Effects of Multicomponent Exercise Training on the Health of Older Women with Osteoporosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Research Question
2.4. Risk of Bias Analysis
2.5. Methodological Quality Analysis
2.6. Data Collection Process
2.7. Meta-Analysis
2.8. Evidence Level Assessment
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Search Phrase |
| MEDLINE (PubMed) | ((“osteoporosis”[MeSH Terms] OR “osteoporosis”[All Fields] OR “osteoporoses”[All Fields] OR “osteoporosis, postmenopausal”[MeSH Terms] OR (“osteoporosis”[All Fields] AND “postmenopausal”[All Fields]) OR “postmenopausal osteoporosis”[All Fields]) AND (“aged”[MeSH Terms] OR “aged”[All Fields] OR “elderly”[All Fields] OR “elderlies”[All Fields] OR “elderly s”[All Fields] OR “elderlys”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “treatments”[All Fields] OR “therapy”[MeSH Subheading] OR “therapy”[All Fields] OR “treatment”[All Fields] OR “treatment s”[All Fields]) AND (“exercise”[MeSH Terms] OR “exercise”[All Fields] OR “exercises”[All Fields] OR “exercise therapy”[MeSH Terms] OR (“exercise”[All Fields] AND “therapy”[All Fields]) OR “exercise therapy”[All Fields] OR “exercise s”[All Fields] OR “exercised”[All Fields] OR “exerciser”[All Fields] OR “exercisers”[All Fields] OR “exercising”[All Fields])) AND (clinicaltrial[Filter] OR randomizedcontrolledtrial[Filter]) |
| Web of Science | (((TS = (osteoporosis)) AND TS = (elderly)) AND TS = (exercise)) AND TS = (treatment) |
| Scopus | (TITLE-ABS-KEY (osteoporosis) OR TITLE-ABS-KEY (bone loss) OR TITLE-ABS-KEY (osteoporosis) AND TITLE-ABS-KEY (therapeutic) OR TITLE-ABS-KEY (therapy) OR TITLE-ABS-KEY (treatment) AND TITLE-ABS-KEY (elderly) OR TITLE-ABS-KEY (aged) AND TITLE-ABS-KEY(exercise) OR TITLE-ABS-KEY(physical activity) OR TITLE-ABS-KEY(physical exercise)) |
| CINHAL | AB (osteoporosis OR bone density OR bone loss) AND AB (elderly OR aged OR older OR elder or geriatric) AND AB (treatment OR intervention OR therapy) AND AB (exercise OR physical activity) |
References
- Marcos-Pardo, P.J.; Orquin-Castrillón, F.J.; Gea-García, G.M.; Menayo-Antúnez, R.; González-Gálvez, N.; Vale, R.G.S.; Martínez-Rodríguez, A. Effects of a moderate-to-high intensity resistance circuit training on fat mass, functional capacity, muscular strength, and quality of life in elderly: A randomized controlled trial. Sci. Rep. 2019, 9, 7830. [Google Scholar] [CrossRef] [PubMed]
- Banitalebi, E.; Ghahfarrokhi, M.M.; Dehghan, M. Effect of 12-weeks elastic band resistance training on MyomiRs and osteoporosis markers in elderly women with osteosarcopenic obesity: A randomized controlled trial. BMC Geriatr. 2021, 21, 433. [Google Scholar] [CrossRef] [PubMed]
- Braz, R.R.S.; Campos, S.L.; Villela, D.W.; Antonino, G.B.; Batista, P.K.A.; Guerino, M.R.; Rodrigues, F.T.M.; Alves, K.F.P.; Duarte, J.V.T.; de Andrade Silva, D.; et al. Effectiveness of whole-body vibration combined with multicomponent training on the risk of falls and quality of life in elderly women with osteoporosis: Study protocol for a randomized controlled clinical trial. Biology 2022, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- DadeMatthews, O.O.; Agostinelli, P.J.; Neal, F.K.; Oladipupo, S.O.; Hirschhorn, R.M.; Wilson, A.E.; Sefton, J.M. Systematic review and meta-analyses on the effects of whole-body vibration on bone health. Complement. Ther. Med. 2022, 65, 102811. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Matthews, B.; Greig, A.; Briggs, A.; Kelly, A.; Sherburn, M.; Larsen, J.; Wark, J. Effects of an exercise and manual therapy program on physical impairments, function and quality-of-life in people with osteoporotic vertebral fracture: A randomised, single-blind controlled pilot trial. BMC Musculoskelet. Disord. 2010, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Borba-Pinheiro, C.J.; Dantas, E.H.M.; Vale, R.G.S.; Drigo, A.J.; Carvalho, M.C.G.; Tonini, T.; Figueiredo, N.M.A. Resistance training programs on bone related variables and functional independence of postmenopausal women in pharmacological treatment: A randomized controlled trial. Arch. Gerontol. Geriatr. 2016, 65, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.D.; Khan, K.M.; McKay, H.A.; Petit, M.A.; Waterman, C.; Heinonen, A.; Janssen, P.A.; Donaldson, M.G.; Mallinson, A.; Riddell, L.; et al. Community-based exercise program reduces risk factors for falls in 65- to 75-year-old women with osteoporosis: Randomized controlled trial. CMAJ 2002, 167, 997–1004. [Google Scholar] [PubMed]
- Silva Sobrinho, A.C.; Almeida, M.L.; Rodrigues, G.S.; Finzeto, L.C.; Silva, V.R.R.; Bernatti, R.F.; Bueno Junior, C.R. Effect of flexibility training associated with multicomponent training on posture and quality of movement in physically inactive older women: A randomized study. Int. J. Environ. Res. Public Health 2021, 18, 10709. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.B.; Rodacki, A.; Costa, S.; Pitta, A.; Bento, P.C. Perceptive-cognitive and physical function in pre-frail older adults: Exergaming vs traditional multicomponent training. Rejuvenation Res. 2020, 24, 28–36. [Google Scholar] [CrossRef]
- Cardoso, A.P.; Pereira, Z.S.; Lopes Júnior, D.B.; Araújo-Gomes, R.C.; Carvalho, P.D.P.; Rivera, L.F.S.; Drigo, A.J.; Borba-Pinheiro, C.J. Efectos de un programa de entrenamiento multicomponente sobre indicadores de salud física y cognitiva de mujeres mayores. Rev. Cien. Actividad Física 2021, 22, 1–19. [Google Scholar] [CrossRef]
- Silva Sobrinho, A.C.; Almeida, M.L.; Rodrigues, G.S.; Bertani, R.F.; Lima, J.G.R.; Bueno Junior, C.R. Stretching and multicomponent training to functional capacities of older women: A randomized study. Int. J. Environ. Res. Public Health 2022, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Nakai, Y.; Tomioka, K.; Taniguchi, Y.; Sato, N.; Wada, A.; Kiyama, R.; Tsutsumimoto, K.; Ohishi, M.; Kiuchi, Y.; et al. Effects of a multicomponent exercise program in physical function and muscle mass in sarcopenic/pre-sarcopenic adults. J. Clin. Med. 2020, 9, 1386. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.S.; Hsieh, C.C.; Cheng, H.S.; Tseng, T.J.; Su, S.C. Association between physical fitness and successful aging in Taiwanese older adults. PLoS ONE 2016, 11, e0150389. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Chang, C.B.; Han, D.S.; Hong, C.H.; Hwang, J.S.; Tsai, K.S.; Yang, R.S. Effects of exercise improves muscle strength and fat mass in patients with high fracture risk: A randomized control trial. J. Formos. Med. Assoc. 2018, 117, 572–582. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Santos, C.M.C.; Pimenta, C.A.M.; Nobre, M.R. The PICO strategy for the research question construction and evidence searches. Rev. Lat. Am. Enfermagem 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Montori, V.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Djulbegovic, B.; Atkins, D.; Falck-Ytter, Y.; et al. GRADE guidelines: 5. Rating the quality of evidence—Publication bias. J. Clin. Epidemiol. 2011, 64, 1277–1282. [Google Scholar] [CrossRef]
- Burke, T.N.; França, F.J.R.; Meneses, S.R.F.; Cardoso, V.I.; Marques, A.P. Postural control in elderly persons with osteoporosis: Efficacy of an intervention program to improve balance and muscle strength. Am. J. Phys. Med. 2010, 89, 549–556. [Google Scholar] [CrossRef]
- Dizdar, M.; Irdesel, J.F.; Dizdar, O.S.; Topsaç, M. Effects of balance-coordination, strengthening, and aerobic exercises to prevent falls in postmenopausal patients with osteoporosis: A 6-month randomized parallel prospective study. J. Aging Phys. Act. 2018, 26, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Evstigneeva, L.; Lesnyak, O.; Bultink, I.E.M.; Lems, W.F.; Kozhemyakina, E.; Negodaeva, E.; Belkin, A. Effect of twelve-month physical exercise program on patients with osteoporotic vertebral fractures: A randomized, controlled trial. Osteoporos. Int. 2016, 27, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- FilipoviĆ, T.N.; LazoviĆ, M.P.; BackoviĆ, A.N.; FilipoviĆ, A.N.; IgnjatoviĆ, A.M.; DimitrijeviĆ, S.S.; GopČeviĆ, K.R. A 12-week exercise program improves functional status in postmenopausal osteoporotic women: Randomized controlled study. Eur. J. Phys. Rehabil. Med. 2021, 57, 120–130. [Google Scholar] [CrossRef] [PubMed]
- García-Gomáriz, C.; Blasco, J.M.; Macián-Romero, C.; Guillem-Hernández, E.; Igual-Camacho, C. Effect of 2 years of endurance and high-impact training on preventing osteoporosis in postmenopausal women. Menopause 2018, 25, 301–306. [Google Scholar] [CrossRef]
- Halvarsson, A.; Franzén, E.; Ståhle, A. Balance training with multi-task exercises improves fall-related self-efficacy, gait, balance performance and physical function in older adults with osteoporosis: A randomized controlled trial. Clin. Rehabil. 2014, 29, 365–375. [Google Scholar] [CrossRef]
- Lord, S.R.; Ward, J.A.; Williams, P.; Zivanovic, E. The effects of a community exercise program on fracture risk factors in older women. Osteoporos. Int. 1996, 6, 361–367. [Google Scholar] [CrossRef]
- Olsen, C.F.; Bergland, A. The effect of exercise and education on fear of falling in elderly women with osteoporosis and a history of vertebral fracture: Results of a randomized controlled trial. Osteoporos. Int. 2014, 25, 2017–2025. [Google Scholar] [CrossRef]
- Paolucci, T.; Morone, G.; Iosa, M.; Grasso, M.R.; Buzi, E.; Zangrando, F.; Fusco, A. Efficacy of group-adapted physical exercises in reducing back pain in women with postmenopausal osteoporosis. Aging Clin. Exp. Res. 2014, 26, 395–402. [Google Scholar] [CrossRef]
- Preisinger, E.; Alacamlioglu, Y.; Pils, K.; Bosina, E.; Metka, M.; Schneider, B.; Ernst, E. Exercise therapy for osteoporosis: Results of a randomised controlled trial. Br. J. Sports Med. 1996, 30, 209–212. [Google Scholar] [CrossRef]
- Stanghelle, B.; Bentzen, H.; Giangregorio, L.; Pripp, A.H.; Skelton, D.A.; Bergland, A. Physical fitness in older women with osteoporosis and vertebral fracture after a resistance and balance exercise programme: 3-month post-intervention follow-up of a randomised controlled trial. BMC Musculoskelet. Disord. 2020, 21, 471. [Google Scholar] [CrossRef]
- Nawrat-Szołtysik, A.; Miodońska, Z.; Opara, J.; Polak, A.; Matyja, B.; Małecki, A. Effect of physical activity on the quality of life in osteoporotic females living in residential facilities. J. Geriatr. Phys. Ther. 2019, 42, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Murtezani, A.; Nevzati, A.; Ibraimi, Z.; Sllamniku, S.; Meka, V.; Abazi, N. The effect of land versus aquatic exercise program on bone mineral density and physical function in postmenopausal women with osteoporosis: A randomized controlled trial. Ortop. Traumatol. Rehabil. 2014, 16, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 115–159. [Google Scholar] [CrossRef]
- Borba-Pinheiro, C.J.; Carvalho, M.C.A.; Silva, N.S.; Drigo, A.J.; Bezerra, J.C.; Dantas, E.H.M. Bone density, balance and quality of life of postmenopausal women taking alendronate participating in different physical activity programs. Ther. Adv. Musculoskelet. Dis. 2010, 2, 175–185. [Google Scholar] [CrossRef]
| Studies | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total |
|---|---|---|---|---|---|---|---|---|
| Burke et al. [20] | Uncertain | Low | Low | Low | Low | Low | Low | Uncertain |
| Carter et al. [7] | Low | Low | Low | Low | Low | Low | Low | Low |
| Dizdar et al. [21] | Low | Low | Low | Low | Low | Low | Low | Low |
| Evstigneeva et al. [22] | Low | Low | Low | Low | Low | Low | Low | Low |
| FilipoviĆ et al. [23] | Low | Low | Low | Low | Low | Low | Low | Low |
| Garcıa-Gomariz et al. [24] | Low | Low | Low | Low | Low | Low | Low | Low |
| Halvarsson et al. [25] | Low | Low | Low | Low | Low | Low | Low | Low |
| Lord et al. [26] | Low | Low | Low | Low | Low | Low | Low | Low |
| Murtezani et al. [23] | Low | Low | Low | Low | Low | Low | Low | Low |
| Olsen and Bergland [27] | Low | Low | Low | Low | Low | Low | Low | Low |
| Paolucci et al. [28] | Low | Low | Low | Low | Low | Low | Low | Low |
| Preisinger et al. [29] | Low | Low | Low | Low | Low | Low | Low | Low |
| Stanghelle et al. [30] | Low | Low | Low | Low | Low | Low | Low | Low |
| Nawrat-Szołtysik et al. [31] | Low | Low | Low | Low | Low | Low | Low | Low |
| Studies | 1a | 1b | 2a | 2b | 3 | Total Score |
|---|---|---|---|---|---|---|
| Burke et al. [20] | 1 | −1 | 0 | 0 | 1 | 1 |
| Carter et al. [7] | 1 | 1 | 0 | 0 | 1 | 3 |
| Dizdar et al. [21] | 1 | 1 | 0 | 0 | 1 | 3 |
| Evstigneeva et al. [22] | 1 | 1 | 0 | 0 | 1 | 3 |
| FilipoviĆ et al. [23] | 1 | 1 | 0 | 0 | 1 | 3 |
| Garcıa-Gomariz et al. [24] | 1 | 1 | 0 | 0 | 1 | 3 |
| Halvarsson et al. [25] | 1 | 1 | 0 | 0 | 1 | 3 |
| Lord et al. [26] | 1 | 1 | 0 | 0 | 1 | 3 |
| Murtezani et al. [32] | 1 | 1 | 0 | 0 | 1 | 3 |
| Olsen and Bergland [27] | 1 | 1 | 0 | 0 | 1 | 3 |
| Paolucci et al. [28] | 1 | 1 | 0 | 0 | 1 | 3 |
| Preisinger et al. [29] | 1 | 1 | 0 | 0 | 1 | 3 |
| Stanghelle et al. [30] | 1 | 1 | 0 | 0 | 1 | 3 |
| Nawrat-Szołtysik et al. [31] | 1 | 1 | 0 | 0 | 1 | 3 |
| Author | Year | Country | Age (Years) | EG (n) | CG (n) | Total (n) | BMD | |
|---|---|---|---|---|---|---|---|---|
| (T-Score) | (g/cm3) | |||||||
| Lord et al. [26] | 1996 | Australia | EG: 71.7 ± 5.4 CG: 71.5 ± 5.5 | 90 | 89 | 179 | NI | Lumbar spine: EG: 1.014 ± 0.2 CG: 0.965 ± 0.2 Femoral Neck: EG: 0.770 ± 0.1 CG: 0.742 ± 0.1 Trochanter EG: 0.689 ± 0.1 CG: 0.652 ± 0.1 |
| Preisinger et al. [29] | 1996 | Austria | EG1: 62.6 ± 5.9 EG2: 60.9 ± 7.8 CG: 59.0 ± 8.0 | EG1: 27 EG2: 34 | 31 | 92 | NI | Distal forearm: EG1: 0.266 EG2: 0.269 CG: 0.305 Mid-forearm: EG1: 0.382 EG2: 0.383 CG: 0.424 |
| Carter et al. [7] | 2002 | Colombia | EG: 69 ± 3.5 CG: 69.6 ± 3.0 | 40 | 40 | 80 | NI | Total hip or lumbar spine ≤−2.5 SD |
| Burke et al. [20] | 2010 | Brazil | EG: 72.8 ± 3.6 CG: 74.4 ± 3.7 | 17 | 16 | 33 | Lumbar spine: EG: −3.69 ± 0.83 CG: −3.53 ± 0.96 | NI |
| Murtezani et al. [32] | 2014 | Switzerland | EG1: 60.68 ± 7.62 EG2: 59.78 ± 5.99 | 31 | 30 | 61 | Lumbar spine: EG1: −3.04 ± 0.4 EG2: −3.10 ± 0.5 | NI |
| Olsen and Bergland [27] | 2014 | Norway | EG: 70.4 ± 5.9 CG: 72 ± 5.6 | 47 | 42 | 89 | NI | NI |
| Paolucci et al. [28] | 2014 | Switzerland | EG: 65.6 ± 5.8 CG: 65.6 ± 5.3 | 40 | 20 | 60 | NI | Lumbar spine ≤−2.5 SD |
| Halvarsson et al. [25] | 2014 | Sweden | EG1: 76 ± 10 EG2: 77 ± 9 CG: 76 ± 10 | EG1: 25 EG2: 18 | 26 | 69 | NI | NI |
| Evstigneeva et al. [22] | 2016 | Russia | EG: 70.7 ± 8.1 CG: 67.6 ± 7.0 | 40 | 38 | 78 | NI | NI |
| García−Gomáriz et al. [24] | 2017 | Spain | EG: 60.3 ± 5.4 CG: 56.5 ± 6.7 | 17 | 17 | 34 | Femoral neck: −0.76 Lumbar spine: 1.93 | NI |
| Dizdar et al. [21] | 2017 | Turkey | EG1: 57.87 ± 4.5 EG2: 59.86 ± 5.5 EG3: 60.91 ± 6.5 | EG1: 25 EG2: 25 EG3: 25 | – | 75 | Lumbar total: EG1: −2.44 ± 0.8 EG2: −2.62 ± 0.8 EG3: −2.54 ± 0.6 Femur neck: EG1: −1.67 ± 0.8 EG2: −1.85 ± 0.8 EG3: −1.97 ± 0.4 Femur total: EG1: −0.63 ± 1.2 EG2: −0.95 ± 0.9 EG3: −0.91 ± 1.1 | NI |
| Nawrat−Szołtysik et al. [31] | 2019 | Poland | EG: 81.5 ± 10 CG: 81.5 ± 10 | EG1: 23 EG2: 21 EG3: 23 | 24 | 91 | ≤1 | NI |
| Stanghelle et al. [30] | 2020 | Norway | EG: 74.2 ± 5.8 CG: 74.7 ± 6.1 | 76 | 73 | 149 | NI | Lumbar spine and femoral neck ≤ −2.5 SD |
| FilipoviĆ et al. [23] | 2021 | Serbia | EG: 64.40 ± 5.45 CG: 64.20 ± 5.08 | 47 | 49 | 96 | Neck: −2.62 ± 0.72 | Lumbar spine and femoral neck ≤ −2.5 SD |
| Study | Intervention | Duration (Weeks) | VT | |
|---|---|---|---|---|
| DT (min) | FT (×/week) | |||
| Lord et al. [26] | EG: RT for upper limbs and balance CG: no physical exercises | 5 | 60 | 4 |
| Preisinger et al. [29] | EG1: regular RT, balance, motor, and postural coordination EG2: irregular RT, balance, motor, and postural coordination CG: no physical exercises | 48 | 20 | 2 |
| Carter et al. [7] | EG: RT, balance, postural exercises, and coordination CG: no physical exercises | 20 | 40 | 2 |
| Burke et al. [20] | EG: RT for lower limbs and balance CG: no physical exercises | 8 | 30 | 2 |
| Murtezani et al. [32] | EG1: RT, balance, and aerobic exercise (land) EG2: aerobic exercise and RT (water) | 40 | 35 | 3 |
| Olsen and Bergland [27] | EG: aerobic circuit training, balance, and flexibility CG: no physical exercises | 12 | 60 | 3 |
| Paolucci et al. [28] | EG: low-impact aerobics training, balance, and flexibility CG: aerobic training | 24 | 60 | 3 |
| Halvarsson et al. [25] | EG1: balance EG2: balance and aerobic training CG: no physical exercises | 12 | 30–45 | 2 |
| Evstigneeva et al. [22] | EG: RT for lower limbs and balance CG: no physical exercises | 48 | 40 | 2 |
| Dizdar et al. [21] | EG1: RT, balance, and coordination EG2: RT EG3: aerobic training | 12–24 | 60 | 3 |
| García-Gomáriz et al. [24] | EG: RT and high-impact training + calcium + vitamin D CG: high-intensity walk | 96 | 60 | 2 |
| Nawrat-Szołtysik et al. [31] | EG1: modified Sinaki exercises EG2: Nordic walking EG3: modified Sinaki exercises + Nordic walking CG: did not perform physical exercises | 12 | 40 | 2 |
| Stanghelle et al. [30] | EG: RT and balance CG: no physical exercises | 24 | 60 | 2 |
| FilipoviĆ et al. [23] | EG: RT, aerobic training, and balance CG: no physical exercises | 4–24 | 50–60 | 5 |
| Study | Evaluation | Results in the EG (p < 0.05) |
|---|---|---|
| Lord et al. [26] | Balance | EG: ↔ Sway (d = −0.30) |
| BMD | EG: ↔ lumbar spine (d = 0.06); ↔ femoral neck (d = 0.08); ↔ trochanter (d = 0.04) | |
| Muscle strength | EG: ↑ quadriceps strength (d = 0.88) | |
| Preisinger et al. [29] | BMD | EG1: ↑ distal forearm; ↔ mid-forearm EG2: ↓ distal forearm; ↓ mid-forearm |
| Carter et al. [7] | Balance | EG: ↔ composite balance score (d = 0.13) |
| Functional fitness | EG: ↔ eight−figure (d = 0.33) | |
| Muscle strength | EG: ↔ knee extension strength (d = 0.09) | |
| QoL | EG: QUALEFFO−41: ↔ total score; ↔ social; ↔ general health perception; ↔ physical function; ↔ pain; ↔ mental state | |
| Burke et al. [20] | Isometric muscle strength | EG: ↑ ankle flexion; ↑ knee extension; ↑ knee flexion |
| Balance | EG: ↑ COP velocity; ↑ endpoint excursion; ↓ maximum excursion; ↓ directional control; ↑ stable surface/open eyes; ↓ stable surface/closed eyes; ↓ unstable surface/open eyes; ↓ unstable surface/closed eyes | |
| Murtezani et al. [32] | Balance | EG1: ↔ BBS (d = 0.21) EG2: ↔ BBS (d = 0.02) |
| Functional fitness | EG1: ↑ six-minute walking test (d = 0.96) EG2: ↑ six-minute walking test (d = 0.71) | |
| Muscle strength | EG1: ↑ quadriceps strength (d = 0.34); ↑grip strength (d = 0.76) EG2: ↑ quadriceps strength (d = 0.11); ↑grip strength (d = 0.02) | |
| BMD | EG1: ↑ BMD (d = 0.51) EG2: ↔ BMD (d = 0.07) | |
| Olsen and Bergland [27] | Fall | EG: ↓ falls efficacy scale (d = −0.70) |
| Functional fitness | EG: ↓ maximum walking speed (d = −0.40) | |
| Flexibility | EG: ↔ functional reach (d = 0.10) | |
| Paolucci et al. [28] | Pain | EG1: ↓ VAS of pain (d = −1.58); ↓ McGill Pain Questionnaire (d = −0.60) EG2: ↑ VAS of pain (d = −2.31); ↑ McGill Pain Questionnaire (d = −2.31) |
| QoL | EG1: ↑ Shortened Osteoporosis Quality of Life Questionnaire (d = 0.63) EG2: ↑ Shortened Osteoporosis Quality of Life Questionnaire (d = 0.88) | |
| Disability | EG1: ↓ Oswestry Disability Questionnaire (d = −0.63) EG2: ↓ Oswestry Disability Questionnaire (d = −0.88) | |
| Halvarsson et al. [25] | Balance | EG: ↑ preferred speed single−task (d = 0.60); ↑ preferred speed dual-task (d = 1.00); ↔ error in the performance of the dual-task in percentage (d = 0.20); ↑ fast speed (d = 0.50); ↔ LLFDI: functional total (d = 0.40); ↔ upper extremity (d = 0.00); ↔ basic lower extremity (d = 0.20); ↑ advanced lower extremity (d = 0.60) |
| Fall | EG: ↓ FES−I: ↑ one leg stance; ↑ modified figure−of−eight test time; ↑ physical activity; ↔ fear of falling; ↑ no percent; ↓ a little percent; ↓ quite a bit; ↓ very much; ↓ gait speed | |
| Evstigneeva et al. [22] | QoL | EG: QUALEFFO−41: ↓ pain (d = −1.20); ↔ADL (0.10); ↓ mobility (d = −1.27), ↓ social function (d = −0.65); ↓ general health perception (d = −1.10); ↓ mental function (d = −0.48) |
| Functional fitness | EG: ↔ Test weight−bearing/squat (d = 0.17); ↑ sit-to-stand weight transfer (d = −0.24); ↔ sit-to-stand left/right weight symmetry (d = −0.12); ↑ Tandem Walk and Sway test (d = −0.48); ↔ TUG (d = 0.03) | |
| Flexibility | EG: ↔ occiput−wall distance (d = 0.24) | |
| Dizdar et al. [21] | Balance | EG: ↓ TUG (12th) (d = −0.33); ↑ BBS (12th) (d = 0.33) |
| Pain | EG: ↓ VAS (12th) (d = −1.21) | |
| QoL | EG: QUALEFFO−41: ↓ total score (d = −0.43); ↓ pain (24th) (d = −0.43); ↔ physical function (d = −0.15); ↓ social function (d = −0.56); ↓ general health (d = −0.46); ↔ mental function (d = 0.06) | |
| García−Gomáriz et al. [24] | BMD | EG: ↑ femoral neck (d = 0.37); ↔ lumbar spine (d = 0.41) |
| Nawrat−Szołtysik et al. [31] | Functional fitness | EG1 vs. EG2: ↑ number of steps and distance per day (d = 3.18); ↔ TUG; ↔ FRT EG2 vs. EG3: no differences (p > 0.05) |
| QoL | EG1/EG2/EG3: QUALEFFO−41: ↔ pain, ↔ ADL; ↔ mobility; ↔ jobs around the house; ↔ mobility; ↔ leisure social activities; ↓ general health perception; ↓ mental function | |
| Stanghelle et al. [30] | Functional fitness | EG: ↔ FRT (d = 0.39); ↓ four square step test (d = −0.32); ↔ grip strength right (d = −0.11); ↑ arm curl (d = −0.69); 30−s sit to stand (d = 0.44); ↔ TUG (d = −0.05); ↔ 6−min walking distance (d = 0.25) |
| QoL | EG: HRQoL (QUALEFFO−41): ↓ FES−I (d = −0.13) | |
| FilipoviĆ et al. [23] | Balance | EG: ↓ TUG (d = −0.63); ↑OLST (d = 0.76) |
| Muscle strength | EG: ↓ STS (d = −0.80) | |
| Osteoporosis | EG: ↑ OKAT−S (d = 2.92) | |
| Fall | EG: ↓ FES−I (d = −1.15) |
| Certainty Assessment | No. of Participants | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | EG | CG | Relative (95% CI) | Absolute (95% CI) | ||
| QoL (analyzed with QUALEFFO-41) | ||||||||||||
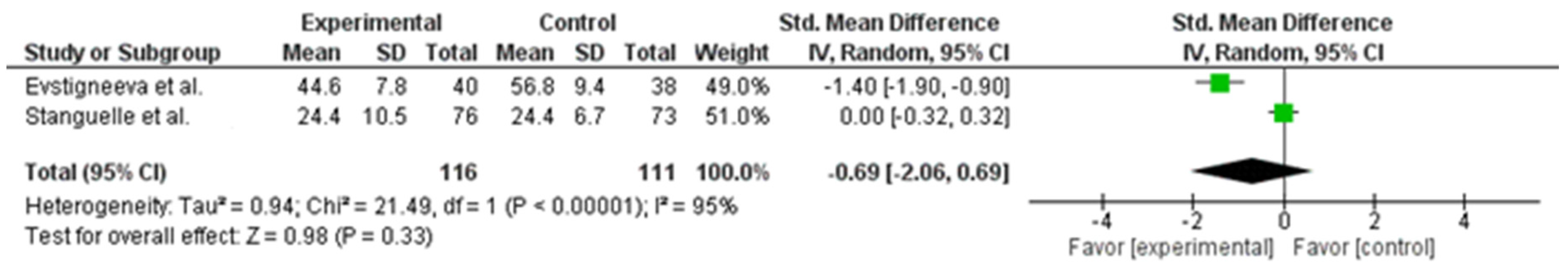
| 2 | RCTs | not serious | not serious | not serious | not serious | none | 116 | 111 | __ | mean −1.09 highest (−2.06 lower to 0.69 higher) | ⨁⨁⨁⨁ HIGH | Important |
| Balance (analyzed with TUG) | ||||||||||||
| 2 | RCTs | not serious | not serious | not serious | not serious | none | 87 | 87 | __ | mean −0.46 highest (−1.41 lower to −0.50 higher) | ⨁⨁⨁⨁ HIGH | Important |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linhares, D.G.; Borba-Pinheiro, C.J.; Castro, J.B.P.d.; Santos, A.O.B.d.; Santos, L.L.d.; Cordeiro, L.d.S.; Drigo, A.J.; Nunes, R.d.A.M.; Vale, R.G.d.S. Effects of Multicomponent Exercise Training on the Health of Older Women with Osteoporosis: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14195. https://doi.org/10.3390/ijerph192114195
Linhares DG, Borba-Pinheiro CJ, Castro JBPd, Santos AOBd, Santos LLd, Cordeiro LdS, Drigo AJ, Nunes RdAM, Vale RGdS. Effects of Multicomponent Exercise Training on the Health of Older Women with Osteoporosis: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(21):14195. https://doi.org/10.3390/ijerph192114195
Chicago/Turabian StyleLinhares, Diego Gama, Claudio Joaquim Borba-Pinheiro, Juliana Brandão Pinto de Castro, Andressa Oliveira Barros dos Santos, Luciano Lima dos Santos, Lilliany de Souza Cordeiro, Alexandre Janotta Drigo, Rodolfo de Alkmim Moreira Nunes, and Rodrigo Gomes de Souza Vale. 2022. "Effects of Multicomponent Exercise Training on the Health of Older Women with Osteoporosis: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 21: 14195. https://doi.org/10.3390/ijerph192114195
APA StyleLinhares, D. G., Borba-Pinheiro, C. J., Castro, J. B. P. d., Santos, A. O. B. d., Santos, L. L. d., Cordeiro, L. d. S., Drigo, A. J., Nunes, R. d. A. M., & Vale, R. G. d. S. (2022). Effects of Multicomponent Exercise Training on the Health of Older Women with Osteoporosis: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(21), 14195. https://doi.org/10.3390/ijerph192114195