Abstract
Saliva is a useful biomarker for diagnosing oral health conditions, including periodontal disease (PD). Smoking is a risk factor for PD. The aim of this systematic review was to summarize the salivary biomarkers associated with PD based on smoking status. A comprehensive search of the MEDLINE (via PubMed), EMBASE, Cochrane, SCOPUS, and Web of Sciences databases was conducted up to 1 January 2021 using key terms relevant to the topic of our research and Cochrane methodology and improved with searching a gray literature resource. The methodological quality of all included studies was assessed with the revised Quality Assessment of Diagnostic Accuracy Studies-2. Seven studies were included. Smokers had increased levels of malondialdehyde, sialic acid, salivary cortisol, salivary interleukin 1β, albumin, tissue inhibitor of matrix metalloproteinase (TIMP), and the pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP), as well as decreased levels of superoxide dismutase, activity of lactate dehydrogenase, activity of enzyme activity of β-glucuronidase, uric acid, matrix metalloproteinase-8 (MMP-8)/TIMP-1 ratio, and combinations of MMP-8 and ICTP. However, mixed results were observed some studies in detecting glutathione peroxidase, MMP-8, and MMP-14. The results were interpreted with caution because of limitations in the number of included studies and the study design. Some salivary biomarkers are potentially useful in combination or alone for diagnosing PD. Methodological and systematic studies are needed to develop more effective biomarkers.
1. Introduction
Periodontal disease (PD) is one of the most common inflammatory diseases of the oral cavity, and it affects up to 90% of the global population [1]. It is caused by inflammation of the surrounding structures of teeth, such as the gingiva, periodontal ligament, and bone; if not treated properly, it can lead to tooth loss and contribute to systemic inflammation [2].
Since PD often progresses without symptoms, many patients do not receive professional dental care until the periodontal destruction that cannot be treated has progressed [3]. In addition, there is an unmet need for diagnosing PD quickly because a PD diagnosis relies on time-consuming clinical measurements [3]. Saliva is an optimal biological fluid to serve as a point-of-care (POC) diagnostic tool for PD. From this point of view, a POC diagnosis simplifies diagnosis and improves prognosis, and the feasibility of PD diagnostic testing has been reported [4].
Many promising salivary biomarkers associated with PD have been reported [3]. The pathogenesis of periodontitis is related to enzymatic alterations such as malondialdehyde (MDA), sialic acid (SA), lactate dehydrogenase (LDH), cortisol, β-glucuronidase (BetaG), interleukin 1β (IL-1β), antioxidants, oxidative stress, superoxide dismutase (SOD), 8-hydroxydeoxyguanosine, glutathione peroxidase (GPx), and 4-hydroxynonenal [5,6,7,8]. SOD is an antioxidant enzyme that is localized within human periodontal ligaments, and it provides an important defense within gingival fibroblasts against superoxide [9]. However, plasma glutathione peroxidase, a selenium-containing peroxidase, comprises a major group of enzymes that remove the hydrogen peroxide created by SOD in the cell [10]. IL-1β stimulates the expression of matrix metalloproteinases (MMPs), which contribute to bone resorption and tissue destruction [11]. To date, 24 different MMPs have been cloned, and three of them have been found in humans. Based on the substrate to be degraded, they are divided into six types: collagenase, gelatinases (type collagenase), stromelysins, matrilysins, membrane-type metalloproteinases, and others [12]. Among the MMPs, MMP-8 and MMP-9 are in the spotlight as biomarkers for periodontal disease. A kit that can test for MMP-8 in 5 min in an office has been developed [13,14].
PD progression can be influenced by various risk factors such as periodontal pathogens, host factors, anatomical factors, and iatrogenic factors [15]. Among the associated risk factors, smoking is the second-largest risk factor for PD after dental plaque [1]. Reports indicate that the prevalence of periodontitis is 3–6 times higher in smokers than in non-smokers, and the increased risk is proportional to the duration of smoking and smoking rate [16,17]. Smokers exhibit more pronounced PD clinical findings than non-smokers, such as deeper pockets, more extensive and severe loss of attachment, higher levels of bone destruction, and higher rates of tooth loss [18,19,20]. In addition, smoking negatively affects successful implant placement and non-surgical and surgical treatment [21].
Meanwhile, saliva contains a unique and complex variety of enzymes and proteins with important oral functions [5]. The use of these enzymes for diagnosing PD has unfortunately been hindered because the relevance of protein and enzymes in saliva and disease etiology remain limited. Furthermore, enzymatic alterations can be caused by various factors such as temperature, pH, enzyme substrates, and the effect of inhibitors and activators [22]. In particular, tobacco compounds the damage activities of salivary enzymes at the molecular level [23]. However, saliva samples are non-invasive, readily available, and inexpensive; therefore, saliva can be a valid alternative to blood as a biomarker [24,25]. Saliva is a favorable oral fluid to determine the health state of the oral cavity, including the presence of PD [26,27]. Therefore, an effective and reproducible salivary biomarker would be preferred over other biomarkers. The aim of this study was to evaluate the evidence, using a systematic review, and to highlight the future directions regarding the diagnostic potential of salivary biomarkers associated with PD based on smoking status.
2. Materials and Methods
2.1. Study Design
This study was a systematic literature review that synthesized 20 years of research by analyzing PD-related salivary biomarker factors associated with smoking status. We conducted this systematic review per the standard method of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [28] and according to the PRISMA 2020 checklist (Supplementary Material) [29].
2.2. Literature Search and Selection
The review questions were formulated in the population, intervention, comparison, outcome, and type of study design (PICOT) format [30] (Table 1). The search strategy consisted of three main concepts: target condition (PD), type of oral sample analyzed (saliva), and index tests (salivary biomarkers). Plain text words (including synonyms or plural forms) and controlled vocabulary of concept (e.g., Medical Subject Headings terms) were combined and used for searches in the title and abstract fields for each database.

Table 1.
Formulated research questions for the systematic review.
The electronic database search was conducted in the MEDLINE (via PubMed), EMBASE, SCOPUS, and Web of Sciences databases. The search was restricted by the following specification: English-language literature published in peer-reviewed journals from January 2000 to January 2021. The complete search strategy is available in Supplementary Materials Table S1. The target conditions were PD, based on the 2017 classification of periodontal and peri-implant diseases and conditions published by the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP), irrespective of the severity or extent of the illness [31,32].
The detailed selection process is presented in Figure 1. The initial database search returned 3702 articles; 2056 duplicates were removed. Among the remaining 1646 articles, 1426 articles were excluded in the abstract evaluation phase. A full-text evaluation was conducted for the 220 retained articles; 213 articles were excluded, based on the criteria for selection (e.g., the articles did not report smoking status-specific values). Finally, seven articles were included for the systematic review.
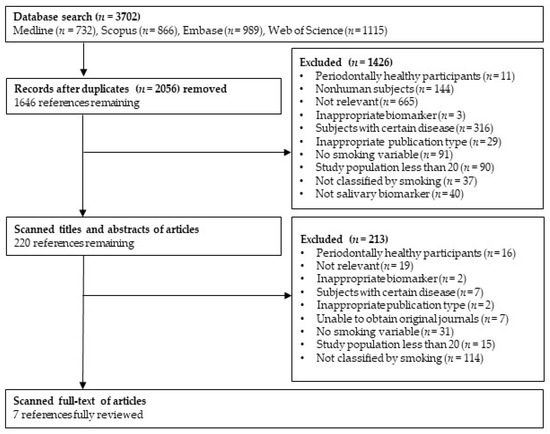
Figure 1.
Flow diagram of the literature search strategy.
The seven studies used for the data analysis were coded based on the year of publication, country, study design, case sample size, age, definition of PD, smoking status, and type of salivary biomarkers for the subgroup analysis on the major categorical variables. After categorizing these factors, the related factors of the group and main conclusions were coded. For effect size, the sign, value, and correlation effect size were coded.
2.3. Study Selection and Exclusion Criteria
We included studies if (1) they included individuals with clinical PD but without explicit systemic disease; (2) they provided individual level, smoking status-specific results of at least 20 individuals; (3) they were prospective studies; and (4) they were written in English and published in a peer-reviewed journal.
We excluded studies if any of the following criteria were present: (1) they explicitly stated that they included individuals with systemic disease, conditions, or syndromes; (2) they were retrospective, prognostic, or predictive accuracy studies; (3) they were published in non-English language literature; (4) they involved non-human subjects; (5) they reported the data of fewer than 20 individuals for one smoking status; (6) they were published in theses, dissertations, reviews, letters, personal opinions, book chapters, short communications, conference abstracts, and patents; and (7) they did not report the smoking status-specific results of salivary biomarkers. In cases of overlapping articles of the same study cohorts, only articles with the largest number of participants were included.
3. Results
The systematic literature review yielded a total of seven papers for analysis. Of these, 213 articles were excluded because smoking status was not reported [9,13,33] or because they entailed a topic unrelated to the study question [18,34].
3.1. Risk of Bias Assessment
The quality assessment of all the included studies was independently reviewed by all authors by using a modified version of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool [35]. Salivary samples can be preserved for several years with little damage and degradation of the salivary molecules [36,37,38]; therefore, the time lag between a reference standard (i.e., clinical measurements) and the index test (e.g., saliva analysis) should not be a high-risk bias. However, standardized storage protocol must be followed completely to conserve the stability of the salivary biomarkers [38,39]. Therefore, we additionally included information on salivary sample storage in the modified version of the QUADAS-2 tool for the assessment of the bias.
In total, 14 salivary protein biomarkers were identified. Each case was described by measurements of the PPD and CAL; however, diverse thresholds and clinical parameters were used for diagnosing PD. The results of our quality assessment with the modified QUADAS-2 tools are summarized in Table 2. Except for two studies [5,40], all seven studies recruited study participants via non-random, convenience-sampling methods. Therefore, the risk of patient selection bias was marked as “high” for five studies. The risk bias of index test was recorded as “unclear” for all seven studies because the blinding assessments for measuring reference tests of interpreting the index test were not mentioned.

Table 2.
QUADAS-2 risk of bias assessment.
3.2. Descriptive Summary of the Studies Included in the Systematic Review
A descriptive summary of the studies included in this systematic review study is presented in Table 3. Most of the selected studies had been published in the previous 10 years and were conducted in three countries: India [5,6,7,40,41], Turkey [8], and Finland [42]. The number of participants with PD ranged from 40 [41] to 100 [5]. Fourteen different types of salivary biomarkers were evaluated. One study [42] reported salivary biomarkers, stand-alone, and combination/ratio, whereas other studies reported the results for only single biomarkers.

Table 3.
Descriptive summary of the included studies in the systematic review.
3.3. Salivary Biomarker Levels Based on Smoking Status
Table 4 summarizes salivary biomarker levels based on smoking status. Compared to non-smokers, smokers had increased levels of MDA [6], SA [6], salivary cortisol, and IL-1β [7] (p < 0.001). The tissue inhibitor of matrix metalloproteinase (TIMP) [42] and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) [42] were higher in smokers than in non-smokers but without statistical significance. In addition, albumin [40] was higher in smokers than in non-smokers, but this was not statistically significant (p > 0.05).

Table 4.
Salivary biomarker levels based on smoking status.
On the other hand, the levels of SOD [6] (p < 0.001) and uric acid (UA) [40] (p < 0.01) using spectrophotometry were significantly higher in non-smokers than in smokers. The levels of activity of LDH [5] and BetaG [5], uric acid (UA) [40], MMP-8/TIMP-1 ratio [42], and combination of MMP-8 and ICTP [42] were higher in non-smokers than in smokers but without statistical significance.
In particular, the salivary biomarkers GPx [6,8] and MMP-8 [41,42] showed conflicting results between smokers and non-smokers when different detection methods were used. These results suggest that further studies are necessary. In addition, the descriptive statistics varied among the studies on MMP-8 using enzyme-linked immunosorbent assay (ELISA), where the level of MMP-14 was higher with APMA than without APMA.
4. Discussion
This systematic review estimated the salivary biomarkers of PD based on smoking status. In our results, seven studies published since 2000 highlighted 14 host-derived salivary biomarkers. Owing to the considerable and growing interest in saliva as a useful tool for biomarker analysis, as highlighted based on our literature search, significant studies have been recently published [3,4,38]. Salivary-derived diagnostic techniques could potentially allow for the timely screening of an entire population for specific diseases [3,33]. However, studies evaluating the effectiveness of salivary biomarkers for diagnosing PD are in their infancy [38].
4.1. Evidence for Salivary Biomarkers Based on Smoking Status
As a result of a systematic literature review on salivary biomarkers for PD diagnosis by smoking status, some markers showed significant differences between smokers and non-smokers. However, certain salivary biomarkers may be potentially useful in combination and alone in the diagnosis of PD, but more systematically robust studies are needed to validate these biomarkers [38]. In our study, higher levels of MDA, SA, salivary cortisol, IL-1β, TIMP, and ICTP were found in smokers compared to non-smokers with PD. In particular, cortisol and IL-1β levels were higher in smokers than in non-smokers. These results were reported by Zhang et al. [43] and are consistent with the report that salivary cortisol levels were significantly higher in smokers with chronic periodontitis than in non-smokers with chronic periodontitis.
However, non-smokers showed high levels of SOD and UA. In addition, although not significant, they had higher levels of activity of LDH and BetaG, MMP-8/TIMP-1 ratio [40], and combined MMP-8 and ICTP. LDH and BetaG activity were significantly decreased in smokers with periodontitis [44], which is similar to the results found in this systematic review, but our results were not statistically significant [5]. IL-1β and MMP-8 were consistent with the diagnostic value of host-derived salivary biomarkers based on the reported sensitivity and specificity in relation to the clinical parameters of the diagnosis of PD in adults [38]. In addition, research results regarding IL-1β are conflicting. Unlike the finding in the current systematic review, another study [45] demonstrated that IL-1β gene expression was lower in smokers with chronic periodontitis than in non-smokers with chronic periodontitis (p = 0.003). Currently, there is limited evidence confirming the diagnostic power of salivary biomarkers in the clinical evaluation of PD. Nevertheless, findings from several studies, including this one, are of growing importance for salivary biomarkers and may guide larger and more well-controlled studies of diagnostic accuracy. Although not conclusive, IL-1β is reported to be a promising biomarker for future studies [43].
Saliva is an easy and non-invasive diagnostic fluid that is useful for the diagnosis of early periodontitis, and the possibility of early diagnosis of periodontitis in adolescents, especially boys, based on elevated salivary MMP-8 levels has been reported [46]. Smoking may affect the usefulness of salivary biomarker assays and should always be considered when interpreting biomarker results. Smoking is a risk factor influencing the inflammatory response leading to PD. Therefore, attention should be paid to the disturbance caused by smoking in the interpretation of potential salivary diagnostic test results [47]. While MMP-8 was mainly affected by smoking pack-years, salivary MMP-9 and TIMP-1 are reported to be mainly affected in current smokers or those who have quit smoking within the last 1 year [47]. In addition, a meta-analysis by Lin et al. [48] showed that MMP-8 is currently considered one of the most promising biomarkers for the early diagnosis of periodontitis, but conflicting results were found in several studies, including this study. Overall salivary MMP-8 levels were significantly higher in periodontitis patients compared with healthy controls. However, they reported that higher quality studies are still needed to confirm the conclusions due to the heterogeneity of studies and publication bias [49].
4.2. Significance and Limitations of This Review
To the extent we have identified so far, this study is the first systematic review to evaluate salivary biomarkers of PD based on smoking status. To utilize saliva as a reliable diagnostic tool, standardized guidelines for procedures for collecting and processing saliva samples are needed [38].
Unfortunately, one limitation of this review is that the systematic literature review process was not performed according to prospero-based registration and protocol. Future research should be conducted according to prospero-based registration for the systematic review protocol. However, we did use the PRISMA 2020 checklist to review the compliance of the systematic literature review studies [29].
Differences in study design and methods, index tests, and reference tests have a significant impact on study results [38]. Unfortunately, five studies had a relatively small sample size (n < 100) and five had no power calculations [6,8,40,41,42]. In addition, in two studies, the statistical interpretation of the association between PD and salivary biomarkers based on smoking status was limited [5,42]. However, all but one of the seven studies analyzed in our study clearly presented the participant selection and exclusion criteria.
Future studies should employ larger sample sizes and use validated power calculations to overcome these limitations [50]. In addition, advanced statistical approaches should be used to ensure the validity of the study results [51]. A good approach for participant selection would be to distinguish patients with milder cases of gingivitis and periodontitis. This approach would overcome spectrum bias and prevent overestimation of the results [52]. All seven studies analyzed in our study were observational studies; longitudinal randomized trials may provide more reliable results [50,51]. However, studies of salivary biomarkers useful for diagnosing PD according to smoking status may be difficult to follow-up on at a large scale due to specific problems such as disease progression [53,54] and disease susceptibility in older people [55]. To address the limits and utilize large-scale and cost-effective biological information that would be unsuitable for a smaller sample size, further studies will need to collaborate with major networks such as big data surveyed at the national level.
Regarding saliva sample handling, six studies [5,6,7,8,41,42] collected and stored saliva before clinical assessment. The other study did not mention the process of saliva sampling and storage [40]. In addition, no information about the blinding process was provided, nor were there details about whether information was provided to examiners interpreting the biomarker analysis results. The saliva treatment technique and collection method will affect the change in saliva composition within and between individuals. The composition of saliva can change rapidly with flow rate, type of stimulation, and time. The flow of saliva also drops to nearly zero during sleep; therefore, bedtime and snacking are important variables [34,56].
In general, smoking is known to cause nicotine-induced gingival keratinization and vasoconstriction, thereby masking gingiva bleeding in patients with PD and affecting systemic conditions and various clinical indicators [57]. The fact that systemic conditions would be high-risk confounders for assessing the biomarkers of PD, based on smoking status, should also be noted. In addition, we divided the participants who had already been diagnosed with PD into groups (n > 20) based on whether they did or did not smoke. This process caused us to analyze a very limited number of studies.
Further studies will need to consider the association between smoking and salivary biomarkers as a diagnostic medium of PD by comparing three groups: healthy smokers, smokers with PD, and non-smokers with PD. Analyzing the relevance between smoking habits and salivary biomarkers associated with PD by distinguishing between periodontitis and gingivitis is also necessary. In addition, the standard for determining smoking status, which was the most important variable in this systemic review, differed between studies, and all relied on self-reported responses. Future studies should overcome these problems to improve the quality of studies. In a previous study [56], the change in bleeding on probing over time was significantly higher for female non-smokers with catalase levels of >225 µg/mL than for male non-smokers or male smokers. Thus, analyzing differences by sex is necessary. Comparing and analyzing light smokers and heavy smokers with chronic periodontitis is also a good approach.
5. Conclusions
This systematic review summarized evidence regarding effective salivary biomarkers for the diagnosis and monitoring of PD based on smoking status. The levels of cortisol, IL-1β, and MMP-8 were higher in smokers with PD compared to non-smokers. Therefore, in the future, these biomarkers could be used as potential salivary biomarkers for assessing the diagnosis and severity of chronic PD, as well as for helping with the early detection of PD progression. However, in this systematic review, it was confirmed that individual studies had limitations regarding study designs and methods. In future studies, advanced salivary biomarker studies using smoking status should be conducted with well-designed, large-scale, randomized controlled trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192114619/s1, Table S1: Search strategy and PRISMA 2020 checklist.
Author Contributions
Conceptualization, J.-H.J.; methodology, J.-w.N. and J.-H.J.; software, H.-S.Y., K.-B.K., M.-H.H., Y.-J.K. and Y.L.; validation, J.-w.N. and J.-H.J.; formal analysis, H.-e.J. and Y.L.; investigation, H.-S.Y., K.-B.K., M.-H.H. and Y.-J.K.; resources, H.-S.Y., K.-B.K., M.-H.H. and Y.-J.K.; data curation, H.-S.Y., K.-B.K., M.-H.H., and Y.-J.K.; writing—original draft preparation, H.-S.Y., J.-H.J. and M.-H.H.; writing—review and editing, J.-H.J. and J.-w.N.; visualization, J.-H.J.; supervision, J.-w.N. and J.-H.J.; project administration, J.-H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2017R1A2B4012865).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, Y.; Zhou, X.; Cheng, L.; Li, M. The impact of smoking on subgingival microflora: From periodontal health to disease. Front. Microbiol. 2020, 11, 66. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Amerio, E.; Farina, R.; Nart, J.; Ramanauskaite, A.; Renvert, S.; Roccuzzo, A.; Salvi, G.E.; Schwarz, F.; Trombelli, L.; et al. Significance of probing for monitoring peri-implant diseases. Int. J. Oral Implantol. 2021, 14, 385–399. [Google Scholar]
- Srivastava, N.; Nayak, P.A.; Rana, S. Point of care: A novel approach to periodontal diagnosis—A review. J. Clin. Diagn. Res. 2017, 11, ZE01–ZE06. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Telgi, R.L.; Tirth, A.; Tantry, I.Q.; Aleem, A. Lactate dehydrogenase and β-glucuronidase as salivary biochemical markers of periodontitis among smokers and non-smokers. Sultan Qaboos Univ. Med. J. 2018, 18, e318–e323. [Google Scholar] [CrossRef] [PubMed]
- Naresh, C.K.; Rao, S.M.; Shetty, P.R.; Ranganath, V.; Patil, A.S.; Anu, A.J. Salivary antioxidant enzymes and lipid peroxidation product malondialdehyde and sialic acid levels among smokers and nonsmokers with chronic periodontitis—A clinico-biochemical study. J. Fam. Med. Prim. Care. 2019, 8, 2960–2964. [Google Scholar] [CrossRef]
- Bawankar, P.V.; Kolte, A.P.; Kolte, R.A. Evaluation of stress, serum and salivary cortisol, and interleukin-1β levels in smokers and non-smokers with chronic periodontitis. J. Periodontol. 2018, 89, 1061–1068. [Google Scholar] [CrossRef]
- Hendek, M.K.; Erdemir, E.O.; Kisa, U.; Ozcan, G. Effect of initial periodontal therapy on oxidative stress markers in gingival crevicular fluid, saliva, and serum in smokers and non-smokers with chronic periodontitis. J. Periodontol. 2015, 86, 273–282. [Google Scholar] [CrossRef]
- Nazaryan, R.; Kryvenko, L. Salivary oxidative analysis and periodontal status in children with atopy. Interv. Med. Appl. Sci. 2017, 9, 199–203. [Google Scholar] [CrossRef]
- Chafik, A.; Essamadi, A.; Çelik, S.Y.; Solak, K.; Mavi, A. Characterization of an interesting selenium-dependent glutathione peroxidase (Se-GPx) protecting cells against environmental stress: The Camelus dromedarius erythrocytes Se-GPx. Biocatal. Agric. Biotechnol. 2019, 18, 101000. [Google Scholar] [CrossRef]
- Sardarian, A.; Andisheh Tadbir, A.; Zal, F.; Amini, F.; Jafarian, A.; Khademi, F.; Mostafavi-Pour, Z. Altered oxidativestatus and integrin expression incyclosporine A-treated oral epithelialcells. Toxicol. Mech. Methods. 2015, 25, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Jiang, X.; Lin, X.; Huang, H.; Wang, J.; Yao, Q.; Chen, R. Matrix metalloproteinase inspired therapeutic strategies for bone diseases. Curr. Pharm. Biotechnol. 2021, 22, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Lähteenmäki, H.; Tervahartiala, T.; Räisänen, I.T.; Pärnänen, P.; Mauramo, M.; Gupta, S.; Sampson, V.; Rathnayake, N.; Heikkinen, A.M.; Alassiri, S.; et al. Active MMP-8 point-of-care (PoC)/chairside enzyme-test as an adjunctive tool for early and real? time diagnosis of peri? implantitis. Clin. Exp. Dent. Res. 2022, 8, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, N.; Gieselmann, D.R.; Heikkinen, A.M.; Tervahartiala, T.; Sorsa, T. Salivary diagnostics—Point-of-care diagnostics of MMP-8 in dentistry and medicine. Diagnostics 2017, 7, 7. [Google Scholar] [CrossRef]
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S78–S94. [Google Scholar] [CrossRef]
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent epidemiologic trends in periodontitis in the USA. Periodontology 2000 2020, 82, 257–267. [Google Scholar] [CrossRef]
- Smith, M.M.; Knight, E.T.; Al-Harthi, L.; Leichter, J.W. Chronic periodontitis and implant dentistry. Periodontology 2000 2017, 74, 63–73. [Google Scholar] [CrossRef]
- Souto, M.L.S.; Rovai, E.S.; Villar, C.C.; Braga, M.M.; Pannuti, C.M. Effect of smoking cessation on tooth loss: A systematic review with meta-analysis. BMC Oral Health 2019, 19, 245. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Anerud, A.; Dulac, M.; Lulic, M.; Cullinan, M.P.; Seymour, G.J.; Faddy, M.J.; Bürgin, W.; Schätzle, M.; Lang, N.P. Natural history of periodontitis: Disease progression and tooth loss over 40 years. J. Clin. Periodontol. 2017, 44, 1182–1191. [Google Scholar] [CrossRef]
- Arrejaie, A.S.; Al-Aali, K.A.; Alrabiah, M.; Vohra, F.; Mokeem, S.A.; Basunbul, G.; Alrahlah, A.; Abduljabbar, T. Proinflammatory cytokine levels and peri-implant parameters among cigarette smokers, individuals vaping electronic cigarettes, and non-smokers. J. Periodontol. 2019, 90, 367–374. [Google Scholar] [CrossRef]
- Alqahtani, F.; Alqahtani, M.; Shafqat, S.S.; Akram, Z.; Al-Kheraif, A.A.; Javed, F. Efficacy of mechanical debridement with adjunctive probiotic therapy in the treatment of peri-implant mucositis in cigarette-smokers and never-smokers. Clin. Implant Dent. Relat. Res. 2019, 21, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ramesh, V.; Locasale, J.W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet. 2020, 21, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, D.; Jain, S.; Jaiswal, S.; Goyal, R.K. Evaluation of salivary alkaline phosphatase levels in tobacco users to determine its role as a biomarker in oral potentially malignant disorders. Int. J. Health Sci. Res. 2022, 6, 12443–12449. [Google Scholar] [CrossRef]
- Ornelas-González, A.; Ortiz-Martínez, M.; González-González, M.; Rito-Palomares, M. Enzymatic methods for salivary biomarkers detection: Overview and current challenges. Molecules 2021, 26, 7026. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kim, S.J.; Hong, S.; Kim, Y. Diagnosis of Alzheimer’s disease utilizing amyloid and tau as fluid biomarkers. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Bostanci, N.; Mitsakakis, K.; Afacan, B.; Bao, K.; Johannsen, B.; Baumgartner, D.; Müller, L.; Kotolová, H.; Emingil, G.; Karpíšek, M. Validation and verification of predictive salivary biomarkers for oral health. Sci. Rep. 2021, 11, 6406. [Google Scholar] [CrossRef]
- Hall, M.W.; Singh, N.; Ng, K.F.; Lam, D.K.; Goldberg, M.B.; Tenenbaum, H.C.; Neufeld, J.D.; Beiko, G.R.; Senadheera, D.B. Interpersonal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. NPJ Biofilms Microbiomes 2017, 3, 1–7. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; PRISMA-DTA Group. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 89. [Google Scholar] [CrossRef]
- Riva, J.J.; Malik, K.M.; Burnie, S.J.; Endicott, A.R.; Busse, J.W. What is your research question? An introduction to the PICOT format for clinicians. J. Can. Chiropr. Assoc. 2012, 56, 167–171. [Google Scholar]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S219–S229. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89 (Suppl. S1), S313–S318. [Google Scholar] [CrossRef] [PubMed]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Blanco-Pintos, T.; Alonso-Sampedro, M.; Relvas, M.; González-Peteiro, M.M.; Balsa-Castro, C.; Tomás, I. Diagnostic accuracy of IL1β in saliva: The development of predictive models for estimating the probability of the occurrence of periodontitis in non-smokers and smokers. J. Clin. Periodontol. 2020, 47, 702–714. [Google Scholar] [CrossRef]
- Chang, C.H.; Han, M.L.; Teng, N.C.; Lee, C.Y.; Huang, W.T.; Lin, C.T.; Huang, Y.K. Cigarette smoking aggravates the activity of periodontal disease by disrupting redox homeostasis-An observational study. Sci. Rep. 2018, 8, 11055. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Shakeeb, N.; Varkey, P.; Ajit, A. Human saliva as a diagnostic specimen for early detection of inflammatory biomarkers by real-time RT-PCR. Inflammation 2021, 44, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Ghizoni, J.S.; Nichele, R.; De Oliveira, M.T.; Pamato, S.; Pereira, J.R. The utilization of saliva as an early diagnostic tool for oral cancer: microRNA as a biomarker. Clin. Transl. Oncol. 2020, 22, 804–812. [Google Scholar] [CrossRef]
- Kc, S.; Wang, X.Z.; Gallagher, J.E. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: Systematic review. J. Clin. Periodontol. 2020, 47, 289–308. [Google Scholar] [CrossRef]
- Tomita, A.; Mori, M.; Hiwatari, K.; Yamaguchi, E.; Itoi, T.; Sunamura, M.; Soga, T.; Tomita, M.; Sugimoto, M. Effect of storage conditions on salivary polyamines quantified via liquid chromatography-mass spectrometry. Sci. Rep. 2018, 8, 12075. [Google Scholar] [CrossRef]
- Sharma, M.D.; Nahar, P.; Singh, M.P.; Bhuvaneshwari, S.; Goel, S.; Mathur, H. Saliva as a diagnostic tool for evaluating oxidative stress in periodontitis and its correlation with tobacco habits: A cross sectional study. J. Indian Acad. Oral Med. Radiol. 2018, 30, 361. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, N.D.; Goyal, L.; Moin, S.; Khan, S.; Gupta, A.; Garg, S. The influence of smoking on the levels of matrix metalloproteinase-8 and periodontal parameters in smoker and nonsmoker patients with chronic periodontitis: A clinicobiochemical study. J. Oral Biol. Craniofac. Res. 2016, 6, S39–S43. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, U.K.; Könönen, E.; Pradhan-Palikhe, P.; Tervahartiala, T.; Pussinen, P.J.; Suominen-Taipale, L.; Sorsa, T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J. Clin. Periodontol. 2010, 37, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, B.; Pan, C.; Zhang, A. To evaluate the serum cortisol, salivary cortisol, and serum interleukin-1 b level in patients of chronic periodontitis with smoking and stress and without smoking and stress. Medicine 2021, 100, e26757. [Google Scholar] [CrossRef] [PubMed]
- Lamster, I.B.; Holmes, L.G.; Gross, K.B.; Oshrain, R.L.; Cohen, D.W.; Rose, L.F.; Peters, L.M.; Pope, M.R. The relationship of beta-glucuronidase activity in crevicular fluid to clinical parameters of periodontal disease. Findings from a multicenter study. J. Clin. Periodontol. 1994, 21, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.F.; Tucci, M.A.; De Siqueira, A.; De Faveri, M.; Figueiredo, L.C.; Vallim, P.C.; Duarte, P.M. Diabetes may affect the expression of matrix metalloproteinases and their inhibitors more than smoking in chronic periodontitis. J. Periodont. Res. 2017, 52, 292–299. [Google Scholar] [CrossRef] [PubMed]
- de Lima, C.L.; Acevedo, A.C.; Grisi, D.C.; Taba, M., Jr.; Guerra, E.; de Luca, C.G. Hosts-derived salivary biomarkers in diagnosing periodontal disease. systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 492–502. [Google Scholar] [CrossRef]
- Lahdentausta, L.; Paju, S.; Mäntylä, P.; Buhlin, K.; Pietiäinen, M.; Tervahartiala, T.; Nieminen, M.S.; Sinisalo, J.; Sorsa, T.; Pussinen, P.J. Smoking confounds the periodontal diagnostics using saliva biomarkers. J. Periodontol. 2019, 90, 475–483. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, Z.; Pan, W.L.; Wang, H.L. Is History of Periodontal Disease Still a Negative Risk Indicator for Peri-implant Health under Supportive Post-implant Treatment Coverage? A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implants 2020, 35, 52–62. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yan, H.; Huang, L. Salivary matrix metalloproteinase (MMP)-8 as a biomarker for periodontitis: A PRISMA-compliant systematic review and meta-analysis. Medicine 2018, 97, e9642. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 010502. [Google Scholar] [CrossRef]
- Willis, B.H.; Riley, R.D. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat. Med. 2017, 36, 3283–3301. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, S.S.; Keusch, G.T.; Babiker, A.G.; Edwards, K.M.; Lewis, R.J.; Lundgren, J.D.; Wells, C.D.; Wabwire-Mangen, F.; McAdam, K.P.W.J. Rigorous clinical trial design in public health emergencies is essential. Clin. Infect. Dis. 2018, 66, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Helman, S.K.; Mummah, R.O.; Gostic, K.M.; Buhnerkempe, M.G.; Prager, K.C.; Lloyd-Smith, J.O. Estimating prevalence and test accuracy in disease ecology: How Bayesian latent class analysis can boost or bias imperfect test results. Ecol. Evol. 2020, 10, 7221–7232. [Google Scholar] [CrossRef] [PubMed]
- Kanmaz, B.; Lamont, G.; Danacı, G.; Gogeneni, H.; Buduneli, N.; Scott, D.A. Microbiological and biochemical findings in relation to clinical periodontal status in active smokers, non-smokers and passive smokers. Tob. Induc Dis. 2019, 17, 20. [Google Scholar] [CrossRef]
- Al-Nasser, L.; Lamster, I.B. Prevention and management of periodontal diseases and dental caries in the older adults. Periodontology 2020, 84, 69–83. [Google Scholar] [CrossRef]
- Armstrong, A.J.S.; Parmar, V.; Blaser, M.J. Assessing saliva microbiome collection and processing methods. NPJ Biofilms Microbiomes 2021, 7, 81. [Google Scholar] [CrossRef]
- Holliday, R.S.; Campbell, J.; Preshaw, P.M. Effect of nicotine on human gingival, periodontal ligament and oral epithelial cells. A systematic review of the literature. J. Dent. 2019, 86, 81–88. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).