Assessment of Children’s Metal Exposure via Hand Wipe, Outdoor Soil and Indoor Dust and Their Associations with Blood Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
2.3. Sample Pretreatment and Analysis
2.4. Quality Assurance and Quality Control
2.5. Exposure Assessment
2.6. Risk Calculations
2.7. Statistic Analysis
3. Results and Discussions
3.1. Metal Concentrations in Different Sample Types
3.1.1. Hand Wipes
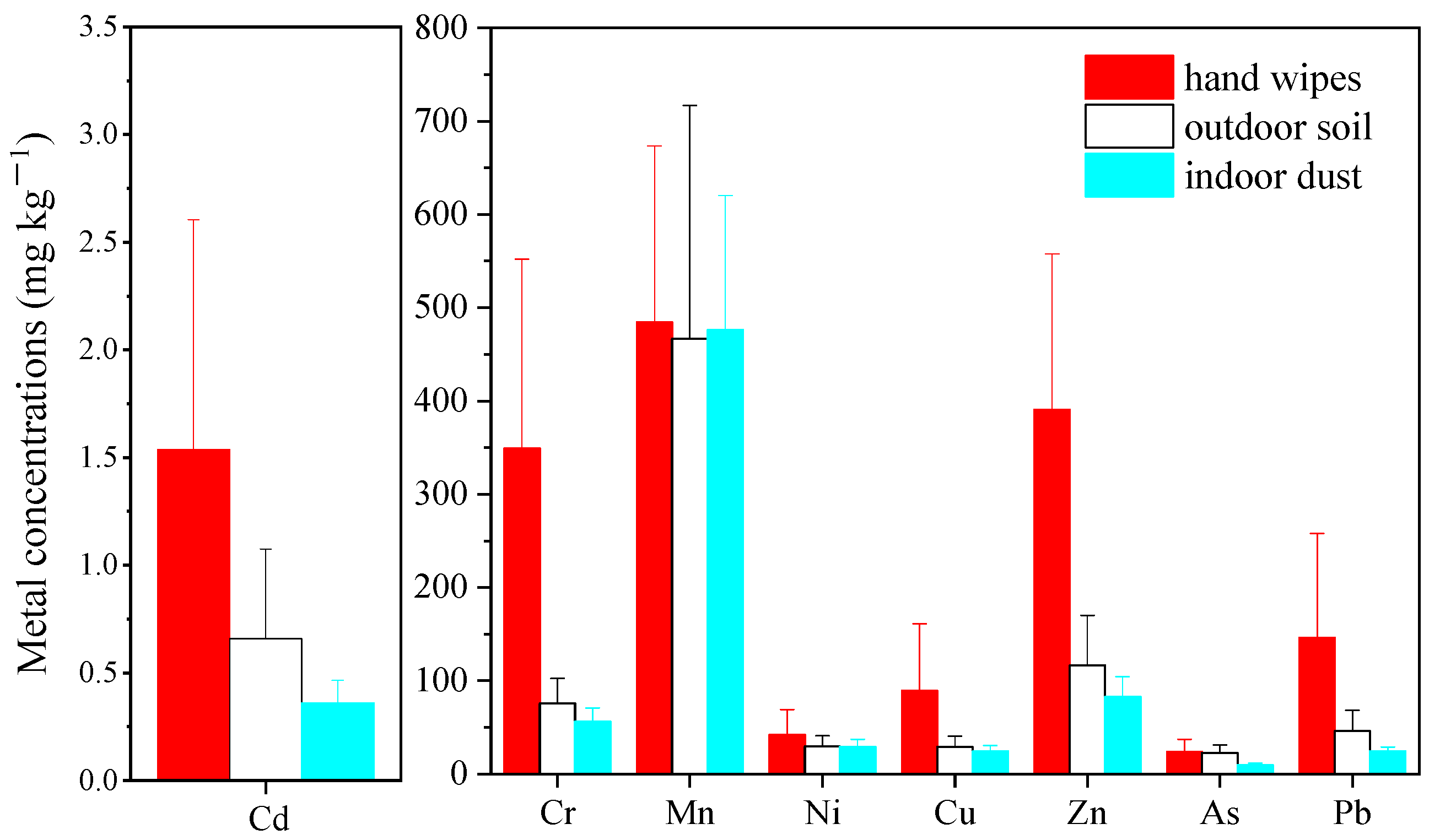
3.1.2. Comparison with Exterior Soil and Interior Dust
3.2. Exposure and Risk Level
3.2.1. Hand Wipes
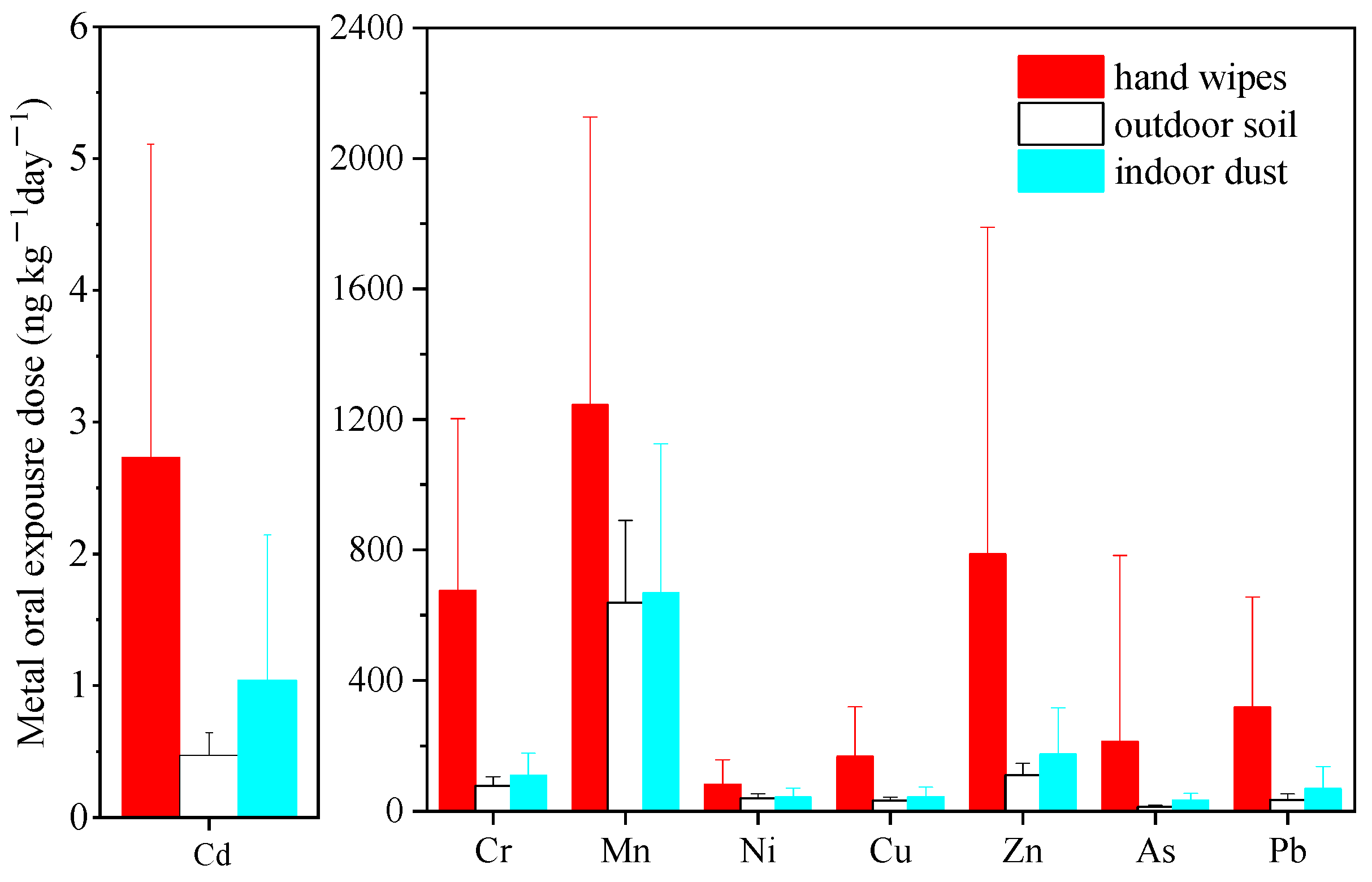
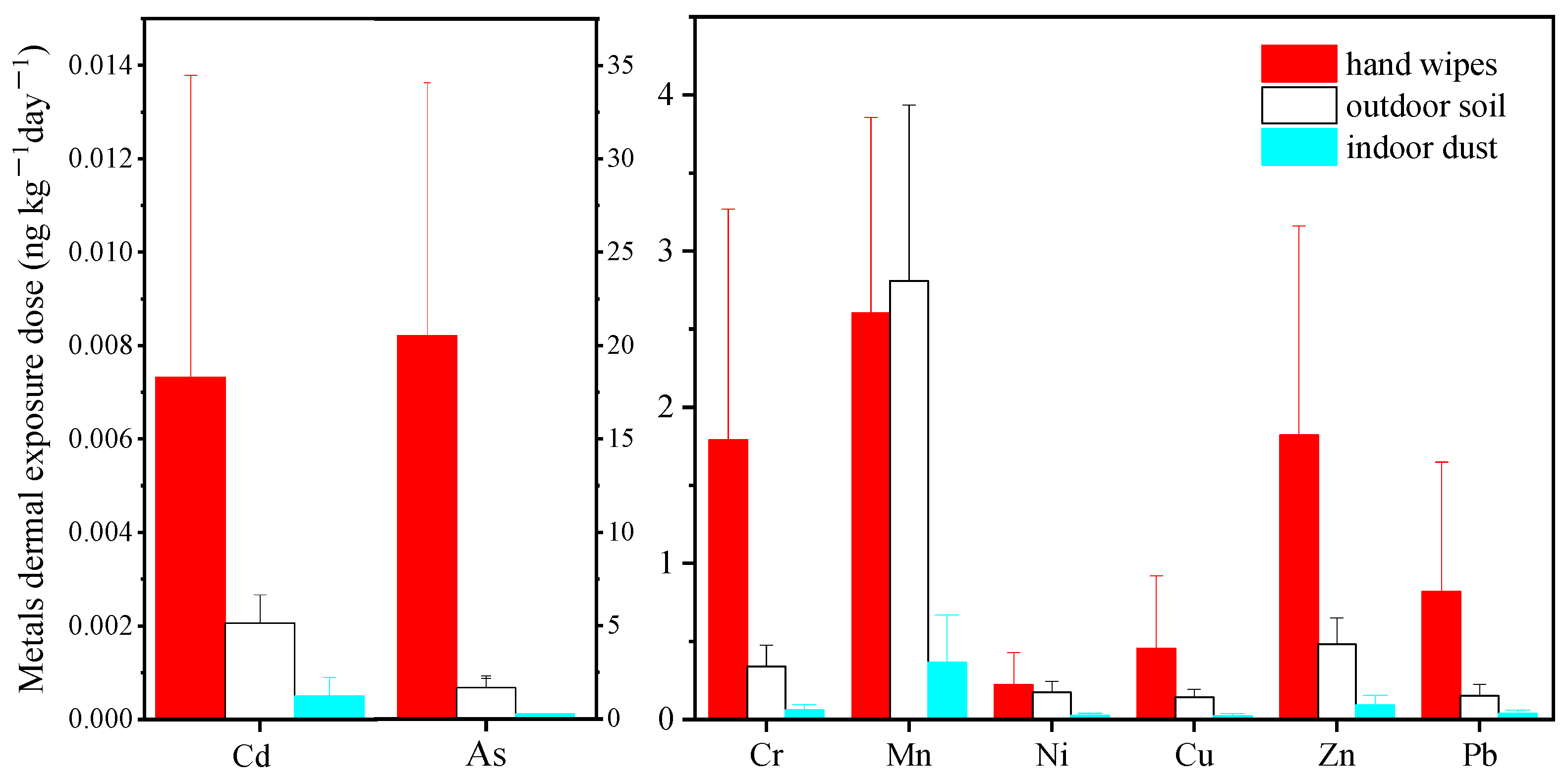
3.2.2. Comparison with Exterior Soil and Interior Dust
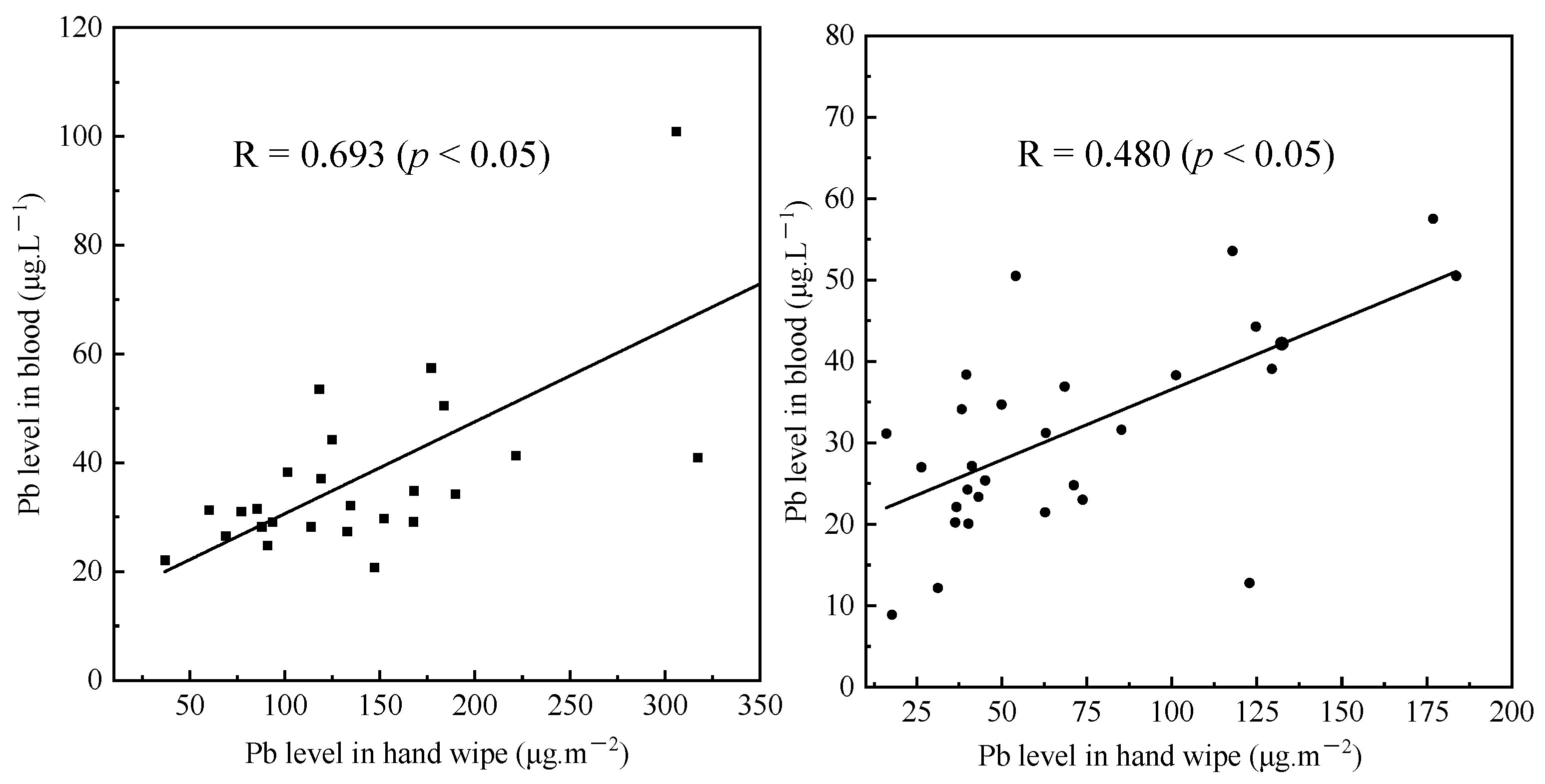
3.3. Association between External and Internal Exposure
3.4. Implication for Sampling and Assessing Method Selection
3.5. Strengths and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, B.; Gao, F.; Qin, N.; Duan, X.; Li, Y.; Cao, S. A comprehensive analysis on source-distribution-bioaccumulation-exposure risk of metal(loid)s in various vegetables in peri-urban areas of Shenzhen, China. Environ. Pollut. 2022, 293, 118613. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Wan, Y.; Wu, R.; Feng, D.; Li, K. Source identification and comprehensive apportionment of the accumulation of soil heavy metals by integrating pollution landscapes, pathways, and receptors. Sci. Total Environ. 2021, 786, 147436. [Google Scholar] [CrossRef]
- Hamadani, J.D.; Tofail, F.; Nermell, B.; Gardner, R.; Shiraji, S.; Bottai, M.; Arifeen, S.; Huda, S.N.; Vahter, M. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: A population-based cohort study. Int. J. Epidemiol. 2011, 40, 1593–1604. [Google Scholar] [CrossRef]
- Cárdenas-González, M.; Osorio-Yáñez, C.; Gaspar-Ramírez, O.; Pavković, M.; Ochoa-Martínez, A.; López-Ventura, D.; Medeiros, M.; Barbier, O.C.; Pérez-Maldonado, I.N.; Sabbisetti, V.S.; et al. Environmental exposure to arsenic and chromium in children is associated with kidney injury molecule-1. Environ. Res. 2016, 150, 653–662. [Google Scholar] [CrossRef]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Carrington, C.; Devleesschauwer, B.; Gibb, H.J.; Bolger, P.M. Global burden of intellectual disability resulting from dietary exposure to lead, 2015. Environ. Res. 2019, 172, 420–429. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, X.; Zhu, Y.; Wang, Q.; Zhang, Y.; Huo, X. Pb and Cd exposure linked with Il-10 and Il-13 gene polymorphisms in asthma risk relevant immunomodulation in children. Chemosphere 2022, 294, 133656. [Google Scholar] [CrossRef]
- Gundacker, C.; Forsthuber, M.; Szigeti, T.; Kakucs, R.; Mustieles, V.; Fernandez, M.F.; Bengtsen, E.; Vogel, U.; Hougaard, K.S.; Saber, A.T. Lead (Pb) and neurodevelopment: A review on exposure and biomarkers of effect (BDNF, HDL) and susceptibility. Int. J. Hyg. Environ. Health 2021, 238, 113855. [Google Scholar] [CrossRef]
- Flannery, B.M.; Schaefer, H.R.; Middleton, K.B. A scoping review of infant and children health effects associated with cadmium exposure. Regul. Toxicol. Pharmacol. 2022, 131, 105155. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, J.; Chen, Y.; Shi, C.; Zhang, Q.; Ning, Z.; Yu, Y.; Li, Y. Urinary cadmium in relation to bone damage: Cadmium exposure threshold dose and health-based guidance value estimation. Ecotoxicol. Environ. Saf. 2021, 226, 112824. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, R.; Jiang, Q.; Wang, Y.; Yu, C. Analysis of cadmium accumulation in community adults and its correlation with low-grade albuminuria. Sci. Total Environ. 2022, 834, 155210. [Google Scholar] [CrossRef]
- Dai, J.; Yang, X.; Yuan, Y.; Jia, Y.; Liu, G.; Lin, N.; Xiao, H.; Zhang, L.; Chen, J. Toxicity, gut microbiota and metabolome effects after copper exposure during early life in SD rats. Toxicology 2020, 433, 152395. [Google Scholar] [CrossRef]
- Wang, B.; Duan, X.; Feng, W.; He, J.; Cao, S.; Liu, S.; Shi, D.; Wang, H.; Wu, F. Health risks to metals in multimedia via ingestion pathway for children in a typical urban area of China. Chemosphere 2019, 226, 381–387. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, Z.; Huang, K.; Wang, X.; Cheng, L.; Zeng, L.; Zhou, Y.; Jing, T. Multiple exposure pathways and health risk assessment of heavy metal(loid)s for children living in fourth-tier cities in Hubei Province. Environ. Int. 2019, 129, 517–524. [Google Scholar] [CrossRef]
- Yang, S.; He, M.; Zhi, Y.; Chang, S.X.; Gu, B.; Liu, X.; Xu, J. An integrated analysis on source-exposure risk of heavy metals in agricultural soils near intense electronic waste recycling activities. Environ. Int. 2019, 133 (Pt B), 105239. [Google Scholar] [CrossRef]
- Cao, P.; Fujimori, T.; Juhasz, A.; Takaoka, M.; Oshita, K. Bioaccessibility and human health risk assessment of metal(loid)s in soil from an e-waste open burning site in Agbogbloshie, Accra, Ghana. Chemosphere 2020, 240, 124909. [Google Scholar] [CrossRef]
- Wu, W.; Wu, P.; Yang, F.; Sun, D.-L.; Zhang, D.-X.; Zhou, Y.-K. Assessment of heavy metal pollution and human health risks in urban soils around an electronics manufacturing facility. Sci. Total Environ. 2018, 630, 53–61. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Q.; Ma, J.; Wu, H.; Qu, Y.; Gong, Y.; Yang, S.; An, Y.; Zhou, Y. Heavy metal(loid)s in the topsoil of urban parks in Beijing, China: Concentrations, potential sources, and risk assessment. Environ. Pollut. 2020, 260, 114083. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Weber, J.; Smith, E. Impact of soil particle size and bioaccessibility on children and adult lead exposure in peri-urban contaminated soils. J. Hazard. Mater. 2011, 186, 1870–1879. [Google Scholar] [CrossRef]
- Poothong, S.; Padilla-Sanchez, J.A.; Papadopoulou, E.; Giovanoulis, G.; Thomsen, C.; Haug, L.S. Hand Wipes: A Useful Tool for Assessing Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) through Hand-to-Mouth and Dermal Contacts. Environ. Sci. Technol. 2019, 53, 1985–1993. [Google Scholar] [CrossRef]
- Hou, M.; Shi, Y.; Na, G.; Cai, Y. A review of organophosphate esters in indoor dust, air, hand wipes and silicone wristbands: Implications for human exposure. Environ. Int. 2021, 146, 106261. [Google Scholar] [CrossRef]
- Tang, J.; Lin, M.; Ma, S.; Yang, Y.; Li, G.; Yu, Y.; Fan, R.; An, T. Identifying Dermal Uptake as a Significant Pathway for Human Exposure to Typical Semivolatile Organic Compounds in an E-Waste Dismantling Site: The Relationship of Contaminant Levels in Handwipes and Urine Metabolites. Environ. Sci. Technol. 2021, 55, 14026–14036. [Google Scholar] [CrossRef]
- Xu, F.; Eulaers, I.; Alves, A.; Papadopoulou, E.; Padilla-Sanchez, J.A.; Lai, F.Y.; Haug, L.S.; Voorspoels, S.; Neels, H.; Covaci, A. Human exposure pathways to organophosphate flame retardants: Associations between human biomonitoring and external exposure. Environ. Int. 2019, 127, 462–472. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Kelly, S.M.; Allen, J.G.; McClean, M.D.; Webster, T.F. Measurement of polyhrominated diphenyl ethers on hand wipes: Estimating exposure from hand-to-mouth contact. Environ. Sci. Technol. 2008, 42, 3329–3334. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Eagle, S.; Sjodin, A.; Webster, T.F. Serum PBDEs in a North Carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 2012, 120, 1049–1054. [Google Scholar] [CrossRef]
- Mohmand, J.; Eqani, S.A.M.A.S.; Fasola, M.; Alamdar, A.; Mustafa, I.; Ali, N.; Liu, L.; Peng, S.; Shen, H. Human exposure to toxic metals via contaminated dust: Bio-accumulation trends and their potential risk estimation. Chemosphere 2015, 132, 142–151. [Google Scholar] [CrossRef]
- Wei, J.X.; Gao, J.Q.; Cen, K. Levels of eight heavy metals and health risk assessment considering food consumption by China’s residents based on the 5th China total diet study. Sci. Total Environ. 2019, 689, 1141–1148. [Google Scholar] [CrossRef]
- Yamamoto, N.; Takahashi, Y.; Yoshinaga, J.; Tanaka, A.; Shibata, Y. Size Distributions of Soil Particles Adhered to Children’s Hands. Arch. Environ. Contam. Toxicol. 2006, 51, 157–163. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xu, R.J.; Wu, G.P.; Wei, F.S. Determination of trace elements in soil in Xuanwei and Fuyuan by microwave digestion ICP-MS. Environ. Monit. China 2010, 26, 6–10. [Google Scholar]
- Liu, R.; He, R.; Cui, X.; Ma, L.Q. Impact of particle size on distribution, bioaccessibility, and cytotoxicity of polycyclic aromatic hydrocarbons in indoor dust. J. Hazard. Mater. 2018, 357, 341–347. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. A Framework for Assessing Health Risks of Environmental Exposures to Children; EPA/600/R-05/093F; U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- Holmes, K.K.; Shirai, J.H.; Richter, K.Y.; Kissel, J.C. Field measurement of dermal soil loadings in occupational and recreational activities. Environ. Res. 1999, 80, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kissel, J.C.; Richter, K.Y.; Fenske, R.A. Factors affecting soil adherence to skin in hand-press trials. Bull. Environ. Contam. Toxicol. 1996, 56, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Kissel, J.C.; Shirai, J.H.; Richter, K.Y.; Fenske, R.A. Empirical investigation of hand-to-mouth transfer of soil. Bull. Environ. Contam. Toxicol. 1998, 60, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pan, L.B.; Wang, Q.; Lin, C.Y.; Duan, X.L.; Hou, H. Estimation of the daily soil/dust (SD) ingestion rate of children from Gansu Province, China via hand-to-mouth contact using tracer elements. Environ. Geochem. Health 2018, 40, 295–301. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); EPA/540/R/99/005; U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
- U.S. Environmental Protection Agency. Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A); EPA/540/I -89/002; U.S. Environmental Protection Agency: Washington, DC, USA, 1989.
- Taylor, M.P.; Mould, S.A.; Kristensen, L.J.; Rouillon, M. Environmental arsenic, cadmium and lead dust emissions from metal mine operations: Implications for environmental management, monitoring and human health. Environ. Res. 2014, 135, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.P.; Camenzuli, D.; Kristensen, L.J.; Forbes, M.; Zahran, S. Environmental lead exposure risks associated with children’s outdoor playgrounds. Environ. Pollut. 2013, 178, 447–454. [Google Scholar] [CrossRef]
- Gorce, J.-P.; Roff, M. Hand Self-Wiping Protocol for the Investigation of Lead Exposure in the Workplace. J. Occup. Environ. Hyg. 2015, 12, 699–707. [Google Scholar] [CrossRef]
- Gulson, B.; Taylor, A.; Stifelman, M. Lead exposure in young children over a 5-year period from urban environments using alternative exposure measures with the US EPA IEUBK model—A trial. Environ. Res. 2018, 161, 87–96. [Google Scholar] [CrossRef]
- Shen, M.; Ren, M.; Wang, Y.; Shen, F.; Du, R.; Quan, L.; Wei, Y.; Zhang, T.; Li, J.; Yan, G.; et al. Identifying dust as the dominant source of exposure to heavy metals for residents around battery factories in the Battery Industrial Capital of China. Sci. Total Environ. 2021, 765, 144375. [Google Scholar] [CrossRef]
- Wu, Y.; Lou, J.; Sun, X.; Ma, L.Q.; Wang, J.; Li, M.; Sun, H.; Li, H.; Huang, L. Linking elevated blood lead level in urban school-aged children with bioaccessible lead in neighborhood soil. Environ. Pollut. 2020, 261, 114093. [Google Scholar] [CrossRef]
- Tay, J.H.; Sellström, U.; Papadopoulou, E.; Padilla-Sánchez, J.A.; Haug, L.S.; de Wit, C.A. Assessment of dermal exposure to halogenated flame retardants: Comparison using direct measurements from hand wipes with an indirect estimation from settled dust concentrations. Environ. Int. 2018, 115, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hughes, L.; Arnot, J.A. Addressing uncertainty in mouthing-mediated ingestion of chemicals on indoor surfaces, objects, and dust. Environ. Int. 2020, 146, 106266. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Duan, X.L. Chinese Environmental Exposure Factors Handbook (Children Aged 0–5 Years); China Environment Press: Beijing, China, 2016. [Google Scholar]
- Zhao, X.G.; Duan, X.L. Reports of Environmental Exposure Related Activity Patterns Research of Chinese Population (Children); China Environment Press: Beijing, China, 2016. [Google Scholar]
- U.S. Environmental Protection Agency. Exposure Factors Handbook: 2011 Edition; EPA/600/R-09/052F; National Center for Environmental Assessment; U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Ikegami, M.; Yoneda, M.; Tsuji, T.; Bannai, O.; Morisawa, S. Effect of particle size on risk assessment of direct soil ingestion and metals adhered to children’s hands at playgrounds. Risk Anal. 2014, 34, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Hsi, H.-C.; Hu, C.-Y.; Tsou, M.-C.; Hu, H.-J.; Özkaynak, H.; Bradham, K.; Hseu, Z.-Y.; Dang, W.; Chien, L.-C. Determination of hand soil loading, soil transfer, and particle size variations after hand-pressing and hand-mouthing activities. Sci. Total Environ. 2018, 627, 844–851. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Liu, Y.; Lin, C.; Cheng, H. Effects of soil particle size on metal bioaccessibility and health risk assessment. Ecotoxicol. Environ. Saf. 2019, 186, 109748. [Google Scholar] [CrossRef]
- Luo, X.-S.; Yu, S.; Li, X.-D. Distribution, availability, and sources of trace metals in different particle size fractions of urban soils in Hong Kong: Implications for assessing the risk to human health. Environ. Pollut. 2011, 159, 1317–1326. [Google Scholar] [CrossRef]
- Lin, C.; Wang, B.; Cui, X.; Xu, D.; Cheng, H.; Wang, Q.; Ma, J.; Chai, T.; Duan, X.; Liu, X.; et al. Estimates of Soil Ingestion in a Population of Chinese Children. Environ. Health Perspect. 2017, 125, 077002. [Google Scholar] [CrossRef]
- Wang, B.; Lin, C.; Zhang, X.; Xu, D.; Cheng, H.; Wang, Q.; Liu, X.; Ma, J. Effects of geography, age, and gender on Chinese children’s soil ingestion rate. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 1983–1989. [Google Scholar] [CrossRef]
- Xu, F.; Giovanoulis, G.; van Waes, S.; Padilla-Sanchez, J.A.; Papadopoulou, E.; Magner, J.; Haug, L.S.; Neels, H.; Covaci, A. Comprehensive Study of Human External Exposure to Organophosphate Flame Retardants via Air, Dust, and Hand Wipes: The Importance of Sampling and Assessment Strategy. Environ. Sci. Technol. 2016, 50, 7752–7760. [Google Scholar] [CrossRef]
- Gu, Y.-G.; Ning, J.-J.; Ke, C.-L.; Huang, H.-H. Bioaccessibility and human health implications of heavy metals in different trophic level marine organisms: A case study of the South China Sea. Ecotoxicol. Environ. Saf. 2018, 163, 551–557. [Google Scholar] [CrossRef]
- Gulson, B.; Mizon, K.; Taylor, A.; Korsch, M.; Davis, J.M.; Louie, H.; Wu, M.; Gomez, L.; Antin, L. Pathways of Pb and Mn observed in a 5-year longitudinal investigation in young children and environmental measures from an urban setting. Environ. Pollut. 2014, 191, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.; Mizon, K.; Taylor, A.; Korsch, M.; Stauber, J.; Davis, J.M.; Louie, H.; Wu, M.; Swan, H. Changes in manganese and lead in the environment and young children associated with the introduction of methylcyclopentadienyl manganese tricarbonyl in gasoline—Preliminary results. Environ. Res. 2006, 100, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
| Metals | Hand Wipes (μg·m−2) | ||||
|---|---|---|---|---|---|
| Mean | Std | Median | P5 | P95 | |
| Cr | 242.2 | 173.9 | 184.1 | 96.6 | 693.4 |
| Mn | 373.4 | 275.0 | 280.2 | 105.1 | 912.9 |
| Ni | 29.8 | 23.8 | 21.4 | 9.9 | 90.5 |
| Cu | 61.2 | 52.3 | 43.2 | 18.0 | 169.5 |
| Zn | 258.4 | 202.4 | 199.5 | 84.7 | 600.7 |
| As | 23.6 | 13.5 | 21.5 | 6.2 | 48.4 |
| Cd | 1.0 | 0.7 | 0.8 | 0.3 | 2.4 |
| Pb | 106.9 | 87.7 | 101.4 | 26.4 | 305.7 |
| Metals | Cr | Mn | Ni | Cu | Zn | As | Cd | Pb |
|---|---|---|---|---|---|---|---|---|
| Cr | 1 | |||||||
| Mn | 0.39 ** | 1 | ||||||
| Ni | 0.69 ** | 0.49 ** | 1 | |||||
| Cu | 0.47 ** | 0.26 | 0.68 ** | 1 | ||||
| Zn | 0.54 ** | 0.57 ** | 0.48 ** | 0.41 ** | 1 | |||
| As | 0.59 ** | 0.59 ** | 0.48 ** | 0.44 ** | 0.53 ** | 1 | ||
| Cd | 0.29 * | 0.39 ** | 0.53 ** | 0.42 ** | 0.21 | 0.30 * | 1 | |
| Pb | 0.12 | 0.61 ** | 0.28 | 0.29 * | 0.37 ** | 0.41 ** | 0.17 | 1 |
| Influencing Factors | n | Cr | Mn | Ni | Cu | Zn | As | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|
| Age of children | |||||||||
| 3–6 years | 30 | 294.4 | 443.8 | 31.8 | 79.4 | 389.5 | 32.9 | 1.1 | 151.9 |
| 7–12 years | 30 | 164.5 | 184.4 | 18.7 | 39.6 | 177.3 | 11.1 | 0.7 | 59.9 |
| p | <0.05 | 0.11 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
| Years of residency | |||||||||
| >5 years | 14 | 226.3 | 397.9 | 21.4 | 44.7 | 253.4 | 18.5 | 0.9 | 107.4 |
| ≤5 years | 46 | 171.0 | 180.9 | 18.5 | 41.7 | 146.7 | 11.6 | 0.6 | 62.0 |
| p | <0.05 | <0.05 | 0.27 | 0.85 | <0.05 | 0.36 | <0.05 | <0.05 | |
| Ground types of children’s regular play areas | |||||||||
| Bare soil | 28 | 266.3 | 443.8 | 31.4 | 44.7 | 244.4 | 15.5 | 1.2 | 107.4 |
| Hard ground surface | 32 | 150.1 | 184.4 | 11.3 | 41.6 | 123.7 | 14.2 | 0.8 | 65.7 |
| p | <0.05 | <0.05 | <0.05 | 0.61 | <0.05 | 0.58 | 0.80 | <0.05 | |
| Hand-washing frequency | |||||||||
| >5 times⸱d−1 | 33 | 180.4 | 241.4 | 21.4 | 49.0 | 200.8 | 11.9 | 0.9 | 72.5 |
| ≤5 times⸱d−1 | 27 | 226.3 | 376.8 | 21.3 | 41.5 | 193.6 | 16.2 | 0.7 | 88.0 |
| p | 0.66 | 0.26 | 0.79 | 0.55 | 0.40 | 0.82 | 0.71 | 0.98 |
| Metals | Hand-to-Mouth Contact (ng kg−1 day−1) | Dermal Absorption (ng kg−1 day−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std | Median | P5 | P95 | Mean | Std | Median | P5 | P95 | |
| Cr | 674.9 | 627.7 | 452.1 | 117.0 | 1662.1 | 1.8 | 1.5 | 1.3 | 0.4 | 5.3 |
| Mn | 1244.2 | 883.0 | 982.7 | 135.2 | 2485.5 | 2.6 | 2.3 | 2.2 | 0.5 | 7.5 |
| Ni | 80.9 | 76.7 | 56.6 | 14.0 | 192.0 | 0.2 | 0.2 | 0.1 | 0.0 | 0.7 |
| Cu | 166.8 | 152.9 | 101.9 | 24.6 | 504.8 | 0.5 | 0.5 | 0.3 | 0.1 | 1.2 |
| Zn | 787.2 | 502.0 | 445.2 | 131.0 | 1299.0 | 1.8 | 1.3 | 1.4 | 0.3 | 4.9 |
| As | 212.2 | 170.7 | 22.9 | 2.1 | 825.0 | 20.5 | 13.5 | 3.3 | 1.0 | 86.5 |
| Cd | 2.7 | 2.4 | 1.9 | 0.5 | 6.1 | 0.007 | 0.006 | 0.005 | 0.002 | 0.018 |
| Pb | 318.3 | 237.4 | 149.3 | 40.7 | 1070.5 | 0.8 | 0.8 | 0.6 | 0.1 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Gao, F.; Li, Y.; Lin, C.; Cheng, H.; Duan, X. Assessment of Children’s Metal Exposure via Hand Wipe, Outdoor Soil and Indoor Dust and Their Associations with Blood Biomarkers. Int. J. Environ. Res. Public Health 2022, 19, 14614. https://doi.org/10.3390/ijerph192114614
Wang B, Gao F, Li Y, Lin C, Cheng H, Duan X. Assessment of Children’s Metal Exposure via Hand Wipe, Outdoor Soil and Indoor Dust and Their Associations with Blood Biomarkers. International Journal of Environmental Research and Public Health. 2022; 19(21):14614. https://doi.org/10.3390/ijerph192114614
Chicago/Turabian StyleWang, Beibei, Fei Gao, Yujie Li, Chunye Lin, Hongguang Cheng, and Xiaoli Duan. 2022. "Assessment of Children’s Metal Exposure via Hand Wipe, Outdoor Soil and Indoor Dust and Their Associations with Blood Biomarkers" International Journal of Environmental Research and Public Health 19, no. 21: 14614. https://doi.org/10.3390/ijerph192114614
APA StyleWang, B., Gao, F., Li, Y., Lin, C., Cheng, H., & Duan, X. (2022). Assessment of Children’s Metal Exposure via Hand Wipe, Outdoor Soil and Indoor Dust and Their Associations with Blood Biomarkers. International Journal of Environmental Research and Public Health, 19(21), 14614. https://doi.org/10.3390/ijerph192114614