REPRO_PL-Polish Mother and Child Cohort—Exposure, Health Status, and Neurobehavioral Assessments in Adolescents—Design and Cohort Update
Abstract
1. Introduction
2. Objectives
- (a)
- The evaluation of the main determinants of occupational (when related to mothers), environmental (air pollution and chemicals) and lifestyle-related (diet, tobacco constituents, physical activity and use of new technologies) exposures on pregnancy outcomes (including gestational age, birth weight, birth length and head circumference) and/or offspring health, with special focus on adiposity, cardiometabolic diseases, respiratory/allergic problems and neurobehavioral outcomes.
- (b)
- The evaluation of simultaneous exposures to a broad spectrum of pre- and postnatal occupational/environmental/lifestyle-related factors, including genetic profiling, on health and neurobehavioral outcomes, in an Exposome-based approach.
- (c)
- The evaluation of outcome associations and trajectories throughout childhood and adolescence derived from occupational/environmental/lifestyle-related exposures.
3. Material and Methods
3.1. General Description of the Cohort
3.1.1. Study Design and Population
3.1.2. Response and Follow-Up
3.1.3. Measurements in REPRO_PL
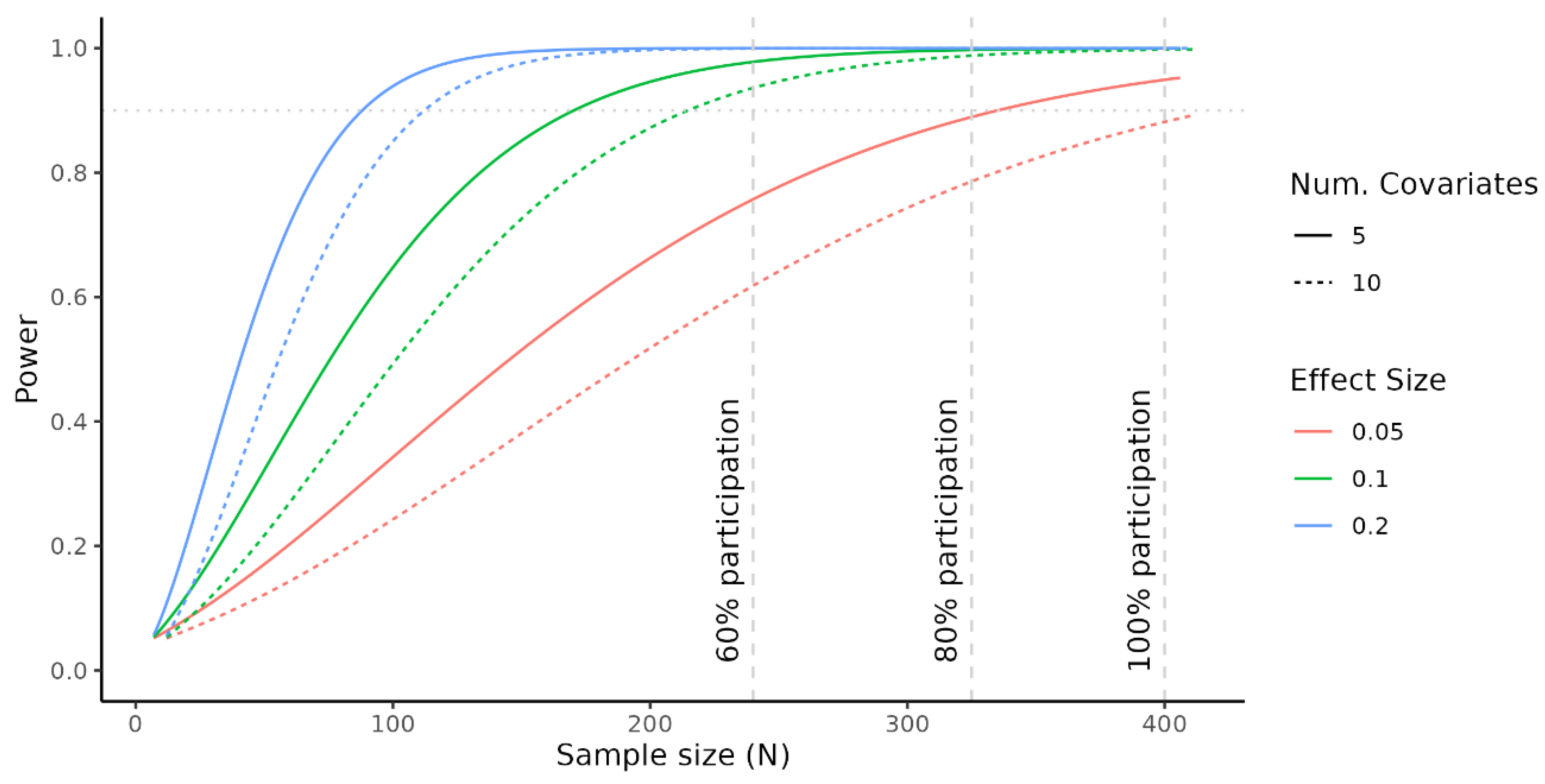
3.1.4. Power/Sample Size Calculation
3.2. Phase IV of REPRO_PL–Adolescence Period
3.2.1. Assessment of Exposure
3.2.2. Adolescents’ Health and Neuropsychomotor Development Assessment
- Pulmonary function tests. Spirometry will be performed using a Master Screen unit (Erich Jaeger GmbhHochberg, Germany) according to the American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines. The results are expressed as percentages of the predicted values. Reversibility testing is performed after administration of salbutamol (400 μg). The percentage change from baseline in FEV1, pre-bronchodilatory FEV1, and PEF will be included in the analysis. The exhaled nitric oxide measurements will be performed according to ATS/ERS recommendations with a chemiluminescence analyzer (model 280i nitric oxide analyzer; Sievers, Boulder, CO, USA) and defined in parts per billion. The mean value of three successive, reproducible recordings of FENO will be retained for the statistical analysis.
- Skin prick testing (SPT) will be performed with the most common inhalant allergens: Dermatophagoides farinae, Dermatophagoides pteronyssinus, Alternaria, Cladosporium, cat dander, dog dander, mixed grass pollen, rye, birch, hazel, ribwort, alder, and mugwort together with a positive (histamine) and negative (glycerol) control (extracts from Allergopharma-Nexter, Reinbeg, Germany). A positive SPT reaction is defined as a mean weal diameter >3 mm in excess of the negative control.
- Weight and height assessment (as well as body composition) will be performed by a nurse using a height analyzer and TANITA-weight/body composition analyzer.
- Systolic and diastolic blood pressure will be measured twice, at least 5 min apart, in a resting position.
- During a separate morning visit, the blood will be collected and analyzed for thyroid stimulating hormone (TSH), triiodothyronine (FT3), thyroxine (FT4), lipid profile (cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides (TG)), and glucose and insulin level.
- Faulty posture, persistent reflexes, balance, and gait parameters will be examined by a physiotherapist. The posture will be assessed using a Zebris system. Visual assessment is made on the frontal and posterior side in the frontal plane and in the sagittal plane on the right and left sides of the subject. The examination also includes tests assessing persistent reflexes that could affect changes in posture and balance. These are: the Asymmetrical Tonic Neck Reflex (ATNR), the Symmetrical Tonic Neck Reflex (STNR), and the Tonic Labyrinthine Reflex (TLR). The last element of the examination is the analysis of balance and gait parameters using MediPost based on inertial sensors—available at no cost for the current study. The static posturography protocol includes assessment of balance in a standing position on a stable surface with eyes open and closed, and on a foam surface with eyes open and closed.
- Sleep quality will be evaluated based on a modified version of the Sleep Disturbance Scales. It is designed to measure length and quality of sleep. The sleep quality is additionally verified by the 7-days smartwatch records.
- Adolescent neuropsychomotor assessment. The behavioral status will be assessed by the Strengths and Difficulties Questionnaire (SDQ), which is filled in by both the parent and the adolescent (https://www.sdqinfo.com, accessed on 23 September 2022). The SDQ is a widely used tool (frequently used in European birth cohorts) for the evaluation of the behavior, with a 25-item questionnaire consisting of four subscales measuring mental health problems (Conduct problems, Emotional symptoms, Peer relationship problems, and Hyperactivity/Inattention problems) and one sub-scale measuring strengths (Pro-social behavior). To assess intelligence and psychomotor abilities, the Intelligence and Development Scales for Children and Adolescents (IDS-2) is applied (https://www.practest.com.pl/ids-2-skale-inteligencji-i-rozwoju-dla-dzieci-i-mlodziezy, (accessed on 23 September 2022). The examination is performed by trained and certified psychologists. The IDS-2 is a tool designed for a thorough evaluation of skills and competences in persons aged 5–20. The battery includes 30 tests to examine cognitive skills (intelligence and executive functions) and competences (psychomotoric, socio-emotional, and school competences as well as work attitude). IDS-2 represent very high internal consistencies and stabilities for the IQ and factor scores, in addition to a >0.70 reliability of intelligence tests. High reliability of the general executive functions index as well as about a 0.80 reliability of tests scores have been achieved in the past.
3.3. Data Management
3.4. Ethics and Privacy Protection
4. Strengths and Weaknesses of the REPRO_PL Cohort Study
5. Collaboration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Jaddoe, V.W.V.; Felix, J.F.; Andersen, A.N.; Charles, M.A.; Chatzi, L.; Corpeleijn, E.; Donner, N.; Elhakeem, A.; Eriksson, J.G.; Foong, R.; et al. The LifeCycle Project-EU Child Cohort Network: A federated analysis infrastructure and harmonized data of more than 250,000 children and parents. Eur. J. Epidemiol. 2020, 35, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Balbus, J.M.; Barouki, R.; Birnbaum, L.S.; Etzel, R.A.; Gluckman, S.P.D.; Grandjean, P.; Hancock, C.; Hanson, M.A.; Heindel, J.J.; Hoffman, K.; et al. Early-life prevention of non-communicable diseases. Lancet 2013, 381, 3–4. [Google Scholar] [CrossRef]
- Vrijheid, M.; Casas, M.; Bergström, A.; Carmichael, A.; Cordier, S.; Eggesbø, M.; Eller, E.; Fantini, M.P.; Fernández, M.F.; Fernández-Somoano, A.; et al. European birth cohorts for environmental health research. Environ. Health Perspect. 2012, 120, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.S.; Kamper-Jørgensen, M.; Adamson, A.; Barros, H.; Bonde, J.P.; Brescianini, S.; Brophy, S.; Casas, M.; Devereux, G.; Eggesbø, M.; et al. Pregnancy and birth cohort resources in europe: A large opportunity for aetiological child health research. Paediatr. Perinat. Epidemiol. 2013, 27, 393–414. [Google Scholar] [CrossRef]
- Ubalde-Lopez, M.; Garani-Papadatos, T.; Scelo, G.; Casas, M.; Lissåker, C.; Peters, S.; Nohr, E.A.; Albin, M.; Lucas, R.; Papantoniou, K.; et al. Working life, health and well-being of parents: A joint effort to uncover hidden treasures in European birth cohorts. Scand. J. Work Environ. Health 2021, 47, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Zugna, D.; Pizzi, C.; Richiardi, L. Sources of confounding in life course epidemiology. J. Dev. Orig. Health Dis. 2019, 10, 299–305. [Google Scholar] [CrossRef]
- Nader, J.L.; López-Vicente, M.; Julvez, J.; Guxens, M.; Cadman, T.; Elhakeem, A.; Järvelin, M.R.; Rautio, N.; Miettunen, J.; El Marroun, H.; et al. Cohort description: Measures of early-life behaviour and later psychopathology in the LifeCycle Project—EU Child Cohort Network. J. Epidemiol. 2021. [Google Scholar] [CrossRef]
- de Prado-Bert, P.; Ruiz-Arenas, C.; Vives-Usano, M.; Andrusaityte, S.; Cadiou, S.; Carracedo, Á.; Casas, M.; Chatzi, L.; Dadvand, P.; González, J.R.; et al. The early-life exposome and epigenetic age acceleration in children. Environ. Int. 2021, 155, 106683. [Google Scholar] [CrossRef]
- Hughes, R.A.; Tilling, K.; Lawlor, D.A. Combining longitudinal data from different cohorts to examine the life-course trajectory. Am. J. Epidemiol. 2021, 190, 2680–2689. [Google Scholar] [CrossRef]
- Polańska, K.; Hanke, W.; Gromadzińska, J.; Ligocka, D.; Gulczyńska, E.; Sobala, W.; Wąsowicz, W. Polish Mother and Child Cohort study—Defining the problem, the aim of the study and methodological assumptions. Int. J. Occup. Med. Environ. Health 2009, 22, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Polańska, K.; Hanke, W.; Jurewicz, J.; Sobala, W.; Madsen, C.; Nafstad, P.; Magnus, P. Polish Mother and Child Cohort study (REPRO_PL)—Methodology of follow-up of the children. Int. J. Occup. Med. Environ. Health 2011, 24, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Polańska, K.; Hanke, W.; Krol, A.; Potocka, A.; Waszkowska, M.; Jacukowicz, A.; Gromadzińska, J.; Wąsowicz, W.; Jerzyńska, J.; Stelmach, W.; et al. Polish Mother and Child Cohort study (REPRO_PL)—Methodology of the follow-up of the children at the age of 7. Int. J. Occup. Med. Environ. Health 2016, 29, 883–893. [Google Scholar] [CrossRef]
- Garí, M.; Koch, H.M.; Palmke, C.; Jankowska, A.; Wesolowska, E.; Hanke, W.; Nowak, D.; Bose-O’Reilly, S.; Polańska, K. Determinants of phthalate exposure and risk assessment in children from Poland. Environ. Int. 2019, 127, 742–753. [Google Scholar] [CrossRef]
- Jankowska, A.; Polańska, K.; Hanke, W.; Wesolowska, E.; Ligocka, D.; Waszkowska, M.; Stanczak, A.; Tartaglione, A.M.; Mirabella, F.; Chiarotti, F.; et al. Prenatal and early postnatal phthalate exposure and child neurodevelopment at age of 7 years—Polish Mother and Child Cohort. Environ. Res. 2019, 177, 108626. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, A.; Polańska, K.; Koch, H.M.; Pälmke, C.; Waszkowska, M.; Stanczak, A.; Wesolowska, E.; Hanke, W.; Bose-O’Reilly, S.; Calamandrei, G.; et al. Phthalate exposure and neurodevelopmental outcomes in early school age children from Poland. Environ. Res. 2019, 179, 108829. [Google Scholar] [CrossRef]
- Sarigiannis, D.A.; Papaioannou, N.; Handakas, E.; Anesti, O.; Polańska, K.; Hanke, W.; Salifoglou, A.; Gabriel, C.; Karakitsios, S. Neurodevelopmental exposome: The effect of in utero co-exposure to heavy metals and phthalates on child neurodevelopment. Environ. Res. 2021, 197, 110949. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, I.; Majak, P.; Jerzynska, J.; Podlecka, D.; Stelmach, W.; Polańska, K.; Ligocka, D.; Hanke, W. The effect of prenatal exposure to phthalates on food allergy and early eczema in inner-city children. Allergy Asthma Proc. 2015, 36, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Polańska, K.; Ligocka, D.; Sobala, W.; Hanke, W. Phthalate exposure and child development: The Polish Mother and Child Cohort study. Early Hum. Dev. 2014, 90, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Polańska, K.; Ligocka, D.; Sobala, W.; Hanke, W. Effect of environmental phthalate exposure on pregnancy duration and birth outcomes. Int. J. Occup. Med. Environ. Health 2016, 29, 683–697. [Google Scholar] [CrossRef]
- Garí, M.; Moos, R.; Bury, D.; Kasper-Sonnenberg, M.; Jankowska, A.; Andysz, A.; Hanke, W.; Nowak, D.; Bose-O’Reilly, S.; Koch, H.M.; et al. Human-Biomonitoring derived exposure and Daily Intakes of Bisphenol A and their associations with neurodevelopmental outcomes among children of the Polish Mother and Child Cohort Study. Environ. Health 2021, 20, 95. [Google Scholar] [CrossRef]
- Garí, M.; Grzesiak, M.; Krekora, M.; Kaczmarek, P.; Jankowska, A.; Krol, A.; Kaleta, D.; Jerzynska, J.; Janasik, B.; Kuras, R.; et al. Prenatal exposure to neurotoxic metals and micronutrients and neurodevelopmental outcomes in early school age children from Poland. Environ. Res. 2022, 204, 112049. [Google Scholar] [CrossRef]
- Polańska, K.; Hanke, W.; Sobala, W.; Trzcinka-Ochocka, M.; Ligocka, D.; Strugala-Stawik, H.; Magnus, P. Predictors of environmental lead exposure among pregnant women—A prospective cohort study in Poland. Ann. Agric. Environ. Med. 2014, 21, 49–54. [Google Scholar]
- Polańska, K.; Hanke, W.; Pawlas, N.; Wesolowska, E.; Jankowska, A.; Jagodic, M.; Mazej, D.; Dominowska, J.; Grzesiak, M.; Mirabella, F.; et al. Sex-dependent impact of low-level lead exposure during prenatal period on child psychomotor functions. Int. J. Environ. Res. Public Health 2018, 15, 2263. [Google Scholar] [CrossRef]
- Calamandrei, G.; Ricceri, L.; Meccia, E.; Tartaglione, A.M.; Horvat, M.; Tratnik, J.S.; Mazej, D.; Spiric, Z.; Prpic, I.; Vlasic-Cicvaric, I.; et al. Pregnancy exposome and child psychomotor development in three European birth cohorts. Environ. Res. 2020, 181, 108856. [Google Scholar] [CrossRef] [PubMed]
- Polańska, K.; Hanke, W.; Sobala, W.; Trzcinka-Ochocka, M.; Ligocka, D.; Brzeznicki, S.; Strugala-Stawik, H.; Magnus, P. Developmental effects of exposures to environmental factors: The Polish Mother and Child Cohort Study. Biomed Res. Int. 2013, 2013, 629716. [Google Scholar] [CrossRef]
- Polańska, K.; Krol, A.; Kaluzny, P.; Ligocka, D.; Mikolajewska, K.; Shaheen, S.; Walton, R.; Hanke, W. Estimation of Saliva Cotinine cut-off points for active and passive smoking during pregnancy-Polish Mother and Child Cohort (REPRO_PL). Int. J. Environ. Res. Public Health 2016, 13, 1216. [Google Scholar] [CrossRef] [PubMed]
- Stragierowicz, J.; Mikolajewska, K.; Zawadzka-Stolarz, M.; Polańska, K.; Ligocka, D. Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res. Int. 2013, 2013, 386784. [Google Scholar] [CrossRef] [PubMed]
- Polańska, K.; Hanke, W.; Krol, A.; Gromadzinska, J.; Kuras, R.; Janasik, B.; Wasowicz, W.; Mirabella, F.; Chiarotti, F.; Calamandrei, G. Micronutrients during pregnancy and child psychomotor development: Opposite effects of zinc and selenium. Environ. Res. 2017, 158, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, I.; Grzelewski, T.; Bobrowska-Korzeniowska, M.; Kopka, M.; Majak, P.; Jerzynska, J.; Stelmach, W.; Polańska, K.; Sobala, W.; Gromadzinska, J.; et al. The role of zinc, copper, plasma glutathione peroxidase enzyme, and vitamins in the development of allergic diseases in early childhood: The Polish mother and child cohort study. Allergy Asthma Proc. 2014, 35, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Podlecka, D.; Stelmach, I.; Jerzynska, J.; Polańska, K.; Janasik, B.; Gromadzinska, J.; Wasowicz, W.; Hanke, W.; Majak, P.; Stelmach, W. Early childhood allergy symptoms in relation to plasma selenium in pregnant mothers. Ann. Allergy Asthma Immunol. 2017, 118, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Polańska, K.; Krol, A.; Sobala, W.; Gromadzinska, J.; Brodzka, R.; Calamandrei, G.; Chiarotti, F.; Wasowicz, W.; Hanke, W. Selenium status during pregnancy and child psychomotor development-Polish Mother and Child Cohort study. Pediatr. Res. 2016, 79, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, A.; Grzesiak, M.; Krekora, M.; Dominowska, J.; Jerzynska, J.; Kaluzny, P.; Wesolowska, E.; Szadkowska-Stanczyk, I.; Trafalska, E.; Kaleta, D.; et al. Determinants of the essential elements and vitamins intake and status during pregnancy: A descriptive study in Polish Mother and Child Cohort. Nutrients 2021, 13, 949. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska-Korzeniowska, M.; Jerzynska, J.; Polańska, K.; Gromadzinska, J.; Hanke, W.; Wasowicz, W.; Stelmach, I. The role of antioxidants and 25-hydroxyvitamin D during pregnancy in the development of allergic diseases in early school-age children—Polish Mother and Child Cohort Study. Allergy Asthma Proc. 2020, 41, e19–e25. [Google Scholar] [CrossRef]
- Gromadzinska, J.; Polańska, K.; Kozlowska, L.; Mikolajewska, K.; Stelmach, I.; Jerzynska, J.; Stelmach, W.; Grzesiak, M.; Hanke, W.; Wasowicz, W. Vitamins A and E during pregnancy and allergy symptoms in an early childhood lack of association with tobacco smoke exposure. Int. J. Environ. Res. Public Health 2018, 15, 1245. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, I.; Majak, P.; Jerzynska, J.; Podlecka, D.; Stelmach, W.; Polańska, K.; Gromadzinska, J.; Wasowicz, W.; Hanke, W. Cord serum 25-hydroxyvitamin D correlates with early childhood viral-induced wheezing. Respir. Med. 2015, 109, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Polańska, K.; Hanke, W.; Dettbarn, G.; Sobala, W.; Gromadzinska, J.; Magnus, P.; Seidel, A. The determination of polycyclic aromatic hydrocarbons in the urine of non-smoking Polish pregnant women. Sci. Total Environ. 2014, 487, 102–109. [Google Scholar] [CrossRef]
- Polańska, K.; Hanke, W.; Sobala, W.; Brzeznicki, S.; Ligocka, D. Exposure of smoking pregnant women to polycyclic aromatic hydrocarbons. Med. Pr. 2009, 60, 103–108. [Google Scholar]
- Jorcano, A.; Lubczynska, M.J.; Pierotti, L.; Altug, H.; Ballester, F.; Cesaroni, G.; El Marroun, H.; Fernandez-Somoano, A.; Freire, C.; Hanke, W.; et al. Prenatal and postnatal exposure to air pollution and emotional and aggressive symptoms in children from 8 European birth cohorts. Environ. Int. 2019, 131, 104927. [Google Scholar] [CrossRef]
- Brzozowska, A.; Podlecka, D.; Jankowska, A.; Krol, A.; Kaleta, D.; Trafalska, E.; Nowakowska-Swirta, E.; Kaluzny, P.; Hanke, W.; Bal-Gieranczyk, K.; et al. Maternal diet during pregnancy and risk of allergic diseases in children up to 7–9 years old from Polish Mother and Child Cohort study. Environ. Res. 2022, 208, 112682. [Google Scholar] [CrossRef] [PubMed]
- Mensink-Bout, S.M.; van Meel, E.R.; de Jongste, J.C.; Annesi-Maesano, I.; Aubert, A.M.; Bernard, J.Y.; Chen, L.W.; Cooper, C.; Crozier, S.R.; Hanke, W.; et al. Maternal diet in pregnancy and child’s respiratory outcomes: An individual participant data meta-analysis of 18000 children. Eur. Respir. J. 2022, 59, 2101315. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, N.; Roumeliotaki, T.; Oken, E.; Barros, H.; Basterrechea, M.; Charles, M.A.; Eggesbø, M.; Forastiere, F.; Gaillard, R.; Gehring, U.; et al. Fish intake in pregnancy and child growth: A pooled analysis of 15 European and US birth cohorts. JAMA Pediatr. 2016, 170, 381–390. [Google Scholar] [CrossRef]
- Polańska, K.; Kaluzny, P.; Aubert, A.M.; Bernard, J.Y.; Duijts, L.; El Marroun, H.; Hanke, W.; Hebert, J.R.; Heude, B.; Jankowska, A.; et al. Dietary quality and dietary inflammatory potential during pregnancy and offspring emotional and behavioral symptoms in childhood: An individual participant data meta-analysis of four European cohorts. Biol. Psychiatry 2021, 89, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Aubert, A.M.; Shivappa, N.; Bernard, J.Y.; Mensink-Bout, S.M.; Geraghty, A.A.; Mehegan, J.; Suderman, M.; Polańska, K.; Hanke, W.; et al. Associations of maternal dietary inflammatory potential and quality with offspring birth outcomes: An individual participant data pooled analysis of 7 European cohorts in the ALPHABET consortium. PLoS Med. 2021, 18, e1003491. [Google Scholar] [CrossRef]
- Chen, L.W.; Aubert, A.; Bernard, J.Y.; Cooper, C.; Duijts, L.; Geraghty, A.A.; Harvey, N.C.; Hebert, J.R.; Heude, B.; Kelleher, C.C.; et al. Maternal dietary quality, inflammatory potential and offspring adiposity throughout childhood: A pooled analysis of 7 European cohorts in the ALPHABET consortium. BMC Med. 2021, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Aubert, A.M.; Forhan, A.; de Lauzon-Guillain, B.; Chen, L.W.; Polańska, K.; Hanke, W.; Jankowska, A.; Mensink-Bout, S.M.; Duijts, L.; Suderman, M.; et al. Deriving the dietary approaches to stop hypertension (DASH) score in women from seven pregnancy cohorts from the European ALPHABET consortium. Nutrients 2019, 11, 2706. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, E.; Jankowska, A.; Trafalska, E.; Kaluzny, P.; Grzesiak, M.; Dominowska, J.; Hanke, W.; Calamandrei, G.; Polańska, K. Sociodemographic, lifestyle, environmental and pregnancy-related determinants of dietary patterns during pregnancy. Int. J. Environ. Res. Public Health 2019, 16, 754. [Google Scholar] [CrossRef]
- Aubert, A.M.; Chen, L.W.; Shivappa, N.; Cooper, C.; Crozier, S.R.; Duijts, L.; Forhan, A.; Hanke, W.; Harvey, N.C.; Jankowska, A.; et al. Predictors of maternal dietary quality and dietary inflammation during pregnancy: An individual participant data meta-analysis of seven European cohorts from the ALPHABET consortium. Clin Nutr. 2022, 41, 1991–2002. [Google Scholar] [CrossRef]
- Polańska, K.; Muszynski, P.; Sobala, W.; Dziankowska, E.; Merecz-Kot, D.; Hanke, W. Maternal lifestyle during pregnancy and child psychomotor development—Polish Mother and Child Cohort study. Early Hum. Dev. 2015, 91, 317–325. [Google Scholar] [CrossRef]
- Philips, E.M.; Santos, S.; Trasande, L.; Aurrekoetxea, J.J.; Barros, H.; von Berg, A.; Bergstrom, A.; Bird, P.K.; Brescianini, S.; Chaoimh, C.N.; et al. Changes in parental smoking during pregnancy and risks of adverse birth outcomes and childhood overweight in Europe and North America: An individual participant data meta-analysis of 229,000 singleton births. PLoS Med. 2020, 17, e1003182. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, S.; Bishop, T.; Crozier, S.R.; Granstrom, C.; Kordas, K.; Kupers, L.K.; O’Brien, E.C.; Polańska, K.; Sauder, K.A.; Zafarmand, M.H.; et al. Associations between maternal physical activity in early and late pregnancy and offspring birth size: Remote federated individual level meta-analysis from eight cohort studies. BJOG 2019, 126, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Smejda, K.; Polańska, K.; Merecz-Kot, D.; Krol, A.; Hanke, W.; Jerzynska, J.; Stelmach, W.; Majak, P.; Stelmach, I. Maternal stress during pregnancy and allergic diseases in children during the first year of life. Respir. Care 2018, 63, 70–76. [Google Scholar] [CrossRef]
- Polańska, K.; Krol, A.; Merecz-Kot, D.; Jurewicz, J.; Makowiec-Dabrowska, T.; Chiarotti, F.; Calamandrei, G.; Hanke, W. Maternal stress during pregnancy and neurodevelopmental outcomes of children during the first 2 years of life. J. Paediatr. Child Health 2017, 53, 263–270. [Google Scholar] [CrossRef]
- Parmes, E.; Pesce, G.; Sabel, C.E.; Baldacci, S.; Bono, R.; Brescianini, S.; D’Ippolito, C.; Hanke, W.; Horvat, M.; Liedes, H.; et al. Influence of residential land cover on childhood allergic and respiratory symptoms and diseases: Evidence from 9 European cohorts. Environ. Res. 2020, 183, 108953. [Google Scholar] [CrossRef] [PubMed]
- Smejda, K.; Polańska, K.; Stelmach, W.; Majak, P.; Stelmach, I. Dog keeping at home before and during pregnancy decreased the risk of food allergy in 1-year-old children. Postępy Dermatol. Alergol. 2020, 37, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska-Korzeniowska, M.; Kapszewicz, K.; Jerzynska, J.; Stelmach, W.; Polańska, K.; Gromadzinska, J.; Mikolajewska, K.; Hanke, W.; Stelmach, I. Early life environmental exposure in relation to new onset and remission of allergic diseases in school children: Polish Mother and Child Cohort Study. Allergy Asthma Proc. 2019, 40, 329–337. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 23 September 2022).
- Champely, S. Basic Functions for Power Analysis. R Package Version 1.3-0. 2020. Available online: https://CRAN.R-project.org/package=pwr (accessed on 23 September 2022).
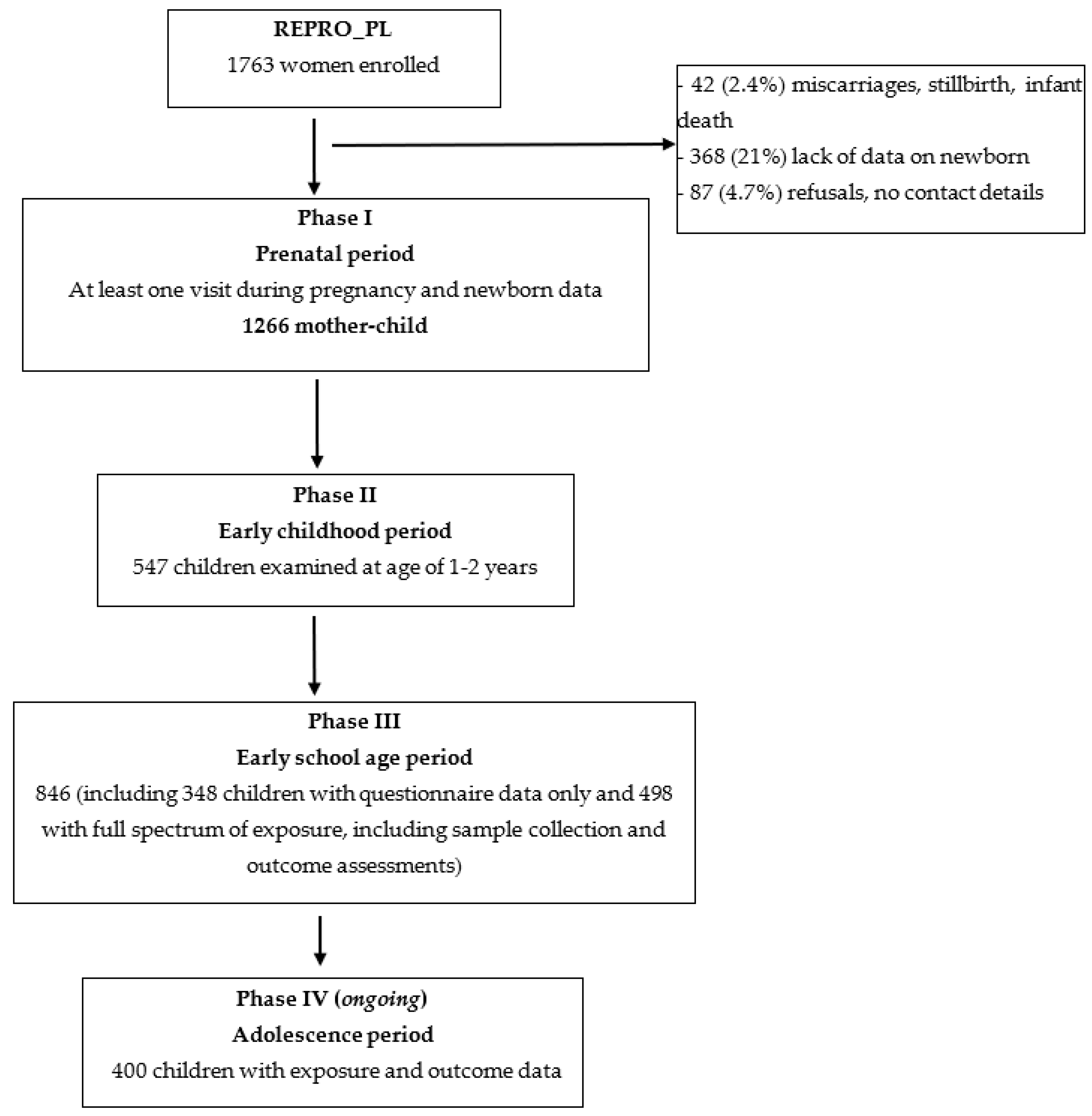
| Prenatal Period (Phase I: T1–T3, Birth) n = 1266 | Early Childhood (Phase II: 1 y/2 ys) n = 547 | Early School Age (Phase III: 7–9 ys) n = 498 | |
|---|---|---|---|
| Mothers | |||
| Age at child birth (years) mean ± SD | 29.2 ± 4.2 | 29.6 ± 4.2 | 29.5 ± 4.0 |
| Education (years of completed education) % | |||
| ≤9 | 2.3 | 2.2 | 2.1 |
| 10–12 | 27.6 | 29.6 | 30 |
| >12 | 69.3 | 68.3 | 67.9 |
| Pre-pregnancy BMI (kg/m2) mean ± SD | 22.3 ± 3.6 | 22.6 ± 3.8 | 22.4 ± 3.7 |
| Smoking (based on cotinine level in 1st trimester of pregnancy) % | |||
| Yes | 14.8 | 14.5 | 14 |
| No | 85.2 | 85.5 | 86 |
| Alcohol consumption during pregnancy % | |||
| Yes | 7.8 | 7.2 | 8.9 |
| No | 92.2 | 92.8 | 91.1 |
| Children | |||
| Sex % | |||
| Male | 51.2 | 49.5 | 49 |
| Female | 48.8 | 50.5 | 51 |
| Birth weight (grams) mean ± SD * | 3367.5 ± 476.9 | 3337.0 ± 478.3 | 3477.2 ± 478.3 |
| Gestational age (weeks) mean ± SD | 39.2 ± 1.4 | 39.2 ± 1.4 | 39.3 ± 1.4 |
| Prenatal Period (Phase I: T1–T3, Birth) | Early Childhood (Phase II: 1 y/2 ys) | Early School Age (Phase III: 7–9 ys) | Adolescent (Phase IV: 14 ys) Ongoing | Main Publications from REPRO_PL | |
|---|---|---|---|---|---|
| Exposure | |||||
| Phthalates (21 metabolites) | Urine (T3) | Urine | Urine | Urine | [14,15,16,17,18,19,20] |
| Bisphenols (BPA, BPS, BPF) | - | - | Urine | Urine | [21] |
| Heavy Metals (Pb, Cd, Hg, As) 1 | Maternal blood, Cord blood, Hair | - | Urine | Urine//Blood/Hair | [17,22,23,24,25,26] |
| Cotinine | Saliva (T1-T3) | Urine | Urine | Urine | [26,27,28] |
| Pesticides (metabolites of pyrethroids, organophosphates, triazoles, neocotinoids) | Urine | Urine | |||
| Persistent Organic Pollutants | Blood | ||||
| Microelements and vitamins (Zn, Cu, Se, vit. A, E, D) | Maternal blood (T1-T3), Cord blood | - | - | - | [22,25,29,30,31,32,33,34,35,36] |
| Air pollution PAH (1-hydroxypyrene, 3-, 4-, 9-hydroxyphenanthrene) Kriging methodology Personal monitoring | Urine (T2) PM10, PM2.5 (T1-T3) - | Urine PM10, PM2,5 - | Urine PM10, PM2,5 |
Urine PM10, PM2,5,, NO2, SO2 GilAir personal aspirators | [26,37,38] [39] |
| Lifestyle | |||||
| Diet | FFQ (T2), | Breastfeeding | 24 HR (parent report) | 24 HR/FFQ(adolescent report) | [33,40,41,42,43,44,45,46,47,48] |
| Alcohol consumption | Questionnaire (T1–T3) | - | - | Questionnaire(adolescent report) | [49] |
| Smoking (active/passive) | Questionnaire (T1–T3) | Questionnaire | Questionnaire (parent report) | Questionnaire (tobacco/e-cigarettes)(adolescent report) | [49,50] |
| Physical activity | Questionnaire (T1–T3) | - | Questionnaire (parent report) | Questionnaire (PAQ), SW(adolescent report) | [49,51] |
| New technologies | Questionnaire (T3) | - | Questionnaire (parent report) | Questionnaire(adolescent report) | |
| Psychosocial conditions | SWCQ, PSS, SRRS, APGAR (T2) | - | Mothers: PAS, EMQ (mother report) | Questionnaire(adolescent report) | [52,53] |
| Outcomes | |||||
| Child health | Questionnaire | Questionnaire | Questionnaire | ||
| Birth outcomes | Medical records | - | - | - | [20,44,50,51] |
| Clinical examination | Neonatologist | Pediatrician | Pediatrician/allergologist | Pediatrician/allergologist | |
| Atopy | - | Questionnaire | Skin prick test | Skin prick test | [18,30,31,34,35,40,52,54,55,56] |
| Pulmonary function | - | - | Spirometry | Spirometry | [34,40] |
| Blood pressure | - | - | 2 measurements | 2 measurements | |
| Thyroid hormones (FT3, FT4, TSH) | - | - | - | Blood | |
| Glucose and insulin | - | - | - | Blood | |
| Lipid profile (cholesterol, LDL, HDL, TG) | - | - | - | Blood | |
| Puberty | - | - | - | Questionnaire (parent/adolescent report) | |
| Adiposity | - | Anthropometric measurements | Anthropometric measurements | Anthropometric measurements | [45] |
| Faulty posture | - | - | - | Zebris system | |
| Persistent reflexes | - | - | - | ATNR, STNR, TLR | |
| Balance and gait parameters | - | - | - | MediPost based on inertial sensors | |
| Sleeping pattern 2 | - | - | - | SDSC (parent/adolescent report) | |
| Neurobehavioral outcomes | |||||
| Behavior | - | - | SDQ (parent report) | SDQ (parent/adolescent report) | [15,16,17,21,22,39,43] |
| Psychomotor and cognitive development | - | BSID-III | IDS | IDS | [15,16,17,21,22,24,25,26,29,32,49,53] |
| Biobanking (−80 °C) | |||||
| DNA | Cord blood | - | Buccal swabs | Buccal swabs | |
| Blood/Plasma | X (Plasma T1–T3, cord blood) | - | X | ||
| Nasopharyngeal microbiome | - | - | - | X | |
| Urine | X (T2,T3) | X | X | X | |
| Saliva | X (T1–T3) | X | X | ||
| Hair | X (T3) | - | - | X | |
| Covariates | |||||
| Personal and sociodemographic data | Questionnaire (T1–T3) | Questionnaire | Questionnaire (parent report) | Questionnaire (parent/adolescent report) | |
| Home environment | Questionnaire (T1–T3) | Questionnaire | Questionnaire (parent report) | Questionnaire (parent report) | |
| Family functioning | Questionnaire (T1–T3) | Questionnaire | Questionnaire (parent report) | Questionnaire (parent report) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janc, M.; Jankowska, A.; Weteska, M.; Brzozowska, A.; Hanke, W.; Jurewicz, J.; Garí, M.; Polańska, K.; Jerzyńska, J. REPRO_PL-Polish Mother and Child Cohort—Exposure, Health Status, and Neurobehavioral Assessments in Adolescents—Design and Cohort Update. Int. J. Environ. Res. Public Health 2022, 19, 14167. https://doi.org/10.3390/ijerph192114167
Janc M, Jankowska A, Weteska M, Brzozowska A, Hanke W, Jurewicz J, Garí M, Polańska K, Jerzyńska J. REPRO_PL-Polish Mother and Child Cohort—Exposure, Health Status, and Neurobehavioral Assessments in Adolescents—Design and Cohort Update. International Journal of Environmental Research and Public Health. 2022; 19(21):14167. https://doi.org/10.3390/ijerph192114167
Chicago/Turabian StyleJanc, Magdalena, Agnieszka Jankowska, Monika Weteska, Agnieszka Brzozowska, Wojciech Hanke, Joanna Jurewicz, Mercè Garí, Kinga Polańska, and Joanna Jerzyńska. 2022. "REPRO_PL-Polish Mother and Child Cohort—Exposure, Health Status, and Neurobehavioral Assessments in Adolescents—Design and Cohort Update" International Journal of Environmental Research and Public Health 19, no. 21: 14167. https://doi.org/10.3390/ijerph192114167
APA StyleJanc, M., Jankowska, A., Weteska, M., Brzozowska, A., Hanke, W., Jurewicz, J., Garí, M., Polańska, K., & Jerzyńska, J. (2022). REPRO_PL-Polish Mother and Child Cohort—Exposure, Health Status, and Neurobehavioral Assessments in Adolescents—Design and Cohort Update. International Journal of Environmental Research and Public Health, 19(21), 14167. https://doi.org/10.3390/ijerph192114167









