Life-Course Persistent Antisocial Behavior and Accelerated Biological Aging in a Longitudinal Birth Cohort
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Assessment of Antisocial Behavior Trajectories
2.3. Measuring Signs of Accelerated Aging in Midlife
2.4. Measuring Childhood Health Indicators
2.5. Measuring Health Issues in Adulthood
2.6. Measuring Adverse Experiences
2.7. Measuring Early Childhood Self-Control Difficulties
2.8. Measuring Health Knowledge
2.9. Statistical Analysis
3. Results
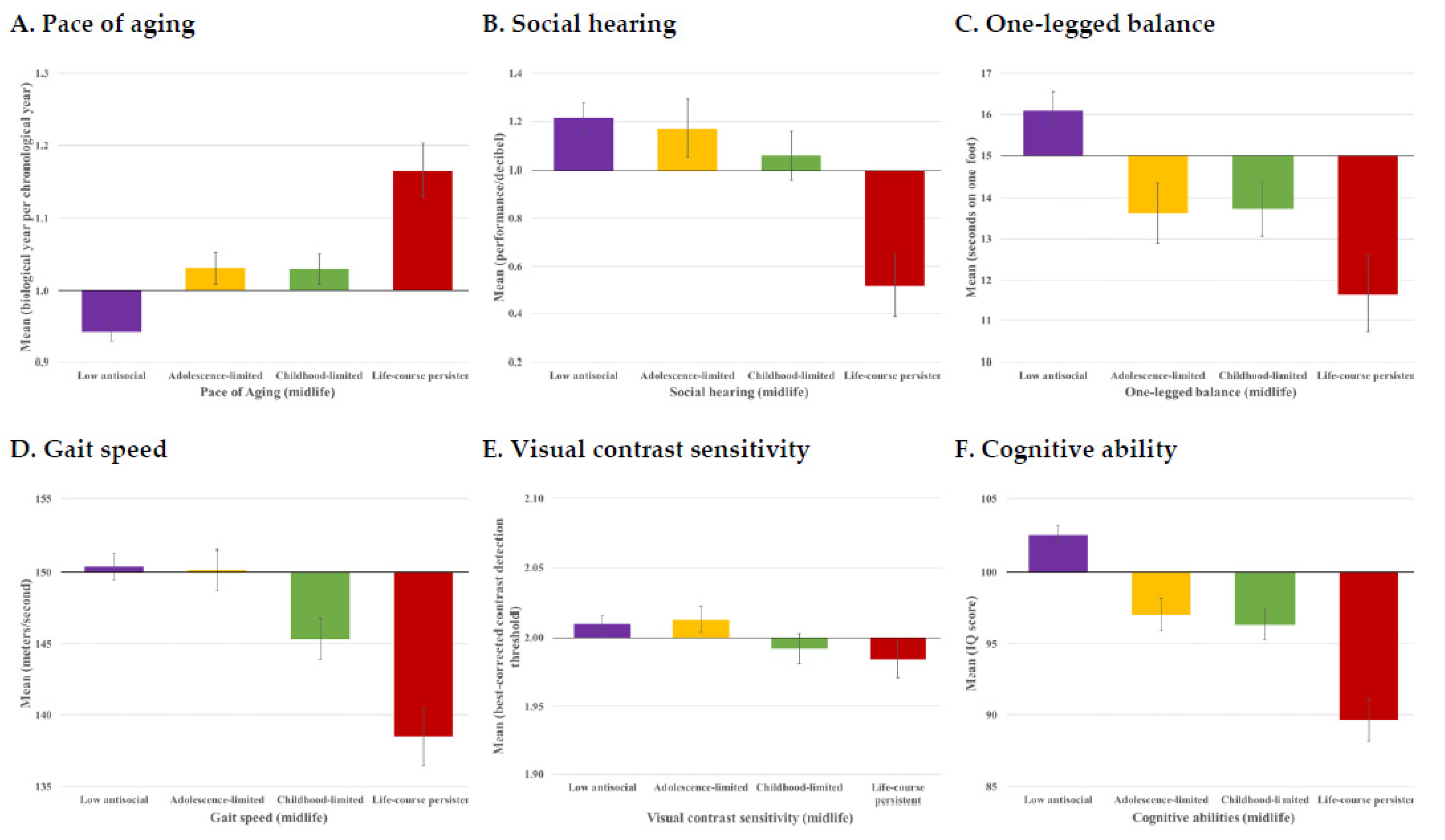
3.1. Are Antisocial Behavior Trajectories Associated with Signs of Accelerated Aging by Midlife?
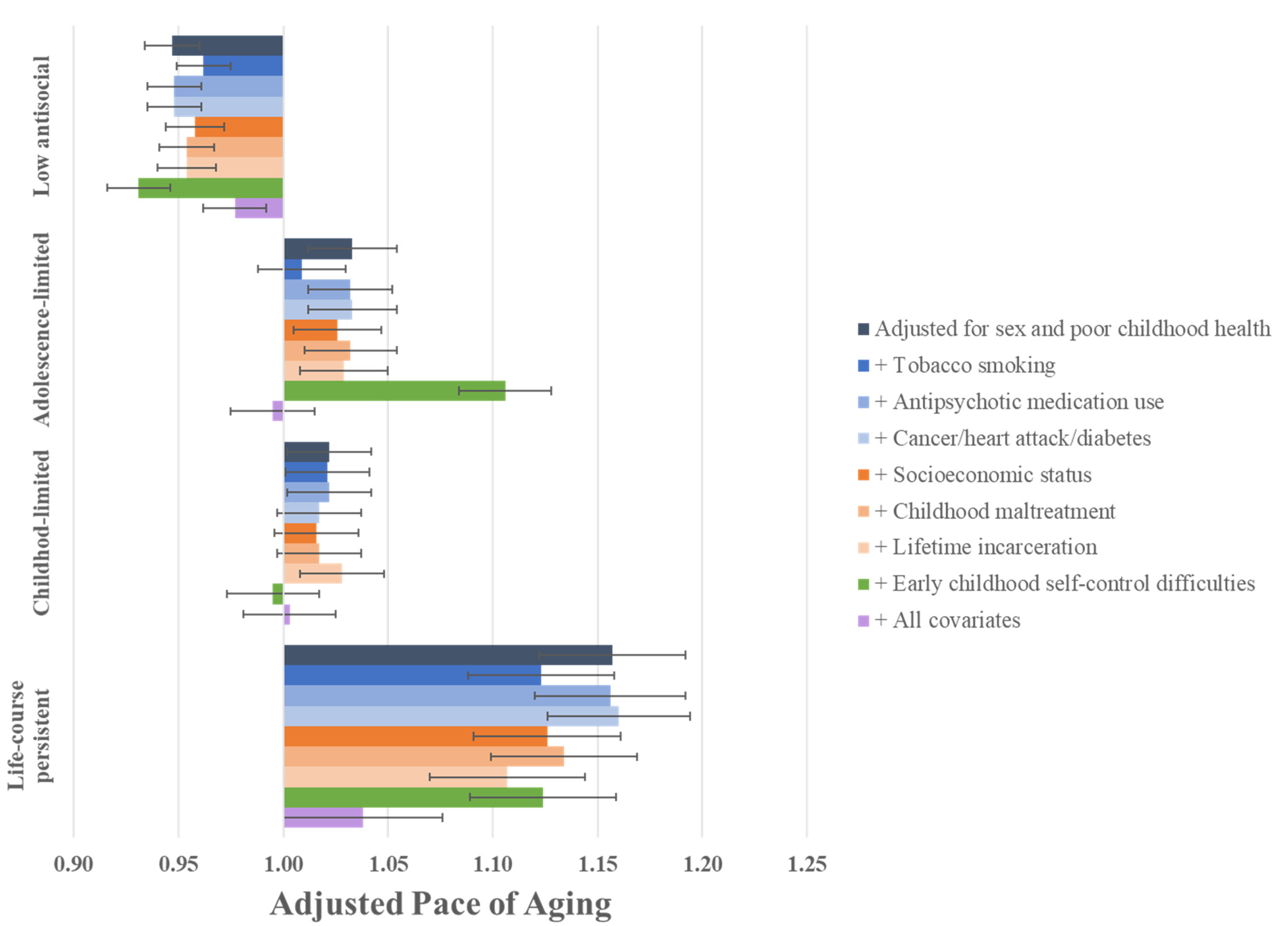
3.2. Are the Associations between Antisocial Behavior Trajectories and Signs of Accelerated Aging in Midlife Capturing a Decline in Health?
3.3. Are the Associations between Antisocial Behavior Trajectories and Signs of Accelerated Aging in Midlife Attributable to Health Problems in Adulthood?
3.4. Are the Associations between Antisocial Behavior Trajectories and Signs of Accelerated Aging in Midlife Due to Adverse Experiences Common to Both Antisocial Behavior and Accelerated Aging?
3.5. Are the Associations between Antisocial Behavior Trajectories and Signs of Accelerated Aging in Midlife Due to Shared Risk from Early Childhood Self-Control Difficulties?
3.6. Are the Associations between Antisocial Behavior Trajectories and Signs of Accelerated Aging in Midlife Due to the Cumulative Impact of Childhood and Adult Health Problems, and Adverse Experiences?
3.7. Do Individuals on Antisocial Behavior Trajectories Differ in Facial Age?
3.8. Are There Group Differences in Health Knowledge According to Antisocial Behavior Trajectories?
3.9. Associations between Antisocial Behavior Trajectories and DunedinPACE in Midlife
4. Discussion
Actionable Recommendations: What Can Be Done?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLisi, M. 4 Age–Crime Curve and Criminal Career Patterns. In The Development of Criminal and Antisocial Behavior; Morizot, J., Kazemian, L., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Quetelet, A. Research on the propensity for crime at different ages. In Crime: Critical Concepts in Sociology; Bean, P., Ed.; Taylor & Francis: London, UK, 2003; pp. 119–135. [Google Scholar]
- Rivenbark, J.G.; Odgers, C.L.; Caspi, A.; Harrington, H.; Hogan, S.; Houts, R.M.; Moffitt, T.E. The high societal costs of childhood conduct problems: Evidence from administrative records up to age 38 in a longitudinal birth cohort. J. Child Psychol. Psychiatry 2018, 59, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Skinner, G.C.; Farrington, D.P. Antisocial personality versus GP reported and self-reported health outcomes. J. Forensic Psychiatry Psychol. 2021, 32, 506–519. [Google Scholar] [CrossRef]
- Skinner, G.C.M.; Farrington, D.P. The healthcare plight of offenders in the community. Crim. Behav. Ment. Health 2022, 32, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.B.; Dawson, D.A.; Chou, S.P.; Ruan, W.J.; Saha, T.D.; Pickering, R.P.; Grant, B.F. Antisocial behavioral syndromes and past-year physical health among adults in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 2008, 69, 368–380. [Google Scholar] [CrossRef]
- Skinner, G.C.; Farrington, D.P. A systematic review and meta-analysis of premature mortality in offenders. Aggress. Violent Behav. 2020, 53, 101431. [Google Scholar] [CrossRef]
- Solares, C.; Dobrosavljevic, M.; Larsson, H.; Cortese, S.; Andershed, H. The mental and physical health of older offenders: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 440–450. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Sierra, F. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Wertz, J.; Caspi, A.; Ambler, A.; Broadbent, J.; Hancox, R.J.; Harrington, H.; Moffitt, T.E. Association of history of psychopathology with accelerated aging at midlife. JAMA Psychiatry 2021, 78, 530–539. [Google Scholar] [CrossRef]
- Kaeberlein, M. Longevity and aging. F1000prime Rep. 2013, 5, 5. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Arseneault, L.; Baccarelli, A.; Corcoran, D.L.; Gao, X.; Moffitt, T.E. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife 2020, 9, e54870. [Google Scholar] [CrossRef]
- Elliott, M.L.; Caspi, A.; Houts, R.M.; Ambler, A.; Broadbent, J.M.; Hancox, R.J.; Moffitt, T.E. Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat. Aging 2021, 1, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, T.E.; Caspi, A. Psychiatry’s opportunity to prevent the rising burden of age-related disease. JAMA Psychiatry 2019, 76, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, T.E. The neuropsychology of conduct disorder. Dev. Psychopathol. 1993, 5, 135–151. [Google Scholar] [CrossRef]
- Odgers, C.L.; Moffitt, T.E.; Broadbent, J.M.; Dickson, N.; Hancox, R.J.; Harrington, H.; Poulton, R.; Sears, M.R.; Thomson, W.M.; Caspi, A. Female and male antisocial trajectories: From childhood origins to adult outcomes. Dev. Psychopathol. 2008, 20, 673–716. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, T.E. Male antisocial behaviour in adolescence and beyond. Nat. Hum. Behav. 2018, 2, 177–186. [Google Scholar] [CrossRef]
- Skinner, G.C.; Farrington, D.P.; Jolliffe, D. Criminal careers and early death: Relationships in the Cambridge study in delinquent development. Br. J. Criminol. 2022, 62, 840–856. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Corcoran, D.L.; Sugden, K.; Poulton, R.; Arseneault, L.; Moffitt, T.E. DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife 2022, 11, e73420. [Google Scholar] [CrossRef]
- Hillary, R.F.; Stevenson, A.J.; McCartney, D.L.; Campbell, A.; Walker, R.M.; Howard, D.M.; Ritchie, C.W.; Horvath, S.; Hayward, C.; McIntosh, A.M.; et al. Epigenetic measures of ageing predict the prevalence and incidence of leading causes of death and disease burden. Clin. Epigenet. 2020, 12, 115. [Google Scholar] [CrossRef]
- Sugden, K.; Caspi, A.; Elliott, M.L.; Bourassa, K.J.; Chamarti, K.; Corcoran, D.L.; Hariri, A.R.; Houts, R.M.; Kothari, M.; Kritchevsky, S.; et al. Association of Pace of Aging measured by blood-based DNA methylation with age-related cognitive impairment and dementia. Neurology 2022, 99, e1402–e1413. [Google Scholar] [CrossRef]
- Correll, C.U.; Frederickson, A.M.; Kane, J.M.; Manu, P. Metabolic syndrome and the risk of coronary heart disease in 367 patients treated with second-generation antipsychotic drugs. J. Clin. Psychiatry 2006, 67, 575–583. [Google Scholar] [CrossRef]
- Danese, A.; McEwen, B.S. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 2012, 106, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Piquero, A.R.; Daigle, L.E.; Gibson, C.; Piquero, N.L.; Tibbetts, S.G. Research Note: Are Life-Course-Persistent Offenders At Risk for Adverse Health Outcomes? J. Res. Crime Delinq. 2007, 44, 185–207. [Google Scholar] [CrossRef]
- Raffington, L.; Belsky, D.W.; Kothari, M.; Malanchini, M.; Tucker-Drob, E.M.; Harden, K.P. Socioeconomic Disadvantage and the Pace of Biological Aging in Children. Available online: https://pubmed.ncbi.nlm.nih.gov/34001641/ (accessed on 1 June 2021).
- Richmond-Rakerd, L.S.; Caspi, A.; Ambler, A.; d’Arbeloff, T.; de Bruine, M.; Elliott, M.; Moffitt, T.E. Childhood self-control forecasts the pace of midlife aging and preparedness for old age. Proc. Natl. Acad. Sci. USA 2021, 118, e2010211118. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.J.H.; Moffitt, T.E.; Eugen-Olsen, J.; Belsky, D.W.; Danese, A.; Harrington, H.; Caspi, A. Cumulative childhood risk is associated with a new measure of chronic inflammation in adulthood. J. Child Psychol. Psychiatry 2019, 60, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.J.H.; Moffitt, T.E.; Arseneault, L.; Danese, A.; Eugen-Olsen, J.; Fisher, H.L.; Caspi, A. Association of adverse experiences and exposure to violence in childhood and adolescence with inflammatory burden in young people. JAMA Pediatr. 2020, 174, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Zaninotto, P. Lower socioeconomic status and the acceleration of aging: An outcome-wide analysis. Proc. Natl. Acad. Sci. USA 2020, 117, 14911–14917. [Google Scholar] [CrossRef]
- Poulton, R.; Moffitt, T.E.; Silva, P.A. The Dunedin Multidisciplinary Health and Development Study: Overview of the first 40 years, with an eye to the future. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 679–693. [Google Scholar] [CrossRef]
- Cameron, S.; Dillon, H. Development of the Listening in Spatialized Noise-Sentences Test (LISN-S). Ear Hear. 2007, 28, 196–211. [Google Scholar] [CrossRef]
- Vereeck, L.; Wuyts, F.; Truijen, S.; Van de Heyning, P. Clinical assessment of balance: Normative data, and gender and age effects. Int. J. Audiol. 2008, 47, 67–75. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Caspi, A.; Ambler, A.; Broadbent, J.M.; Cohen, H.J.; d’Arbeloff, T.; Moffitt, T.E. Association of neurocognitive and physical function with gait speed in midlife. JAMA Netw. Open 2019, 2, e1913123. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale: WAIS-IV: Technical and Interpretive Manual; Pearson: New York, NY, USA, 2008. [Google Scholar]
- Belsky, D.W.; Caspi, A.; Cohen, H.J.; Kraus, W.E.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Impact of early personal-history characteristics on the Pace of Aging: Implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell 2017, 16, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.; Dawes, P.J.D. Variation in the normal hearing threshold predicts childhood IQ, linguistic, and behavioral outcomes. Pediatr. Res. 2007, 61, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Arnheim, D.D.; Sinclair, W.A. The Clumsy Child; VC Mosby Co.: Maryland Heights, MI, USA, 1974. [Google Scholar]
- Bayley, N. The Bayley Scale of Infant Development; Psychological Corporation: San Antonio, TX, USA, 1969. [Google Scholar]
- Wechsler, D. Manual for the Wechsler Intelligence Scale for Children–Revised; Psychological Corporation: New York, NY, USA, 1974. [Google Scholar]
- Elley, W.B.; Irving, J.C. Revised socioeconomic index for New-Zealand. N. Z. J. Educ. Stud. 1976, 11, 25–36. [Google Scholar]
- Poulton, R.; Caspi, A.; Milne, B.J.; Thomson, W.M.; Taylor, A.; Sears, M.R.; Moffitt, T.E. Association between children’s experience of socioeconomic disadvantage and adult health: A life-course study. Lancet 2002, 360, 1640–1645. [Google Scholar] [CrossRef]
- Caspi, A.; McClay, J.; Moffitt, T.E.; Mill, J.; Martin, J.; Craig, I.W.; Taylor, A.; Poulton, R. Role of genotype in the cycle of violence in maltreated children. Science 2002, 297, 851–854. [Google Scholar] [CrossRef]
- Caspi, A.; Henry, B.; McGee, R.O.; Moffitt, T.E.; Silva, P.A. Temperamental origins of child and adolescent behavior problems: From age three to age fifteen. Child Dev. 1995, 66, 55–68. [Google Scholar] [CrossRef]
- Moffitt, T.E.; Arseneault, L.; Belsky, D.; Dickson, N.; Hancox, R.J.; Harrington, H.; Caspi, A. A gradient of childhood self-control predicts health, wealth, and public safety. Proc. Natl. Acad. Sci. USA 2011, 108, 2693–2698. [Google Scholar] [CrossRef]
- Muthén, L.; Muthén, B.O. Mplus User’s Guide, 7th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2015. [Google Scholar]
- Skinner, G.C.; Farrington, D.P.; Shepherd, J.P. Offender trajectories, health and hospital admissions: Relationships and risk factors in the longitudinal Cambridge Study in Delinquent Development. J. R. Soc. Med. 2020, 113, 110–118. [Google Scholar] [CrossRef]
- Testa, A.; Semenza, D. Criminal offending and health over the life-course: A dual-trajectory approach. J. Crim. Justice 2020, 68, 101691. [Google Scholar] [CrossRef]
- Binswanger, I.A.; Stern, M.F.; Deyo, R.A.; Heagerty, P.J.; Cheadle, A.; Elmore, J.G.; Koepsell, T.D. Release from prison—A high risk of death for former inmates. N. Engl. J. Med. 2007, 356, 157–165. [Google Scholar] [CrossRef]
- Schnittker, J.; John, A. Enduring stigma: The long-term effects of incarceration on health. J. Health Soc. Behav. 2007, 48, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.K.; Berg, M.T.; Simons, R.L.; Beach, S.R. Neighborhood structural disadvantage and biological aging in a sample of Black middle age and young adults. Soc. Sci. Med. 2022, 293, 114654. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.L.; Ong, M.L.; Lei, M.K.; Klopack, E.; Berg, M.; Zhang, Y.; Beach, S.S. Unstable childhood, adult adversity, and smoking accelerate biological aging among middle-age African Americans: Similar findings for GrimAge and PoAm. J. Aging Health 2021, 34, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.A.; Colich, N.L.; Uddin, M.; Armstrong, D.; McLaughlin, K.A. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol. Psychiatry 2019, 85, 268–278. [Google Scholar] [CrossRef]
- Beckley, A.L.; Caspi, A.; Arseneault, L.; Barnes, J.C.; Fisher, H.L.; Harrington, H.; Houts, R.; Morgan, N.; Odgers, C.L.; Wertz, J.; et al. The Developmental Nature of the Victim-Offender Overlap. J. Dev. Life Course Criminol. 2018, 4, 24–49. [Google Scholar] [CrossRef]
- Bourassa, K.J.; Caspi, A.; Harrington, H.; Houts, R.; Poulton, R.; Ramrakha, S.; Moffitt, T.E. Intimate partner violence and lower relationship quality are associated with faster biological aging. Psychol. Aging 2020, 35, 1127–1139. [Google Scholar] [CrossRef]
- Bourassa, K.J.; Moffitt, T.E.; Ambler, A.; Hariri, A.R.; Harrington, H.; Houts, R.M.; Caspi, A. Association of Treatable Health Conditions During Adolescence With Accelerated Aging at Midlife. JAMA Pediatr. 2022, 176, 392–399. [Google Scholar] [CrossRef]
- Simons, R.L.; Lei, M.K.; Beach, S.R.; Simons, L.G.; Barr, A.B.; Gibbons, F.X.; Philibert, R.A. Testing life course models whereby juvenile and adult adversity combine to influence speed of biological aging. J. Health Soc. Behav. 2019, 60, 291–308. [Google Scholar] [CrossRef]
- Skinner, G.C.; Farrington, D.P. Health of convicted persons in the third generation of the Longitudinal Cambridge Study in Delinquent Development. Int. J. Offender Ther. Comp. Criminol. 2021, 0306624X211066837. [Google Scholar] [CrossRef]
- Kim, Y.; Huan, T.; Joehanes, R.; McKeown, N.M.; Horvath, S.; Levy, D.; Ma, J. Higher diet quality relates to decelerated epigenetic aging. Am. J. Clin. Nutr. 2022, 115, 163–170. [Google Scholar] [CrossRef]
- Waziry, R.; Corcoran, D.L.; Huffman, K.M.; Kobor, M.S.; Kothari, M.; Kraus, V.B.; Belsky, D.W. Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults: CALERIE™ trial analysis. MedRxiv 2021. [Google Scholar] [CrossRef]
- Song, Z.; Baicker, K. Effect of a workplace wellness program on employee health and economic outcomes: A randomized clinical trial. JAMA 2019, 321, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Clouse, M.L.; Mannino, D.; Curd, P.R. Investigation of the correlates and effectiveness of a prison-based wellness program. J. Correct. Health Care 2012, 18, 184–197. [Google Scholar] [CrossRef][Green Version]
- Farrell, G.; Laycock, G.; Tilley, N. Debuts and legacies: The crime drop and the role of adolescence-limited and persistent offending. Crime Sci. 2015, 4, 16. [Google Scholar] [CrossRef]
- Ruch, D.A.; Steelesmith, D.L.; Brock, G.; Boch, S.J.; Quinn, C.R.; Bridge, J.A.; Fontanella, C.A. Mortality and cause of death among youths previously incarcerated in the juvenile legal system. JAMA Netw. Open 2021, 4, e2140352. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Guralnik, J. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Israel, S.; Blumenthal, J.A.; Poulton, R.; Moffitt, T.E. Cardiorespiratory fitness and cognitive function in midlife: Neuroprotection or neuroselection? Ann. Neurol. 2015, 77, 607–617. [Google Scholar] [CrossRef]
- Belsky, D.W.; Moffitt, T.E.; Corcoran, D.L.; Domingue, B.; Harrington, H.; Hogan, S.; Houts, R.; Ramrakha, S.; Sugden, K.; Williams, B.S.; et al. The genetics of success: How singlenucleotide polymorphisms associated with educational attainment relate to life-course development. Psychol. Sci. 2016, 27, 957–972. [Google Scholar] [CrossRef]
- Belsky, D.W.; Moffitt, T.E.; Houts, R.; Bennett, G.G.; Biddle, A.K.; Blumenthal, J.A.; Evans, J.P.; Harrington, H.; Sugden, K.; Williams, B.; et al. Polygenic risk, rapid childhood growth, and the development of obesity: Evidence from a 4-decade longitudinal study. Arch. Pediatr. Adolesc. Med. 2012, 166, 515–521. [Google Scholar] [CrossRef]
- Williams, S.; Poulton, R. Birth size, growth, and blood pressure between the ages of 7 and 26 years: Failure to support the fetal origins hypothesis. Am. J. Epidemiol. 2002, 155, 849–852. [Google Scholar] [CrossRef]
- Sears, M.R.; Greene, J.M.; Willan, A.R.; Wiecek, E.M.; Taylor, D.R.; Flannery, E.M.; Cowan, J.O.; Herbison, G.P.; Silva, P.A.; Poulton, R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 2003, 349, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Pronk, M.; Lissenberg-Witte, B.I.; van der Aa, H.P.A.; Comijs, H.C.; Smits, C.; Lemke, U.; Zekveld, A.Z.; Kramer, S.E. Longitudinal relationships between decline in speech-in-noise recognition ability and cognitive functioning: The longitudinal aging study amsterdam. J. Speech Lang. Hear. Res. 2019, 62, 1167–1187. [Google Scholar] [CrossRef] [PubMed]
- Dubno, J.R.; Dirks, D.D.; Morgan, D.E. Effects of age and mild hearing loss on speech recognition in noise. J. Acoust. Soc. Am. 1984, 76, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd, A. Developments in speech audiometry. Br. J. Audiol. 1968, 2, 3–10. [Google Scholar] [CrossRef]
- Nomura, H.; Ando, F.; Niino, N.; Shimokata, H.; Miyake, Y. Age-related change in contrast sensitivity among Japanese adults. Jpn. J. Ophthalmol. 2003, 47, 299–303. [Google Scholar] [CrossRef]
- Leat, S.J.; Legge, G.E.; Bullimore, M.A. What is low vision? A re-evaluation of definitions. Optom. Vis. Sci. 1999, 76, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Marron, J.A.; Bailey, I.L. Visual factors and orientation-mobility performance. Optom. Vis. Sci. 1982, 59, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.A.; Welch, D. Does amblyopia have a functional impact? Findings from the © 2022 Wertz J et al. JAMA Psychiatry. Dunedin Multidisciplinary Health and Development Study. Clin. Exp. Ophthalmol. 2013, 41, 127–134. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Larkin, P.A.; Cook, A.C.; Gear, J.; Singer, J. Decrease in timed balance test scores with aging. Phys. Ther. 1984, 64, 1067–1070. [Google Scholar] [CrossRef]
- Hurvitz, E.A.; Richardson, J.K.; Werner, R.A.; Ruhl, A.M.; Dixon, M.R. Unipedal stance testing as an indicator of fall risk among older outpatients. Arch. Phys. Med. Rehabil. 2000, 81, 587–591. [Google Scholar] [CrossRef]
- Springer, B.A.; Marin, R.; Cyhan, T.; Roberts, H.; Gill, N.W. Normative values for the unipedal stance test with eyes open and closed. J. Geriatr. Phys. Ther. 2007, 30, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J. Am. Geriatr. Soc. 2012, 60, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D. McCarthy Scales of Children’s Abilities; Psychological Corp: New York, NY, USA, 1972. [Google Scholar]
- Deary, I.J.; Corley, J.; Gow, A.J.; Harris, H.E.; Houlihan, L.M.; Marioni, R.E.; Penke, L.; Rafnsson, S.B.; Starr, J.M. Age-associated cognitive decline. Br. Med. Bull. 2009, 92, 135–152. [Google Scholar] [CrossRef]
- Marioni, R.E.; Strachan, M.W.J.; Reynolds, R.M.; Lowe, G.D.O.; Mitchell, R.J.; Fowkes, F.G.R.; Frier, B.M.; Lee, A.J.; Butcher, I.; Rumley, A.; et al. Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: The Edinburgh Type 2 Diabetes Study. Diabetes 2010, 59, 710–713. [Google Scholar] [CrossRef]
- Aichele, S.; Rabbitt, P.; Ghisletta, P. Life span decrements in fluid intelligence and processing speed predict mortality risk. Psychol. Aging 2015, 30, 598–612. [Google Scholar] [CrossRef]
- Sugden, K.; Hannon, E.J.; Arseneault, L.; Belsky, D.W.; Corcoran, D.L.; Fisher, H.L.; Houts, R.M.; Kandaswamy, R.; Moffitt, T.E.; Poulton, R.; et al. Patterns of Reliability: Assessing the Reproducibility and Integrity of DNA Methylation Measurement. Available online: https://www.sciencedirect.com/science/article/pii/S2666389920300143#! (accessed on 8 May 2020).

| Developmental Trajectories of Antisocial Behavior | |||||
|---|---|---|---|---|---|
| Life-Course Persistent | Childhood- Limited | Adolescence- Limited | Low Antisocial | ||
| (n = 83) | (n = 207) | (n = 173) | (n = 471) | ||
| Means (SD)/% | |||||
| Childhood health indicators | |||||
| Poor health * | 0.15 (1.03) | 0.15 (1.02) | −0.03 (1.03) | −0.08 (0.97) | |
| Hearing * | −0.53 (1.25) | −0.02 (0.94) | 0.00 (1.02) | 0.10 (0.94) | |
| Balance * | −0.08 (0.94) | −0.03 (1.00) | 0.07 (1.09) | 0.00 (0.98) | |
| Motor development * | −0.12 (1.01) | −0.20 (1.21) | 0.07 (0.95) | 0.09 (0.90) | |
| Vision * | 0.06 (0.58) | 0.01 (0.93) | −0.07 (1.17) | 0.01 (1.02) | |
| Cognitive ability (IQ) * | −0.46 (0.95) | −0.31 (1.04) | 0.00 (0.97) | 0.22 (0.94) | |
| Health problems in adulthood | |||||
| Tobacco smoking | 83.1% | 52.2% | 74.1% | 37.8% | |
| Antipsychotic medication use | 2.4% | 2.4% | 2.3% | 1.5% | |
| Diabetes/heart attack/cancer | 4.8% | 8.3% | 5.8% | 5.3% | |
| Adverse experiences | |||||
| Childhood socioeconomic status * | −0.64 (0.87) | −0.14 (0.95) | −0.15 (0.97) | 0.23 (0.99) | |
| Childhood maltreatment | 27.7% | 13.0% | 11.0% | 3.8% | |
| Lifetime incarceration | 24.1% | 2.0% | 5.8% | 0.6% | |
| Early childhood self-control difficulties * | 0.51 (1.30) | 0.30 (1.24) | −0.09 (0.86) | −0.19 (0.79) | |
| Model 1 | Model 2 | |
|---|---|---|
| β (95%CI) | ||
| A. Pace of Aging | ||
| Life-course persistent | 0.22 *** (0.14, 0.28) | 0.20 *** (0.13, 0.27) |
| Childhood-limited | 0.12 *** (0.06, 0.20) | 0.11 ** (0.04, 0.17) |
| Adolescence-limited | 0.12 *** (0.05, 0.18) | 0.11 ** (0.05, 0.18) |
| Poor childhood health | 0.20 *** (0.13, 0.27) | |
| B. Social hearing | ||
| Life-course persistent | −0.14 *** (−0.21, −0.08) | −0.13 *** (−0.19, −0.07) |
| Childhood-limited | −0.05 (−0.12, 0.02) | −0.04 (−0.11, 0.03) |
| Adolescence-limited | −0.01 (−0.09, 0.06) | −0.01 (−0.09, 0.06) |
| Childhood hearing | 0.09 (0.00, 0.18) | |
| C. One-legged balance | ||
| Life-course persistent | −0.13 *** (−0.18, −0.06) | −0.13 *** (−0.18, −0.06) |
| Childhood-limited | −0.10 ** (−0.17, −0.03) | −0.10 ** (−0.17, −0.03) |
| Adolescence-limited | −0.10 ** (−0.16, −0.03) | −0.10 ** (−0.17, −0.04) |
| Childhood balance | 0.15 *** (0.08, 0.21) | |
| D. Gait speed | ||
| Life-course persistent | −0.18 *** (−0.24, −0.10) | −0.16 *** (−0.21, −0.09) |
| Childhood-limited | −0.11 ** (−0.18, −0.04) | −0.08 * (−0.15, −0.01) |
| Adolescence-limited | −0.01 (−0.07, 0.06) | 0.00 (−0.07, 0.06) |
| Childhood motor development | 0.28 *** (0.21, 0.35) | |
| E. Visual contrast sensitivity | ||
| Life-course persistent | −0.06 (−0.12, 0.00) | −0.06 (−0.12, 0.00) |
| Childhood-limited | −0.06 (−0.13, 0.02) | −0.06 (−0.13, 0.02) |
| Adolescence-limited | 0.01 (−0.05, 0.07) | 0.01 (−0.05, 0.08) |
| Childhood vision | 0.12 * (0.02, 0.23) | |
| F. Cognitive functioning | ||
| Life-course persistent | −0.25 *** (−0.31, −0.18) | −0.09 *** (−0.13, −0.04) |
| Childhood-limited | −0.17 *** (−0.24, −0.11) | −0.01 (−0.06, 0.03) |
| Adolescence-limited | −0.14 *** (−0.21, −0.07) | −0.07 ** (−0.12, −0.03) |
| Childhood IQ | 0.77 *** (0.74, 0.80) | |
| Health Problems in Adulthood | Adverse Experiences | Early Childhood Self-Control Difficulties | |||||
|---|---|---|---|---|---|---|---|
| Tobacco Smoking | Antipsychotic Medication Use | Cancer/ Heart Attack/Diabetes | Childhood SES | Childhood Maltreatment | Lifetime Incarceration | ||
| Pace of Aging | 0.23 *** | 0.19 *** | 0.18 *** | −0.24 *** | 0.19 *** | 0.23 *** | 0.15 *** |
| Social Hearing | −0.11 *** | −0.10 ** | −0.04 | 0.13 *** | −0.08 * | −0.11 *** | −0.18 *** |
| One-legged Balance | −0.16 *** | −0.12 *** | −0.12 *** | 0.19 *** | −0.14 *** | −0.08 ** | −0.15 *** |
| Gait Speed | −0.08 * | −0.09 * | −0.12 ** | 0.20 *** | −0.14 *** | −0.11 *** | −0.17 *** |
| Visual Contrast Sensitivity | −0.07 * | −0.12 ** | −0.03 | 0.06 | −0.14 *** | 0.02 | −0.10 ** |
| Cognitive Functioning | −0.2 1*** | −0.15 *** | −0.07 * | 0.36 *** | −0.17 *** | −0.15 *** | −0.33 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langevin, S.; Caspi, A.; Barnes, J.C.; Brennan, G.; Poulton, R.; Purdy, S.C.; Ramrakha, S.; Tanksley, P.T.; Thorne, P.R.; Wilson, G.; et al. Life-Course Persistent Antisocial Behavior and Accelerated Biological Aging in a Longitudinal Birth Cohort. Int. J. Environ. Res. Public Health 2022, 19, 14402. https://doi.org/10.3390/ijerph192114402
Langevin S, Caspi A, Barnes JC, Brennan G, Poulton R, Purdy SC, Ramrakha S, Tanksley PT, Thorne PR, Wilson G, et al. Life-Course Persistent Antisocial Behavior and Accelerated Biological Aging in a Longitudinal Birth Cohort. International Journal of Environmental Research and Public Health. 2022; 19(21):14402. https://doi.org/10.3390/ijerph192114402
Chicago/Turabian StyleLangevin, Stephanie, Ashalom Caspi, J. C. Barnes, Grace Brennan, Richie Poulton, Suzanne C. Purdy, Sandhya Ramrakha, Peter T. Tanksley, Peter R. Thorne, Graham Wilson, and et al. 2022. "Life-Course Persistent Antisocial Behavior and Accelerated Biological Aging in a Longitudinal Birth Cohort" International Journal of Environmental Research and Public Health 19, no. 21: 14402. https://doi.org/10.3390/ijerph192114402
APA StyleLangevin, S., Caspi, A., Barnes, J. C., Brennan, G., Poulton, R., Purdy, S. C., Ramrakha, S., Tanksley, P. T., Thorne, P. R., Wilson, G., & Moffitt, T. E. (2022). Life-Course Persistent Antisocial Behavior and Accelerated Biological Aging in a Longitudinal Birth Cohort. International Journal of Environmental Research and Public Health, 19(21), 14402. https://doi.org/10.3390/ijerph192114402






