean schoolchildren: A pilot trial. Eur. J. Pediatr. 2018, 177, 1019–1027. [Google Scholar] [CrossRef]
- Popowczak, M.; Rokita, A.; Domaradzki, J. Effects of Tabata training on health-related fitness components among secondary school students. Kinesiology 2022, 54, 221–229. [Google Scholar] [CrossRef]
- Domaradzki, J.; Koźlenia, D.; Popowczak, M. Prevalence of Positive Effects on Body Fat Percentage, Cardiovascular Parameters, and Cardiorespiratory Fitness after 10-Week High-Intensity Interval Training in Adolescents. Biology 2022, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Afyon, Y.A.; Mülazimoğlu, O.; Altun, M. The effect of 6 weekly Tabata training on some physical and motor characteristics on female volleyball players. Eur. J. Phys. Educ. Sport Sci. 2018, 5, 223–229. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Rosenblad, A. A comparison of blood pressure indices as predictors of all-cause mortality among middle-aged men and women during 701,707 person-years of follow-up. J. Hum. Hypertens. 2018, 32, 660–667. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Mosley, W.J., 2nd; Greenland, P.; Garside, D.B.; Lloyd-Jones, D.M. Predictive utility of pulse pressure and other blood pressure measures for cardiovascular outcomes. Hypertension 2007, 49, 1256–1264. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Krzepota, J.; Zwierko, T.; Puchalska-Niedbał, L.; Markiewicz, M.; Florkiewicz, B.; Lubiński, W. The Efficiency of a Visual Skills Training Program on Visual Search Performance. J. Hum. Kinet. 2015, 46, 231–240. [Google Scholar] [CrossRef]
- Zwierko, T.; Głowacki, T.; Osiński, W. The effect of specific anaerobic exercises on peripheral perception in handball players. Kinesiol. Slov. 2008, 14, 68–76. [Google Scholar]
- Blackwell, J.; Doleman, B.; Herrod, P.; Ricketts, S.; Phillips, B.E.; Lund, J.N.; Williams, J.P. Short-Term (<8 wk) High-Intensity Interval Training in Diseased Cohorts. Med. Sci. Sport. Exerc. 2018, 50, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Alkhawam, H.; Madanieh, R.; Shah, N.; Kosmas, C.E.; Vittorio, T.J. Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J. Cardiol. 2017, 9, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Olea, M.A.; Mancilla, R.; Martínez, S.; Díaz, E. Effects of high intensity interval training on blood pressure in hypertensive subjects. Rev. Med. De Chile 2017, 145, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Tjønna, A.E.; Stølen, T.O.; Bye, A.; Volden, M.; Slørdahl, S.A.; Ødegård, R.; Wisløff, U. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin. Sci. 2009, 116, 317–326. [Google Scholar] [CrossRef]
- Farah, B.Q.; Ritti-Dias, R.M.; Balagopal, P.; Hill, J.O.; Prado, W.L. Does exercise intensity affect blood pressure and heart rate in obese adolescents? A 6-month multidisciplinary randomized intervention study. Pediatr. Obes. 2014, 9, 111–120. [Google Scholar] [CrossRef]
- Corte de Araujo, A.C.; Roschel, H.; Picanço, A.R.; do Prado, D.M.L.; Villares, S.M.F.; de Sa Pinto, A.L.; Gualano, B. Similar health benefits of endurance and high-intensity interval training in obese children. PLoS ONE 2012, 7, e42747. [Google Scholar] [CrossRef]
- Koubaa, A.; Trabelsi, H.; Masmoudi, L.; Elloumi, M.; Sahnoun, Z.; Zeghal, K.M.; Hakim, A. Effect of intermittent and continuous training on body composition cardiorespiratory fitness and lipid profile in obese adolescents. IOSR-JPBS 2013, 3, 31–37. [Google Scholar] [CrossRef]
- Baquet, G.; Berthoin, S.; Gerbeaux, M.; Van Praagh, E. High-intensity aerobic training during a 10 week one-hour physical education cycle: Effects on physical fitness of adolescents aged 11 to 16. Int. J. Sports Med. 2001, 22, 295–300. [Google Scholar] [CrossRef]
- Ouerghi, N.; Fradj, M.K.B.; Bezrati, I.; Khammassi, M.; Feki, M.; Kaabachi, N.; Bouassida, A. Effects of high-intensity interval training on body composition, aerobic and anaerobic performance and plasma lipids in overweight/obese and normal-weight young men. Biol. Sport 2017, 34, 385–392. [Google Scholar] [CrossRef]
- Mazurek, K.; Zmijewski, P.; Krawczyk, K.; Czajkowska, A.; Kęska, A.; Kapuściński, P.; Mazurek, T. High intensity interval and moderate continuous cycle training in a physical education programme improves health-related fitness in young females. Biol. Sport 2016, 33, 139–144. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Tosato, M.; Martone, A.M.; Ortolani, E.; Sisto, A.; D’Angelo, E.; Serafini, E.; Desideri, G.; et al. Body Mass Index is Strongly Associated with Hypertension: Results from the Longevity Check-up 7+ Study. Nutrients 2018, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, R.; Zheng, Q.; Yan, X.; Wu, S.; Chen, Y. Impact of body mass index on long-term blood pressure variability: A cross-sectional study in a cohort of Chinese adults. BMC Public Health 2018, 18, 1193. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.S.; Asuzu, M.C.; Mufunda, J.; Forrester, T.; Wilks, R.; Luke, A.; Cooper, R.S. Relationship between blood pressure and body mass index in lean populations. Hypertension 1997, 30, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Collazo-Clavell, M.L.; Korinek, J.; Allison, T.G.; Batsis, J.A.; Sert-Kuniyoshi, F.H.; Lopez-Jimenez, F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int. J. Obes. 2008, 32, 959–966. [Google Scholar] [CrossRef]
- Bintvihok, W.; Chaikittisilpa, S.; Panyakamlerd, K.; Jaisamrarn, U.; Taechakraichana, N. Cut-off value of body fat in association with metabolic syndrome in Thai peri- and postmenopausal women. Climacteri J. Int. Menopause Soc. 2013, 16, 393–397. [Google Scholar] [CrossRef]
- Wang, H.; Necheles, J.; Carnethon, M.; Wang, B.; Li, Z.; Wang, L.; Liu, X.; Yang, J.; Tang, G.; Xing, H.; et al. Adiposity measures and blood pressure in Chinese children and adolescents. Arch. Dis. Child. 2008, 93, 738–744. [Google Scholar] [CrossRef]
- Korhonen, P.E.; Mikkola, T.; Kautiainen, H.; Eriksson, J.G. Both lean and fat body mass associate with blood pressure. Eur. J. Intern. Med. 2021, 91, 40–44. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, S.R. Relation of body mass index, fat mass index and fat-free mass index to blood pressure in children aged 7–12 in Shandong, China. Ann. Hum. Biol. 2011, 38, 313–316. [Google Scholar] [CrossRef]

| EG | CG | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | p | ||||
| males | BMI [kg/m2] | 20.90 | 19.46 | 22.33 | 20.89 | 19.54 | 22.23 | 0.992 |
| FMI [body fat kg/m2] | 3.51 | 2.62 | 4.41 | 3.24 | 2.29 | 4.20 | 0.681 | |
| SMI [muscle kg/m2] | 7.39 | 6.80 | 7.97 | 8.15 | 7.27 | 9.03 | 0.127 | |
| MFR [muscle kg/fat kg] | 2.92 | 2.29 | 3.56 | 3.13 | 2.50 | 3.75 | 0.654 | |
| ΔSBP [mm/Hg] | −8.19 | −11.41 | −4.98 | 0.48 | −1.42 | 2.37 | 0.000 * | |
| ΔDBP [mm/Hg] | −1.71 | −4.45 | 1.03 | −1.29 | −3.77 | 1.20 | 0.825 | |
| ΔPP [mm/Hg] | −6.48 | −10.25 | −2.72 | 1.76 | −1.63 | 5.15 | 0.003 * | |
| ΔMBP [mm/Hg] | −4.95 | −7.27 | −2.63 | −0.40 | −1.82 | 1.01 | 0.004 * | |
| ΔMAP [mm/Hg] | −3.85 | −6.16 | −1.54 | −0.70 | −2.36 | 0.96 | 0.044 * | |
| females | BMI [kg/m2] | 20.57 | 19.97 | 21.17 | 21.25 | 20.12 | 22.37 | 0.304 |
| FMI [body fat kg/m2] | 5.55 | 5.11 | 5.98 | 6.30 | 5.54 | 7.06 | 0.097 | |
| SMI [muscle kg/m2] | 9.47 | 8.78 | 10.17 | 9.78 | 9.10 | 10.47 | 0.528 | |
| MFR [muscle kg/fat kg] | 1.80 | 1.62 | 1.97 | 1.77 | 1.54 | 2.00 | 0.865 | |
| ΔSBP [mm/Hg] | −4.93 | −7.49 | −2.36 | 0.87 | −0.57 | 2.31 | 0.000 * | |
| ΔDBP [mm/Hg] | −2.83 | −5.68 | 0.01 | 0.34 | −2.12 | 2.80 | 0.091 | |
| ΔPP [mm/Hg] | −2.10 | −5.10 | 0.91 | 0.53 | −1.68 | 2.74 | 0.153 | |
| ΔMBP [mm/Hg] | −3.88 | −6.14 | −1.63 | 0.61 | −1.08 | 2.29 | 0.002 * | |
| ΔMAP [mm/Hg] | −3.53 | −5.90 | −1.17 | 0.52 | −1.39 | 2.42 | 0.008 * | |
| EG Males | EG Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BCI | CVP | AUC | −95% CI | +95% CI | p | AUC | −95% CI | +95% CI | p |
| SBP | 0.42 | 0.21 | 0.63 | 0.476 | 0.47 | 0.30 | 0.65 | 0.767 | |
| DBP | 0.59 | 0.38 | 0.80 | 0.392 | 0.60 | 0.41 | 0.79 | 0.301 | |
| BMI | PP | 0.46 | 0.23 | 0.69 | 0.712 | 0.42 | 0.24 | 0.59 | 0.366 |
| MBP | 0.49 | 0.27 | 0.71 | 0.922 | 0.60 | 0.40 | 0.80 | 0.349 | |
| MAP | 0.49 | 0.29 | 0.70 | 0.962 | 0.55 | 0.34 | 0.75 | 0.642 | |
| SBP | 0.45 | 0.22 | 0.68 | 0.648 | 0.44 | 0.25 | 0.63 | 0.540 | |
| DBP | 0.61 | 0.40 | 0.81 | 0.305 | 0.59 | 0.39 | 0.78 | 0.384 | |
| FMI | PP | 0.46 | 0.23 | 0.68 | 0.705 | 0.47 | 0.29 | 0.65 | 0.754 |
| MBP | 0.48 | 0.25 | 0.70 | 0.849 | 0.57 | 0.37 | 0.76 | 0.488 | |
| MAP | 0.51 | 0.29 | 0.72 | 0.963 | 0.56 | 0.36 | 0.76 | 0.560 | |
| SBP | 0.82 | 0.65 | 1.00 | 0.000 * | 0.70 | 0.47 | 0.92 | 0.086 | |
| DBP | 0.49 | 0.27 | 0.71 | 0.938 | 0.69 | 0.49 | 0.89 | 0.057 | |
| SMI | PP | 0.57 | 0.34 | 0.80 | 0.578 | 0.42 | 0.24 | 0.59 | 0.342 |
| MBP | 0.69 | 0.47 | 0.91 | 0.095 | 0.59 | 0.33 | 0.85 | 0.482 | |
| MAP | 0.60 | 0.35 | 0.85 | 0.425 | 0.71 | 0.51 | 0.91 | 0.040 * | |
| SBP | 0.70 | 0.51 | 0.89 | 0.039 * | 0.72 | 0.52 | 0.93 | 0.035 * | |
| DBP | 0.41 | 0.20 | 0.61 | 0.371 | 0.62 | 0.43 | 0.81 | 0.220 | |
| MFR | PP | 0.62 | 0.39 | 0.85 | 0.304 | 0.44 | 0.27 | 0.62 | 0.516 |
| MBP | 0.64 | 0.42 | 0.85 | 0.218 | 0.60 | 0.36 | 0.83 | 0.419 | |
| MAP | 0.59 | 0.37 | 0.80 | 0.441 | 0.68 | 0.48 | 0.88 | 0.082 | |
| CG males | CG females | ||||||||
| SBP | 0.63 | 0.40 | 0.87 | 0.268 | 0.42 | 0.26 | 0.59 | 0.355 | |
| DBP | 0.38 | 0.13 | 0.62 | 0.314 | 0.51 | 0.33 | 0.70 | 0.903 | |
| BMI | PP | 0.69 | 0.46 | 0.93 | 0.102 | 0.42 | 0.26 | 0.59 | 0.348 |
| MBP | 0.60 | 0.35 | 0.85 | 0.435 | 0.42 | 0.25 | 0.59 | 0.343 | |
| MAP | 0.55 | 0.30 | 0.81 | 0.677 | 0.45 | 0.28 | 0.62 | 0.554 | |
| SBP | 0.64 | 0.40 | 0.89 | 0.247 | 0.43 | 0.27 | 0.60 | 0.442 | |
| DBP | 0.51 | 0.25 | 0.77 | 0.943 | 0.54 | 0.36 | 0.71 | 0.688 | |
| FMI | PP | 0.68 | 0.44 | 0.92 | 0.150 | 0.44 | 0.27 | 0.60 | 0.462 |
| MBP | 0.71 | 0.47 | 0.94 | 0.082 | 0.46 | 0.29 | 0.63 | 0.621 | |
| MAP | 0.68 | 0.44 | 0.93 | 0.145 | 0.50 | 0.33 | 0.67 | 0.983 | |
| SBP | 0.57 | 0.30 | 0.83 | 0.616 | 0.55 | 0.38 | 0.73 | 0.563 | |
| DBP | 0.53 | 0.26 | 0.79 | 0.832 | 0.55 | 0.39 | 0.72 | 0.527 | |
| SMI | PP | 0.48 | 0.22 | 0.74 | 0.889 | 0.39 | 0.23 | 0.56 | 0.211 |
| MBP | 0.47 | 0.21 | 0.73 | 0.837 | 0.62 | 0.46 | 0.78 | 0.137 | |
| MAP | 0.47 | 0.20 | 0.74 | 0.843 | 0.59 | 0.43 | 0.76 | 0.271 | |
| SBP | 0.35 | 0.11 | 0.58 | 0.199 | 0.53 | 0.36 | 0.70 | 0.755 | |
| DBP | 0.52 | 0.26 | 0.78 | 0.884 | 0.51 | 0.34 | 0.69 | 0.881 | |
| MFR | PP | 0.38 | 0.13 | 0.62 | 0.335 | 0.51 | 0.33 | 0.67 | 0.999 |
| MBP | 0.31 | 0.07 | 0.55 | 0.118 | 0.62 | 0.45 | 0.78 | 0.163 | |
| MAP | 0.33 | 0.09 | 0.57 | 0.161 | 0.56 | 0.40 | 0.73 | 0.445 |
| SBP | DBP | PP | MBP | MAP | |
|---|---|---|---|---|---|
| Pair-Wise Comparison | EG Males | ||||
| BMI–FMI | 0.957 | 0.574 | 1.000 | 0.273 | 0.481 |
| BMI–SMI | 0.041 * | 0.342 | 0.231 | 0.219 | 0.431 |
| BMI–MFR | 0.134 | 0.244 | 0.167 | 0.189 | 0.315 |
| FMI–SMI | 0.014 * | 0.438 | 0.173 | 0.044 * | 0.217 |
| FMI–MFR | 0.134 | 0.356 | 0.168 | 0.084 | 0.211 |
| SMI–MFR | 0.823 | 0.550 | 0.486 | 0.669 | 0.611 |
| EG females | |||||
| BMI–FMI | 0.599 | 0.831 | 0.427 | 0.632 | 0.833 |
| BMI–SMI | 0.107 | 0.545 | 0.979 | 0.985 | 0.315 |
| BMI–MFR | 0.139 | 0.907 | 0.887 | 1.000 | 0.501 |
| FMI–SMI | 0.050 * | 0.409 | 0.643 | 0.873 | 0.282 |
| FMI–MFR | 0.107 | 0.848 | 0.860 | 0.888 | 0.523 |
| SMI–MFR | 0.791 | 0.455 | 0.755 | 0.974 | 0.729 |
| CG males | |||||
| BMI–FMI | 0.917 | 0.274 | 0.829 | 0.246 | 0.180 |
| BMI–SMI | 0.287 | 0.433 | 0.248 | 0.524 | 0.688 |
| BMI–MFR | 0.206 | 0.543 | 0.178 | 0.219 | 0.343 |
| FMI–SMI | 0.186 | 0.886 | 0.215 | 0.139 | 0.212 |
| FMI–MFR | 0.203 | 0.969 | 0.212 | 0.082 | 0.141 |
| SMI–MFR | 0.588 | 0.955 | 0.526 | 0.287 | 0.396 |
| CG females | |||||
| BMI–FMI | 0.731 | 0.473 | 0.657 | 0.326 | 0.205 |
| BMI–SMI | 0.838 | 0.755 | 0.831 | 0.092 | 0.243 |
| BMI–MFR | 0.509 | 0.991 | 0.619 | 0.208 | 0.468 |
| FMI–SMI | 0.920 | 0.899 | 0.732 | 0.200 | 0.466 |
| FMI–MFR | 0.574 | 0.896 | 0.703 | 0.328 | 0.686 |
| SMI–MFR | 0.293 | 0.607 | 0.162 | 0.941 | 0.719 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domaradzki, J.; Koźlenia, D.; Popowczak, M. Prognostic Potential of the Body Composition Indices in Predicting Positive Changes in Resting Blood Pressure after High-Intensity Interval Training in Adolescents. Int. J. Environ. Res. Public Health 2022, 19, 14658. https://doi.org/10.3390/ijerph192214658
Domaradzki J, Koźlenia D, Popowczak M. Prognostic Potential of the Body Composition Indices in Predicting Positive Changes in Resting Blood Pressure after High-Intensity Interval Training in Adolescents. International Journal of Environmental Research and Public Health. 2022; 19(22):14658. https://doi.org/10.3390/ijerph192214658
Chicago/Turabian StyleDomaradzki, Jarosław, Dawid Koźlenia, and Marek Popowczak. 2022. "Prognostic Potential of the Body Composition Indices in Predicting Positive Changes in Resting Blood Pressure after High-Intensity Interval Training in Adolescents" International Journal of Environmental Research and Public Health 19, no. 22: 14658. https://doi.org/10.3390/ijerph192214658
APA StyleDomaradzki, J., Koźlenia, D., & Popowczak, M. (2022). Prognostic Potential of the Body Composition Indices in Predicting Positive Changes in Resting Blood Pressure after High-Intensity Interval Training in Adolescents. International Journal of Environmental Research and Public Health, 19(22), 14658. https://doi.org/10.3390/ijerph192214658








