Accuracy of Machine Learning Classification Models for the Prediction of Type 2 Diabetes Mellitus: A Systematic Survey and Meta-Analysis Approach
Highlights
- We reviewed soft-computing and statistical learning methods for predicting type 2 diabetes mellitus.
- We searched for papers published between 2010 and 2021 on three academic search engines, obtaining 34 relevant documents for the final meta-analysis.
- We analyzed the data extracted, compared the results and models, discussed their performance, and highlighted the issues related to T2DM.
- Finally, the decision trees model has the best prediction performances, with excellent accuracy compared to other soft-computing models in this systematic meta-analysis.
Abstract
1. Introduction
2. Materials and Methods
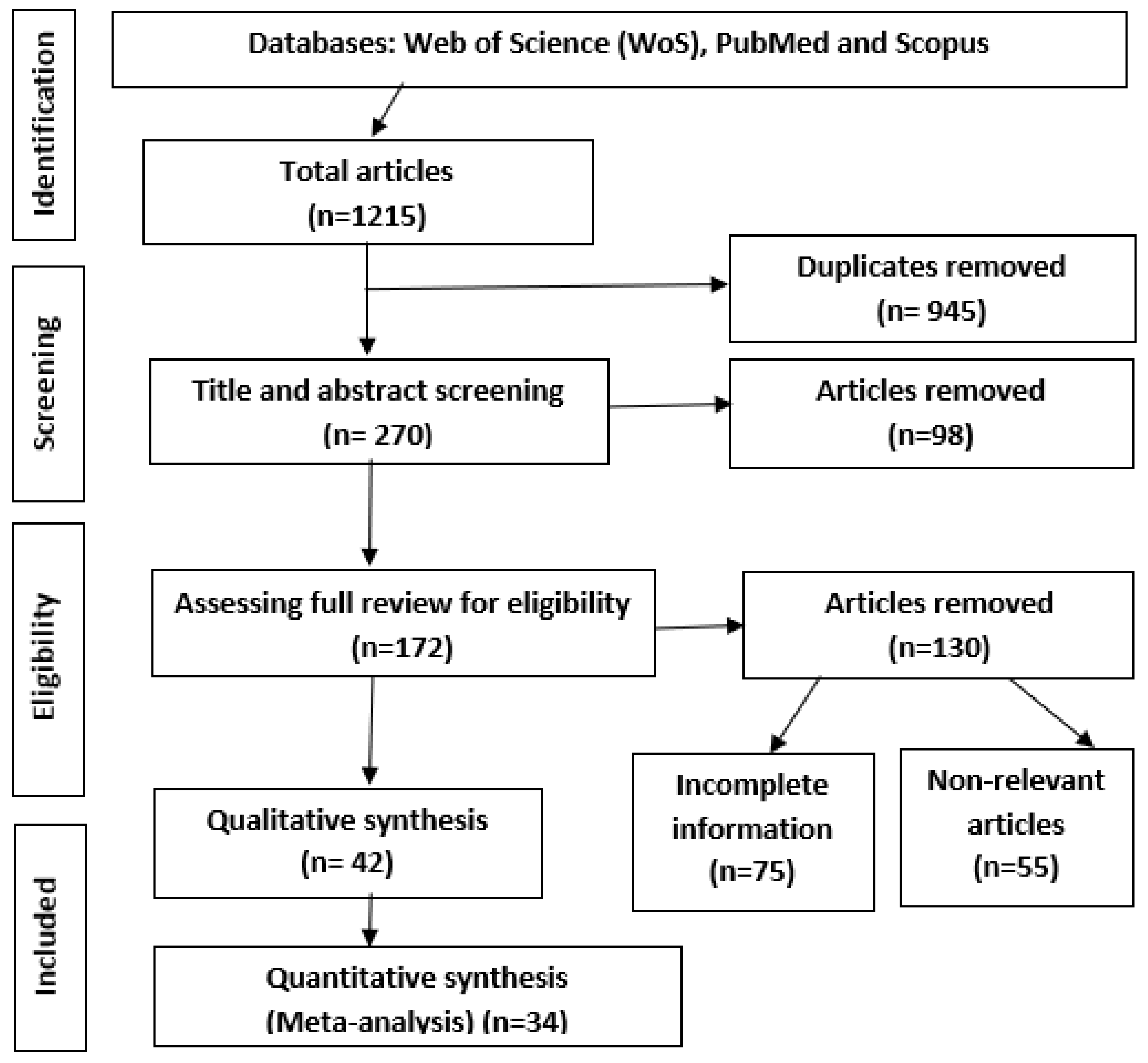
2.1. Search Strategy and Selection Process
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Assessments of Methodological Quality
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Selected Studies
3.2. Meta-Analyses Methods
3.3. Spatial Distribution of Articles and Soft-Computing Models
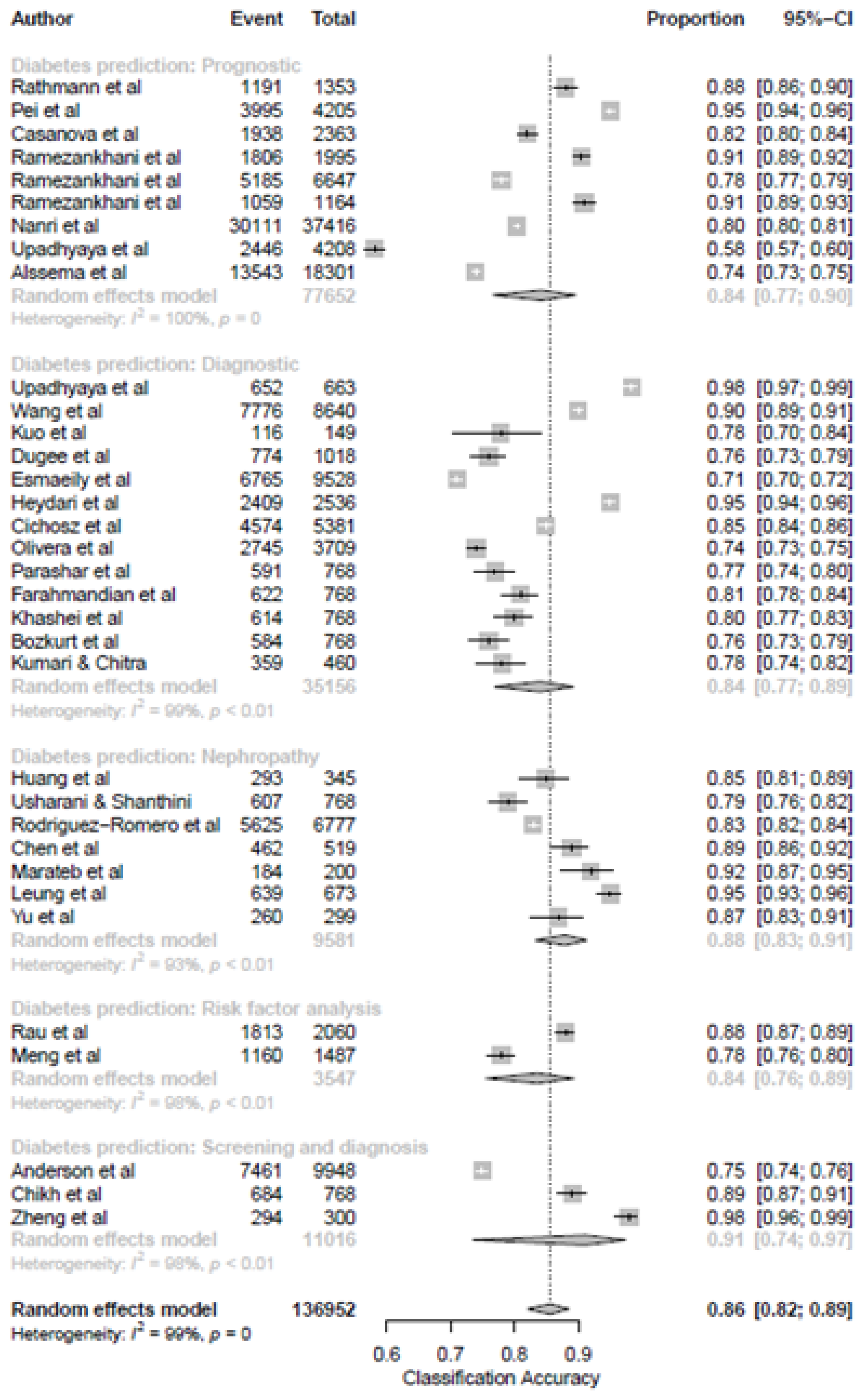
3.4. Results of the Meta-Analysis
Proportions of Classification Accuracy
3.5. ML Models and Diabetes Prediction
3.6. ML Models and Prediction of T2DM
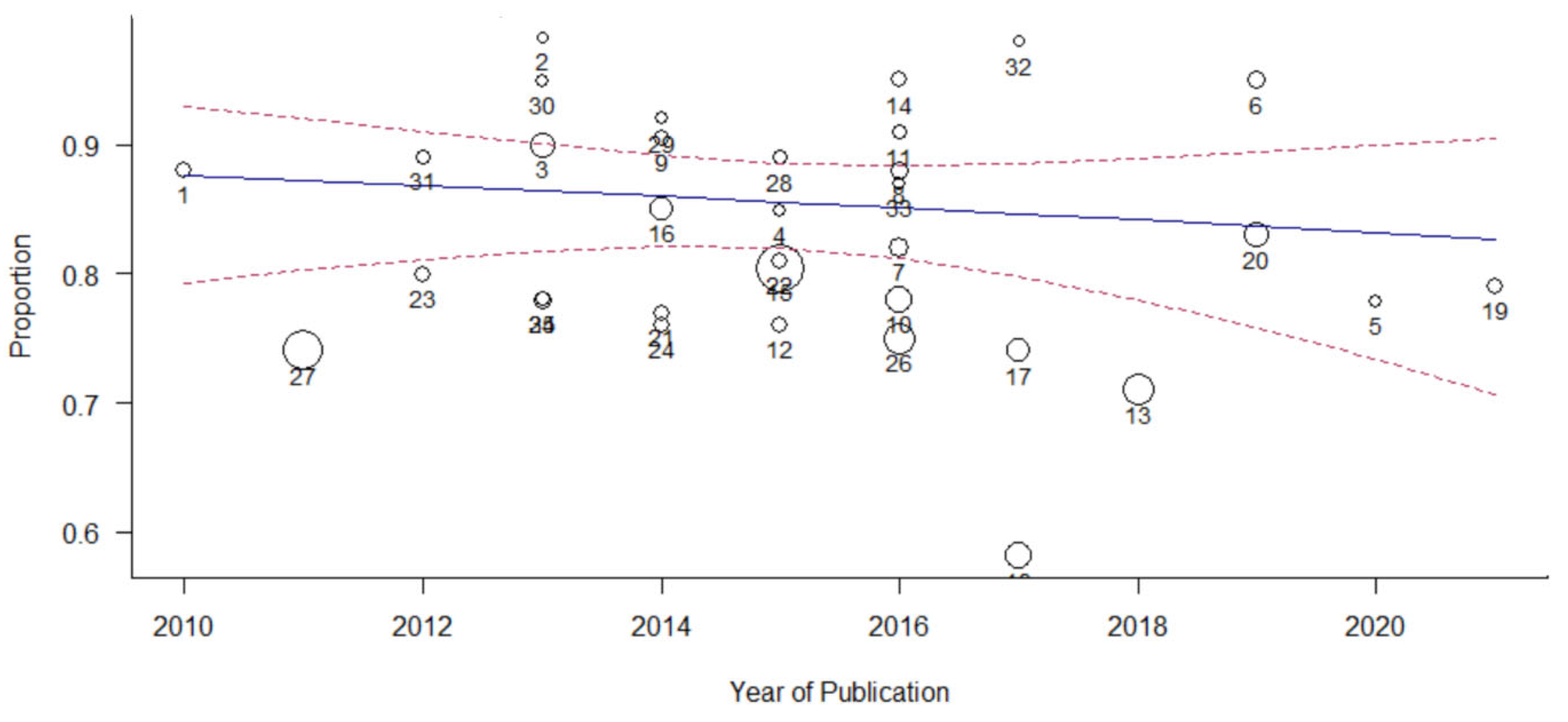
3.7. Moderator Analysis
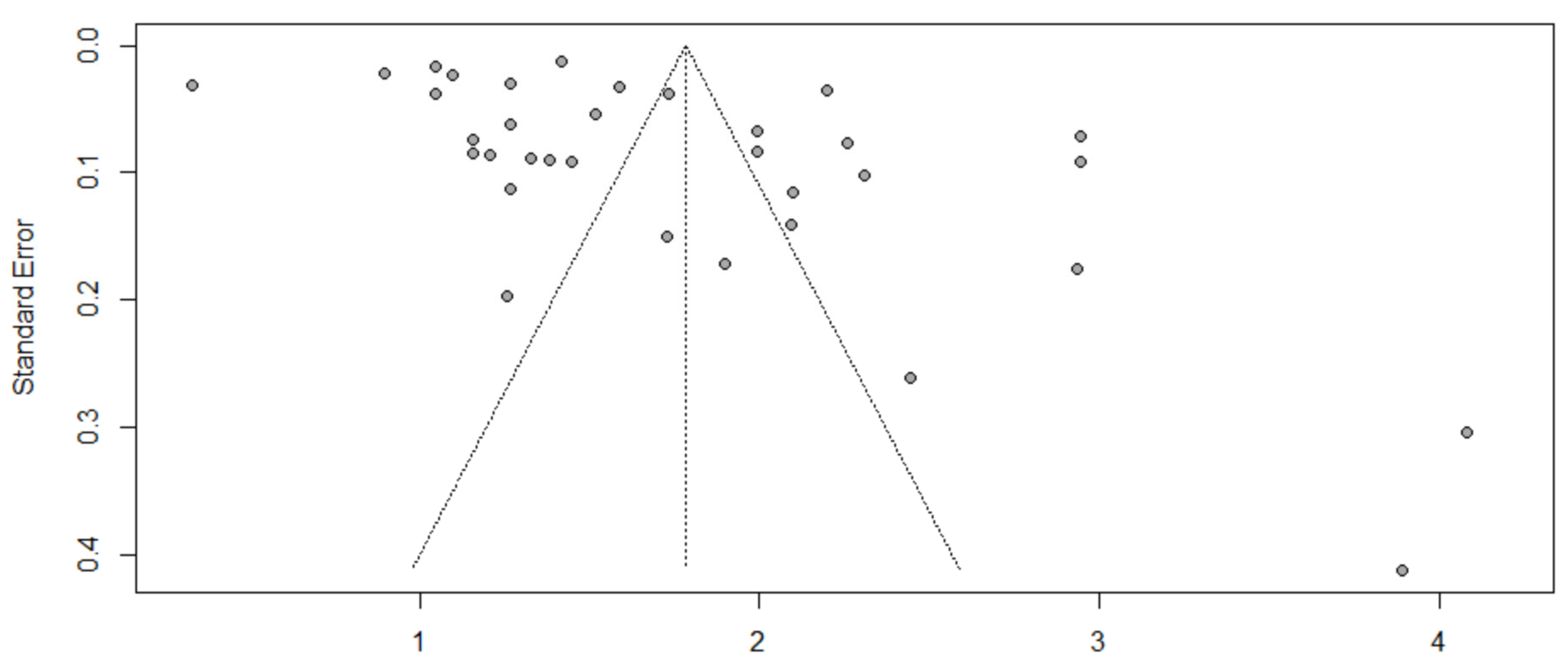
3.8. Evaluation of Publication Bias
4. Discussion
4.1. Synopsis of Evidence
4.2. Policy Implications
4.3. Limitations of the Overview Study
4.4. Concluding Remarks and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rigla, M.; García-Sáez, G.; Pons, B.; Hernando, M.E. Artificial Intelligence Methodologies and Their Application to Diabetes. J. Diabetes Sci. Technol. 2017, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Rau, H.-H.; Hsu, C.-Y.; Lin, Y.-A.; Atique, S.; Fuad, A.; Wei, L.-M.; Hsu, M.-H. Development of a web-based liver cancer prediction model for type II diabetes patients by using an artificial neural network. Comput. Methods Programs Biomed. 2016, 125, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, L.J.; Algehyne, E.A.; Usman, S.S. Predictive supervised machine learning models for diabetes mellitus. SN Comput. Sci. 2020, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, S.G.; Murphree, D.H., Jr.; Ngufor, C.G.; Knight, A.M.; Cronk, D.J.; Cima, R.R.; Curry, T.B.; Pathak, J.; Carter, R.E.; Kor, D.J. Automated diabetes case identification using electronic health record data at a tertiary care facility. Mayo Clin. Proc. Innov. Qual. Outcomes 2017, 1, 100–110. [Google Scholar] [CrossRef]
- Rathmann, W.; Kowall, B.; Heier, M.; Herder, C.; Holle, R.; Thorand, B.; Strassburger, K.; Peters, A.; Wichmann, H.E.; Giani, G.; et al. Prediction models for incident type 2 diabetes mellitus in the older population: KORA S4/F4 cohort study. Diabet. Med. 2010, 27, 1116–1123. [Google Scholar] [CrossRef]
- Wang, C.; Li, L.; Wang, L.; Ping, Z.; Flory, M.T.; Wang, G.; Xi, Y.; Li, W. Evaluating the risk of type 2 diabetes mellitus using artificial neural network: An effective classification approach. Diabetes Res. Clin. Pract. 2013, 100, 111–118. [Google Scholar] [CrossRef]
- Huang, G.-M.; Huang, K.-Y.; Lee, T.-Y.; Weng, J.T.-Y. An interpretable rule-based diagnostic classification of diabetic nephropathy among type 2 diabetes patients. BMC Bioinform. 2015, 16 (Suppl. S1), S5. [Google Scholar] [CrossRef]
- Kuo, K.-M.; Talley, P.; Kao, Y.; Huang, C.H. A multi-class classification model for supporting the diagnosis of type II diabetes mellitus. PeerJ 2020, 8, e9920. [Google Scholar] [CrossRef]
- Pei, D.; Gong, Y.; Kang, H.; Zhang, C.; Guo, Q. Accurate and rapid screening model for potential diabetes mellitus. BMC Med. Inform. Decis. Mak. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Casanova, R.; Saldana, S.; Simpson, S.L.; Lacy, M.E.; Subauste, A.R.; Blackshear, C.; Wagenknecht, L.; Bertoni, A.G. Prediction of Incident Diabetes in the Jackson Heart Study Using High-Dimensional Machine Learning. PLoS ONE 2016, 11, e0163942. [Google Scholar] [CrossRef]
- Ramezankhani, A.; Pournik, O.; Shahrabi, J.; Khalili, D.; Azizi, F.; Hadaegh, F. Applying decision tree for identification of a low risk population for type 2 diabetes. Tehran Lipid and Glucose Study. Diabetes Res. Clin. Pract. 2014, 105, 391–398. [Google Scholar] [CrossRef]
- Ramezankhani, A.; Hadavandi, E.; Pournik, O.; Shahrabi, J.; Azizi, F.; Hadaegh, F. Decision tree-based modelling for identification of potential interactions between type 2 diabetes risk factors: A decade follow-up in a Middle East prospective cohort study. BMJ Open 2016, 6, e013336. [Google Scholar] [CrossRef]
- Ramezankhani, A.; Pournik, O.; Shahrabi, J.; Azizi, F.; Hadaegh, F.; Khalili, D. The Impact of Oversampling with SMOTE on the Performance of 3 Classifiers in Prediction of Type 2 Diabetes. Med. Decis. Mak. 2014, 36, 137–144. [Google Scholar] [CrossRef]
- Dugee, O.; Janchiv, O.; Jousilahti, P.; Sakhiya, A.; Palam, E.; Nuorti, J.P.; Peltonen, M. Adapting existing diabetes risk scores for an Asian population: A risk score for detecting undiagnosed diabetes in the Mongolian population. BMC Public Health 2015, 15, 938. [Google Scholar] [CrossRef]
- Esmaily, H.; Tayefi, M.; Doosti, H.; Ghayour-Mobarhan, M.; Nezami, H.; Amirabadizadeh, A. A Comparison between Decision Tree and Random Forest in Determining the Risk Factors Associated with Type 2 Diabetes. J. Res. Health Sci. 2018, 18, e00412. [Google Scholar]
- Baum, A.; Scarpa, J.; Bruzelius, E.; Tamler, R.; Basu, S.; Faghmous, J. Targeting weight loss interventions to reduce cardiovascular complications of type 2 diabetes: A machine learning-based post-hoc analysis of heterogeneous treatment effects in the Look AHEAD trial. Lancet Diabetes Endocrinol. 2017, 5, 808–815. [Google Scholar] [CrossRef]
- Wilkinson, J.; Arnold, K.F.; Murray, E.J.; van Smeden, M.; Carr, K.; Sippy, R.; de Kamps, M.; Beam, A.; Konigorski, S.; Lippert, C.; et al. time to reality check the promises of machine learning-powered precision medicine. Lancet Digit. Health 2020, 2, e677–e680. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Ogunsakin, R.E.; Olugbara, O.O.; Moyo, S.; Israel, C. Meta-analysis of studies on depression prevalence among diabetes mellitus patients in Africa. Heliyon 2021, 7, e07085. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Upadhyaya, S.; Farahmand, K.; Baker-Demaray, T. Comparison of NN and LR classifiers in the context of screening native American elders with diabetes. Expert Syst. Appl. 2013, 40, 5830–5838. [Google Scholar] [CrossRef]
- Heydari, M.; Teimouri, M.; Heshmati, Z.; Alavinia, M. Comparison of various classification algorithms in the diagnosis of type 2 diabetes in Iran. Int. J. Diabetes Dev. Ctries. 2015, 36, 167–173. [Google Scholar] [CrossRef]
- Nanri, A.; Nakagawa, T.; Kuwahara, K.; Yamamoto, S.; Honda, T.; Okazaki, H.; Uehara, A.; Yamamoto, M.; Miyamoto, T.; Kochi, T.; et al. Correction: Development of Risk Score for Predicting 3-Year Incidence of Type 2 Diabetes: Japan Epidemiology Collaboration on Occupational Health Study. PLoS ONE 2018, 13, e0199075. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.L.; Johansen, M.D.; Ejskjaer, N.; Hansen, T.K.; Hejlesen, O.K. A novel model enhances HbA1c-based diabetes screening using simple anthropometric, anamnestic, and demographic information. J. Diabetes 2014, 6, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.R.; Roesler, V.; Iochpe, C.; Schmidt, M.I.; Vigo, Á.; Barreto, S.M.; Duncan, B.B. Comparison of ma-chine-learning algorithms to build a predictive model for detecting undiagnosed diabetes-ELSA-Brasil: Accuracy study. Sao Paulo Med. J. 2017, 135, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Usharani, R.; Shanthini, A. Neuropathic complications: Type II diabetes mellitus and other risky parameters using machine learning algorithms. J. Ambient. Intell. Humaniz. Comput. 2021, 1–23. [Google Scholar] [CrossRef]
- Rodriguez-Romero, V.; Bergstrom, R.F.; Decker, B.S.; Lahu, G.; Vakilynejad, M.; Bies, R.R. Prediction of nephropathy in type 2 diabetes: An analysis of the ACCORD trial applying machine learning techniques. Clin. Transl. Sci. 2019, 12, 519–528. [Google Scholar] [CrossRef]
- Parashar, A.; Burse, K.; Rawat, K. A Comparative approach for Pima Indians diabetes diagnosis using lda-support vector machine and feed forward neural network. Int. J. Adv. Res. Comput. Sci. Softw. Eng. 2014, 4, 378–383. [Google Scholar]
- Farahmandian, M.; Lotfi, Y.; Maleki, I. Data mining algorithms application in diabetes diseases diagnosis: A case study. MAGNT Res. Tech. Rep. 2015, 3, 989–997. [Google Scholar]
- Khashei, M.; Eftekhari, S.; Parvizian, J. Diagnosing diabetes type II using a soft intelligent binary classification model. Rev. Bioinform. Biom. 2012, 1, 9–23. [Google Scholar]
- Bozkurt, M.R.; Yurtay, N.; Yilmaz, Z.; Sertkaya, C. Comparison of different methods for determining diabetes. Turk. J. Electr. Eng. Comput. Sci. 2014, 22, 1044–1055. [Google Scholar] [CrossRef]
- Kumari, V.A.; Chitra, R. Classification of diabetes disease using support vector machine. Int. J. Eng. Res. Appl. 2013, 3, 1797–1801. [Google Scholar]
- Anderson, A.E.; Kerr, W.T.; Thames, A.; Li, T.; Xiao, J.; Cohen, M.S. Electronic health record phenotyping improves detection and screening of type 2 diabetes in the general United States population: A cross-sectional, unselected, retrospective study. J. Biomed. Inform. 2016, 60, 162–168. [Google Scholar] [CrossRef]
- Alssema, M.; Vistisen, D.; Heymans, M.W.; Nijpels, G.; Glümer, C.; Zimmet, P.Z.; Shaw, J.E.; Eliasson, M.; Stehouwer, C.D.; Tabák, A.G.; et al. The Evaluation of Screening and Early Detection Strategies for Type 2 Diabetes and Im-paired Glucose Tolerance (DETECT-2) update of the Finnish diabetes risk score for prediction of incident type 2 diabetes. Diabetologia 2011, 54, 1004–1012. [Google Scholar] [CrossRef]
- Chen, J.; Tang, H.; Huang, H.; Lv, L.; Wang, Y.; Liu, X.; Lou, T. Development and validation of new glomerular filtration rate predicting models for Chinese patients with type 2 diabetes. J. Transl. Med. 2015, 13, 317. [Google Scholar] [CrossRef]
- Marateb, H.R.; Mansourian, M.; Faghihimani, E.; Amini, M.; Farina, D. A hybrid intelligent system for diagnosing microalbumi-nuria in type 2 diabetes patients without having to measure urinary albumin. Comput. Biol. Med. 2014, 45, 34–42. [Google Scholar] [CrossRef]
- Leung, R.K.; Wang, Y.; Ma, R.C.; Luk, A.O.; Lam, V.; Ng, M.; So, W.Y.; Tsui, S.K.; Chan, J. Using a multi-staged strategy based on machine learning and mathematical modeling to predict genotype-phenotype risk patterns in diabetic kidney disease: A prospective case–control cohort analysis. BMC Nephrol. 2013, 14, 162. [Google Scholar] [CrossRef]
- Chikh, M.A.; Saidi, M.; Settouti, N. Diagnosis of Diabetes Diseases Using an Artificial Immune Recognition System2 (AIRS2) with Fuzzy K-nearest Neighbor. J. Med. Syst. 2011, 36, 2721–2729. [Google Scholar] [CrossRef]
- Zheng, T.; Xie, W.; Xu, L.; He, X.; Zhang, Y.; You, M.; Yang, G.; Chen, Y. A machine learning-based framework to identify type 2 diabetes through electronic health records. Int. J. Med. Inform. 2016, 97, 120–127. [Google Scholar] [CrossRef]
- Yu, C.S.; Liu, C.S.; Chen, R.S.; Lin, C.W. Artificial neural networks for estimating glomerular filtration rate by urinary dipstick for type 2 diabetic patients. Biomed Eng Singap. 2016, 28, 1650016. [Google Scholar]
- Meng, X.H.; Huang, Y.X.; Rao, D.P.; Zhang, Q.; Liu, Q. Comparison of three data mining models for predicting diabetes or pre-diabetes by risk factors. Kaohsiung J. Med. Sci. 2013, 29, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lary, D.J.; Alavi, A.H.; Gandomi, A.H.; Walker, A.L. Machine learning in geosciences and remote sensing. Geosci. Front. 2016, 7, 3–10. [Google Scholar] [CrossRef]
- Dou, J.; Yunus, A.P.; Bui, D.T.; Merghadi, A.; Sahana, M.; Zhu, Z.; Chen, C.-W.; Han, Z.; Pham, B.T. Improved landslide assessment using support vector machine with bagging, boosting, and stacking ensemble machine learning framework in a mountainous watershed, Japan. Landslides 2019, 17, 641–658. [Google Scholar] [CrossRef]
- Lee, Y.; Ragguett, R.M.; Mansur, R.B.; Boutilier, J.J.; Rosenblat, J.D.; Trevizol, A.; Brietzke, E.; Lin, K.; Pan, Z.; Subramaniapillai, M.; et al. Applications of machine learning algorithms to predict therapeutic outcomes in depression: A me-ta-analysis and systematic review. J. Affect. Disord. 2018, 241, 519–532. [Google Scholar] [CrossRef] [PubMed]
- De Silva, K.; Lee, W.K.; Forbes, A.; Demmer, R.T.; Barton, C.; Enticott, J. Use and performance of machine learning models for type 2 diabetes prediction in community settings: A systematic review and meta-analysis. Int. J. Med Inform. 2020, 143, 104268. [Google Scholar] [CrossRef]
- Levy, O.; Goldberg, Y.; Dagan, I. Improving Distributional Similarity with Lessons Learned from Word Embeddings. Trans. Assoc. Comput. Linguist. 2015, 3, 211–225. [Google Scholar] [CrossRef]
- Lucic, M.; Kurach, K.; Michalski, M.; Gelly, S.; Bousquet, O. Are gans created equal? a large-scale study. arXiv 2017, arXiv:1711.10337. [Google Scholar]
- Krittanawong, C.; Virk, H.U.H.; Bangalore, S.; Wang, Z.; Johnson, K.W.; Pinotti, R.; Zhang, H.; Kaplin, S.; Narasimhan, B.; Kitai, T.; et al. Machine learning prediction in cardiovascular diseases: A meta-analysis. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Zou, Q.; Qu, K.; Luo, Y.; Yin, D.; Ju, Y.; Tang, H. Predicting Diabetes Mellitus With Machine Learning Techniques. Front. Genet. 2018, 9, 515. [Google Scholar] [CrossRef]
- Ouyang, F.S.; Guo, B.L.; Ouyang, L.Z.; Liu, Z.W.; Lin, S.J.; Meng, W.; Huang, X.Y.; Chen, H.X.; Qiu-Gen, H.; Yang, S.M. Comparison between linear and non-linear machine-learning algorithms for the classification of thyroid nodules. Eur. J. Radiol. 2019, 113, 251–257. [Google Scholar] [CrossRef]







| Author | Reference | Year | Diabetes Prediction | Sample Size | Sensitivity (%) | Specificity (%) | Overall Classification Accuracy (%) | Classification Technique | Country First Author | Impact Factor |
|---|---|---|---|---|---|---|---|---|---|---|
| Rathmann et al. | [5] | 2010 | Prognostic | 1353 | 88 | LR | Germany | 3.11 | ||
| Upadhyaya et al. | [21] | 2013 | Diagnostic | 663 | 97 | 99 | 98 | NN | USA | 5.45 |
| Wang et al. | [6] | 2013 | Diagnostic | 8640 | 87 | 79 | 90 | ANN | China | 3.24 |
| Huang et al. | [7] | 2015 | Nephropathy | 345 | 85 | 83 | 85 | DT | China | 3.24 |
| Kuo et al. | [8] | 2020 | Diagnostic | 149 | 78 | DT | China | 2.38 | ||
| Pei et al. | [9] | 2019 | Prognostic | 4205 | 95 | DT | China | 2.07 | ||
| Casanova et al. | [10] | 2016 | Prognostic | 2363 | 82 | RF | USA | 2.78 | ||
| Rau et al. | [2] | 2016 | Risk factor analysis | 2060 | 75 | 75 | 88 | ANN | Taiwan | 3.63 |
| Ramezankhani et al. | [11] | 2014 | Prognostic | 1995 | 31 | 98 | 91 | DT | Iran | 3.24 |
| Ramezankhani et al. | [12] | 2016 | Prognostic | 6647 | 70 | 79 | 78 | DT | Iran | 2.38 |
| Ramezankhani et al. | [13] | 2016 | Prognostic | 1164 | 22 | 99 | 91 | DT | Iran | 2.79 |
| Dugee et al. | [14] | 2015 | Diagnostic | 1018 | 76 | LR | Mongolia & Finland | 2.57 | ||
| Esmaeily et al. | [15] | 2018 | Diagnostic | 9528 | 71 | 70 | 71 | RF | Iran | 1.51 |
| Heydari et al. | [22] | 2016 | Diagnostic | 2536 | 98 | 67 | 95 | DT | Iran | 0.59 |
| Nanri et al. | [23] | 2015 | Prognostic | 37,416 | 84 | 80 | 80 | LR | Japan | 2.78 |
| Cichosz et al. | [24] | 2014 | Diagnostic | 5381 | 85 | LR | Denmark | 3.30 | ||
| Olivera et al. | [25] | 2017 | Diagnostic | 3709 | 66 | 69 | 74 | ANN | Brazil | 0.13 |
| Upadhyaya et al. | [4] | 2017 | Prognostic | 4208 | 99 | 99 | 58 | Phenotyping | USA | 0.00 |
| Usharani & Shanthini | [26] | 2021 | Nephropathy | 768 | 79 | LR | India | 4.59 | ||
| Rodriguez-Romero et al. | [27] | 2019 | Nephropathy | 6777 | 83 | RF | USA | 3.99 | ||
| Parashar et al. | [28] | 2014 | Diagnostic | 768 | 77 | SVM | China | 2.5 | ||
| Farahmandian et al. | [29] | 2015 | Diagnostic | 768 | 81 | SVM | Iran | 0.00 | ||
| Khashei et al. | [30] | 2012 | Diagnostic | 768 | 80 | SVM | Iran | 0.00 | ||
| Bozkurt et al. | [31] | 2014 | Diagnostic | 768 | 53 | 89 | 76 | ANN | India | 0.68 |
| Kumari & Chitra | [32] | 2013 | Diagnostic | 460 | 78 | SVM | India | 1.45 | ||
| Anderson et al. | [33] | 2016 | Screening and diagnosis | 9948 | 80 | 73 | 75 | LR | USA | 2.95 |
| Alssema et al. | [34] | 2011 | Prognostic | 18,301 | 74 | LR | The Netherlands | 7.11 | ||
| Chen et al. | [35] | 2015 | Nephropathy | 519 | 89 | ANN | China | 4.19 | ||
| Marateb et al. | [36] | 2014 | Nephropathy | 200 | 95 | 85 | 92 | Hybrid model | Iran | 3.43 |
| Leung et al. | [37] | 2013 | Nephropathy | 673 | 95 | SVM | China | 2.03 | ||
| Chikh et al. | [38] | 2012 | Screening and diagnosis | 768 | 85 | 92 | 89 | CRISP | Algeria | 3.06 |
| Zheng et al. | [39] | 2017 | Screening and diagnosis | 300 | 98 | LR | China | 3.03 | ||
| Yu et al. | [40] | 2016 | Nephropathy | 299 | 83 | 88 | 87 | ANN | Taiwan | 0.43 |
| Meng et al. | [41] | 2013 | Risk factor analysis | 1487 | 81 | 75 | 78 | DT | China | 1.74 |
| Model | SE | p-Values | QM | df | p-Values | |
|---|---|---|---|---|---|---|
| Publication year | −0.0359 | 0.0532 | 0.5001 | 0.4546 | 1 | 0.5001 |
| Impact factor | 0.1297 | 0.0809 | 0.1086 | 2.5747 | 1 | 0.1086 |
| Diabetes prediction | 2.2366 | 4 | 0.6923 | |||
| Diagnostic | Ref | |||||
| Nephropathy | 0.3391 | 0.3516 | 0.3348 | |||
| Prognostic | 0.0219 | 0.3213 | 0.9456 | |||
| Risk factor analysis | −0.0210 | 0.5617 | 0.9701 | |||
| Screening and diagnosis | 0.5811 | 0.4905 | 0.2361 | |||
| Model types | 26.0392 | 8 | 0.0010 | |||
| ANN | Ref | |||||
| CRISP method | 0.3714 | 0.6090 | 0.5420 | |||
| Decision trees | 0.2786 | 0.3035 | 0.3586 | |||
| Hybrid model | 0.7166 | 0.6523 | 0.2720 | |||
| Linear regression | −0.1191 | 0.3047 | 0.6959 | |||
| Neural network | 2.3564 | 0.6708 | 0.0004 * | |||
| Phenotyping | −1.3977 | 0.5988 | 0.0196 * | |||
| Random forest | −0.3935 | 0.3933 | 0.3171 | |||
| Support vector machine | −0.0946 | 0.3409 | 0.7813 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olusanya, M.O.; Ogunsakin, R.E.; Ghai, M.; Adeleke, M.A. Accuracy of Machine Learning Classification Models for the Prediction of Type 2 Diabetes Mellitus: A Systematic Survey and Meta-Analysis Approach. Int. J. Environ. Res. Public Health 2022, 19, 14280. https://doi.org/10.3390/ijerph192114280
Olusanya MO, Ogunsakin RE, Ghai M, Adeleke MA. Accuracy of Machine Learning Classification Models for the Prediction of Type 2 Diabetes Mellitus: A Systematic Survey and Meta-Analysis Approach. International Journal of Environmental Research and Public Health. 2022; 19(21):14280. https://doi.org/10.3390/ijerph192114280
Chicago/Turabian StyleOlusanya, Micheal O., Ropo Ebenezer Ogunsakin, Meenu Ghai, and Matthew Adekunle Adeleke. 2022. "Accuracy of Machine Learning Classification Models for the Prediction of Type 2 Diabetes Mellitus: A Systematic Survey and Meta-Analysis Approach" International Journal of Environmental Research and Public Health 19, no. 21: 14280. https://doi.org/10.3390/ijerph192114280
APA StyleOlusanya, M. O., Ogunsakin, R. E., Ghai, M., & Adeleke, M. A. (2022). Accuracy of Machine Learning Classification Models for the Prediction of Type 2 Diabetes Mellitus: A Systematic Survey and Meta-Analysis Approach. International Journal of Environmental Research and Public Health, 19(21), 14280. https://doi.org/10.3390/ijerph192114280






