Use It or Lose It? A Meta-Analysis on the Effects of Resistance Training Cessation (Detraining) on Muscle Size in Older Adults
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Data Extraction
2.4. Methodological Quality
2.5. Statistical Analysis
3. Results
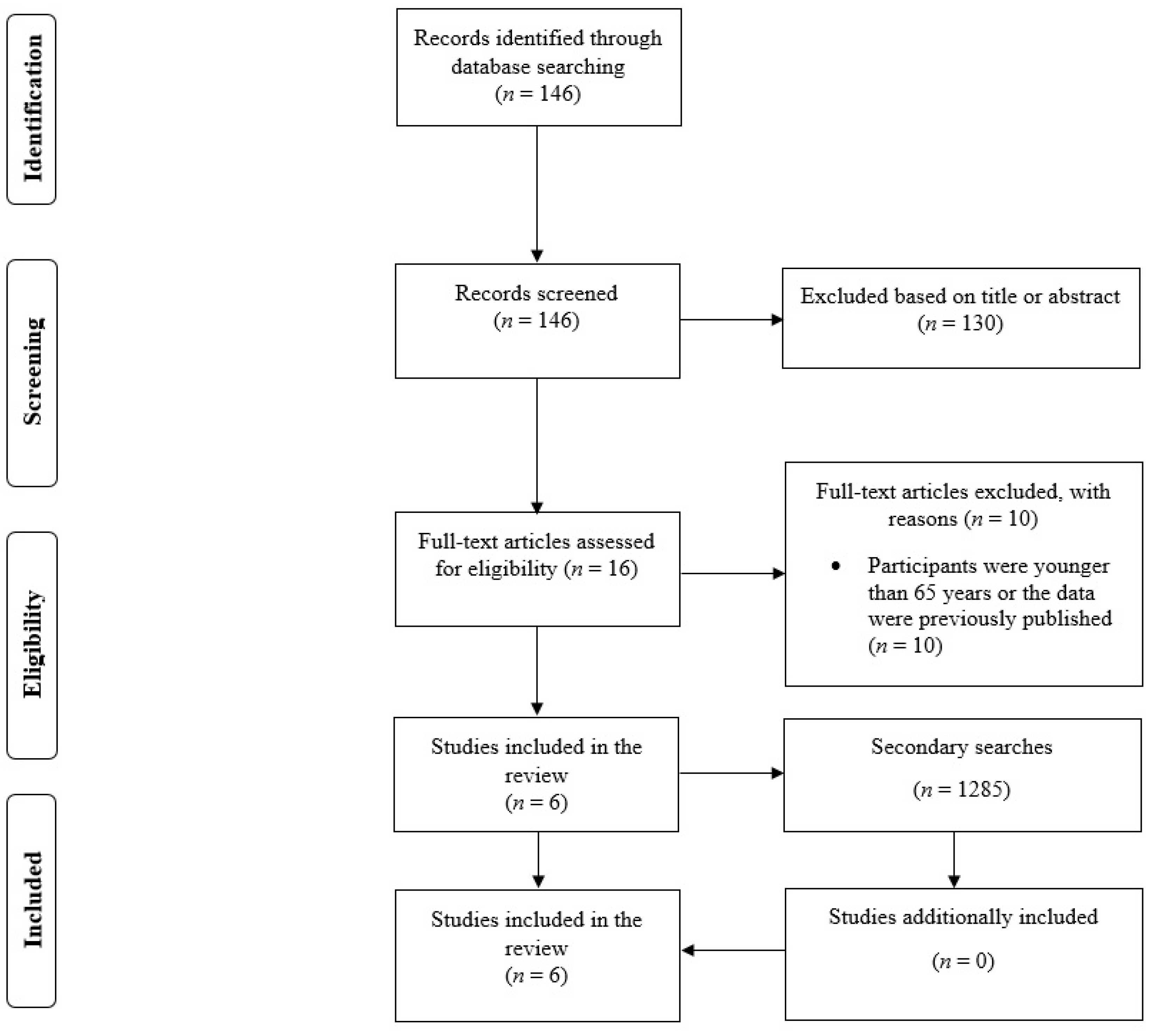
3.1. Search Results
3.2. Summary of Studies
3.3. Methodological Quality
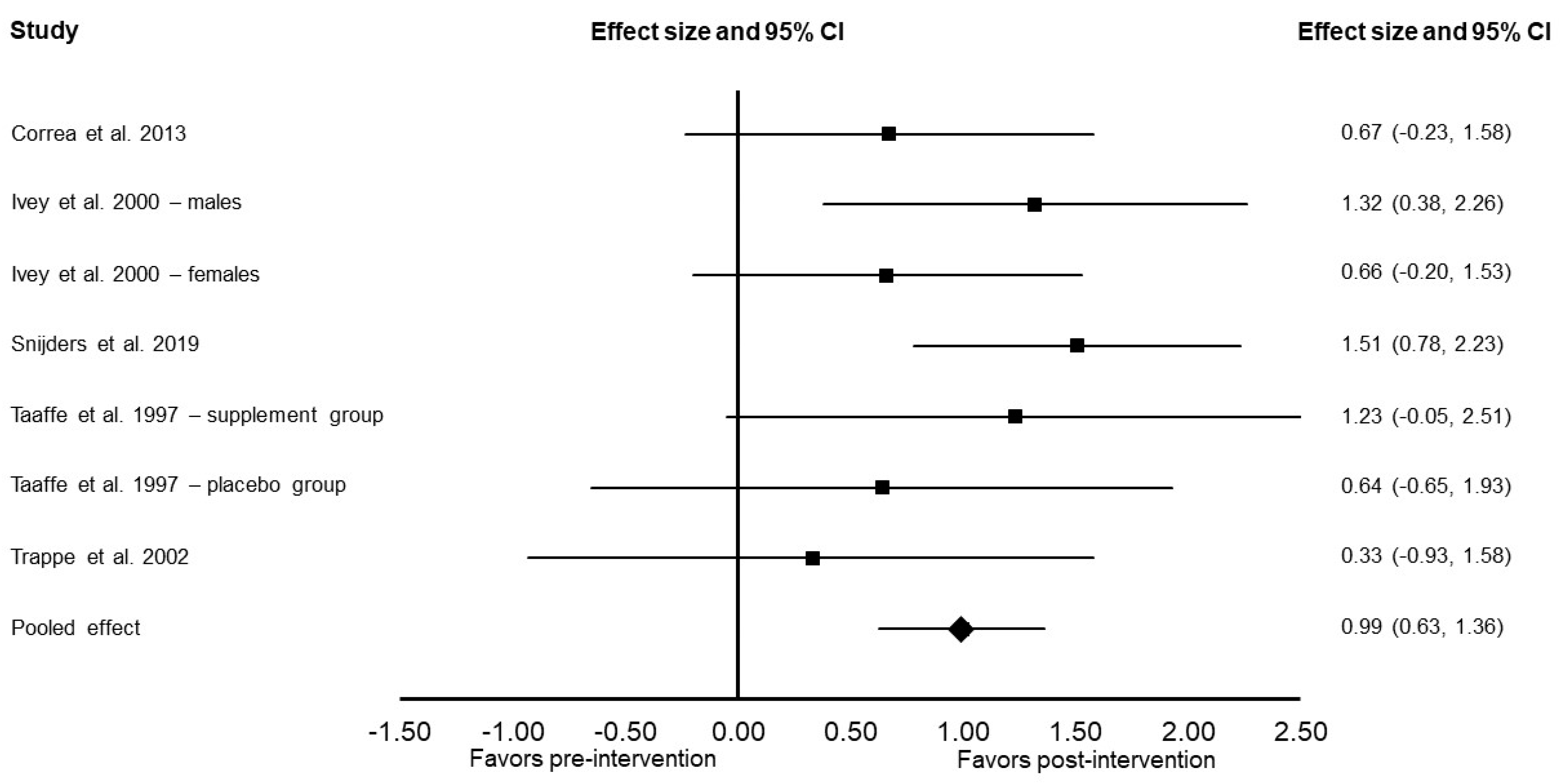
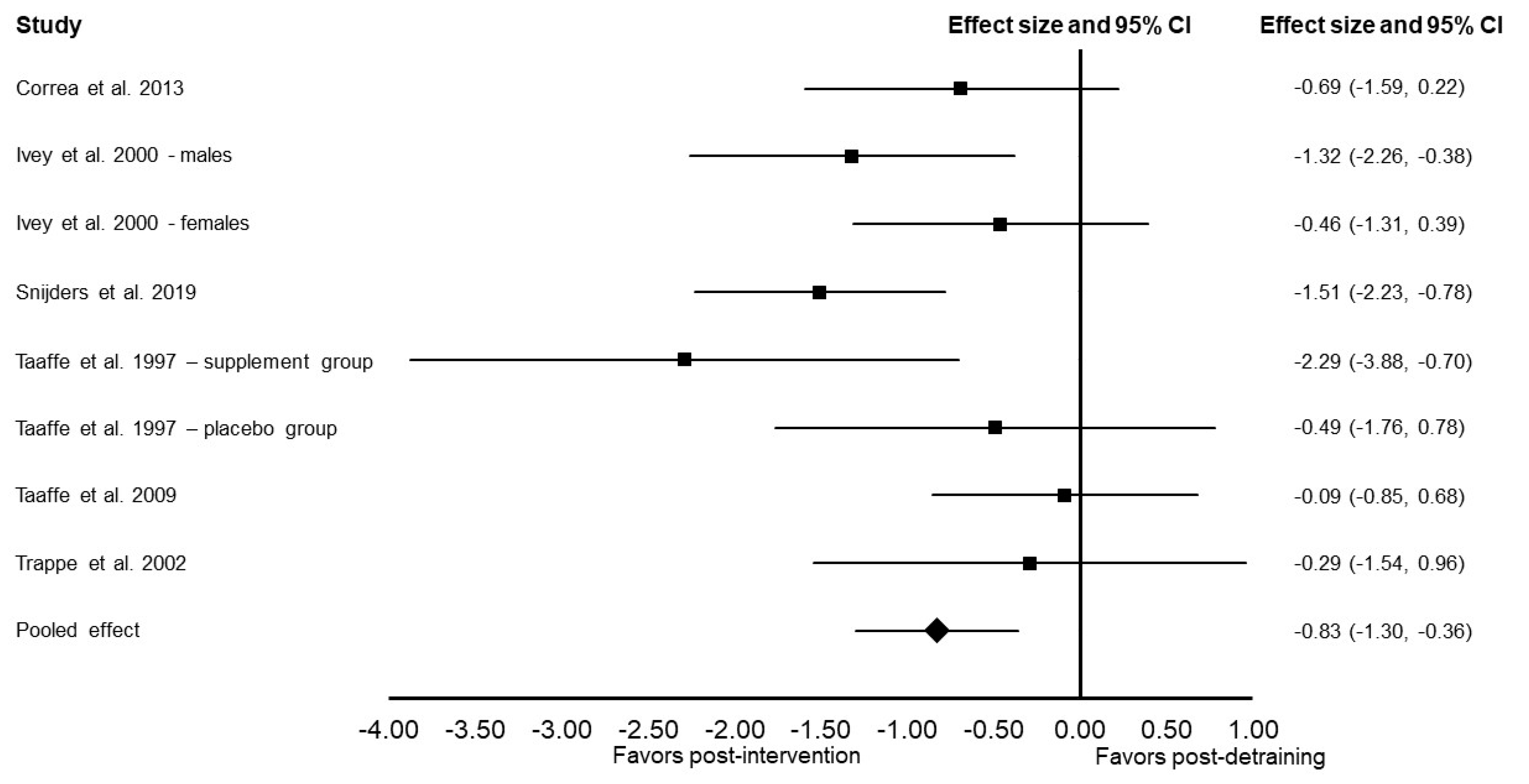
3.4. Meta-Analysis Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet. 2009, 374, 1196–1208. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M. What is dynapenia? Nutrition 2012, 28, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Law, T.D.; Clark, L.A.; Clark, B.C. Resistance exercise to prevent and manage sarcopenia and dynapenia. Annu. Rev. Gerontol. Geriatr. 2016, 36, 205–228. [Google Scholar] [CrossRef]
- Faigenbaum, A.D.; Kraemer, W.J.; Blimkie, C.J.; Jeffreys, I.; Micheli, L.J.; Nitka, M.; Rowland, T.W. Youth resistance training: Updated position statement paper from the national strength and conditioning association. J. Strength Cond. Res. 2009, 23, S60–S79. [Google Scholar] [CrossRef]
- Liu, C.J.; Latham, N.K. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009, 3, 002759. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; McCarthy, J.P.; Bamman, M.M. Effects of resistance training on older adults. Sports Med. 2004, 34, 329–348. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, T.; Arya, Y.K.; Mittal, S. Physical fitness and exercise during the COVID-19 pandemic: A qualitative enquiry. Front. Psychol. 2020, 11, 590172. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.S.; Baroni, B.M.; Radaelli, R.; Lanferdini, F.J.; Cunha Gdos, S.; Reischak-Oliveira, Á.; Vaz, M.A.; Pinto, R.S. Effects of strength training and detraining on knee extensor strength, muscle volume and muscle quality in elderly women. Age 2013, 35, 1899–1904. [Google Scholar] [CrossRef]
- Ivey, F.M.; Tracy, B.L.; Lemmer, J.T.; NessAiver, M.; Metter, E.J.; Fozard, J.L.; Hurley, B.F. Effects of strength training and detraining on muscle quality: Age and gender comparisons. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, B152–B157. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Leenders, M.; de Groot, L.C.P.G.M.; van Loon, L.J.C.; Verdijk, L.B. Muscle mass and strength gains following 6 months of resistance type exercise training are only partly preserved within one year with autonomous exercise continuation in older adults. Exp. Gerontol. 2019, 121, 71–78. [Google Scholar] [CrossRef]
- Taaffe, D.R.; Marcus, R. Dynamic muscle strength alterations to detraining and retraining in elderly. Clin. Physiol. 1997, 17, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Taaffe, D.R.; Henwood, T.R.; Nalls, M.A.; Walker, D.G.; Lang, T.F.; Harris, T.B. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology 2009, 55, 217–223. [Google Scholar] [CrossRef]
- Trappe, S.; Williamson, D.; Godard, M. Maintenance of whole muscle strength and size following resistance training in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B138–B143. [Google Scholar] [CrossRef]
- Haun, C.T.; Vann, C.G.; Roberts, B.M.; Vigotsky, A.D.; Schoenfeld, B.J.; Roberts, M.D. A critical evaluation of the biological construct skeletal muscle hypertrophy: Size matters but so does the measurement. Front. Physiol. 2019, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Pickering, C. The effects of caffeine ingestion on isokinetic muscular strength: A meta-analysis. J. Sci. Med. Sport 2019, 22, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Burd, N.A.; Gorissen, S.H.; van Loon, L.J. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Garofolini, A.; Orazem, J.; Sabol, F.; Schoenfeld, B.J.; Pedisic, Z. Effects of resistance training on muscle size and strength in very elderly adults: A systematic review and meta-analysis of randomized controlled trials. Sports Med. 2020, 50, 1983–1999. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014, 44, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Fisher, J.P.; Grgic, J.; Haun, C.T.; Helms, E.T.; Phillips, S.M.; Steele, J.; Vigotsky, A.D. Resistance training recommendations to maximize muscle hypertrophy in an athletic population: Position stand of the IUSCA. Int. J. Strength Cond. 2021, 1, 1–30. [Google Scholar] [CrossRef]
- Kadi, F.; Schjerling, P.; Andersen, L.L.; Charifi, N.; Madsen, J.L.; Christensen, L.R.; Andersen, J.L. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J. Physiol. 2004, 558, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. Metab. 1997, 273, E99–E107. [Google Scholar] [CrossRef]
- Gavanda, S.; Geisler, S.; Quitmann, O.J.; Bauhaus, H.; Schiffer, T. Three weeks of detraining does not decrease muscle thickness, strength or sport performance in adolescent athletes. Int. J. Exerc. Sci. 2020, 13, 633–644. [Google Scholar] [PubMed]
- Loenneke, J.P.; Rossow, L.M.; Fahs, C.A.; Thiebaud, R.S.; Grant Mouser, J.; Bemben, M.G. Time-course of muscle growth, and its relationship with muscle strength in both young and older women. Geriatr. Gerontol. Int. 2017, 17, 2000–2007. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.C.; Chen, C.N.; Hsu, C.W.; Zhou, W.S.; Chien, K.Y. Training session and detraining duration affect lower limb muscle strength maintenance in middle-aged and older adults: A systematic review and meta-analysis. J. Aging Phys. Act. 2022, 30, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Bosquet, L.; Berryman, N.; Dupuy, O.; Mekary, S.; Arvisais, D.; Bherer, L.; Mujika, I. Effect of training cessation on muscular performance: A meta-analysis. Scand. J. Med. Sci. Sports 2013, 23, e140–e149. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Grgic, I. Resistance training–induced gains in rate of force development are maintained during training cessation: A meta-analysis. Strength Cond. J. 2022. [Google Scholar] [CrossRef]
- Ahmadiahangar, A.; Javadian, Y.; Babaei, M.; Heidari, B.; Hosseini, S.; Aminzadeh, M. The role of quadriceps muscle strength in the development of falls in the elderly people, a cross-sectional study. Chiropr. Man. Ther. 2018, 26, 31. [Google Scholar] [CrossRef]
| Study | Participants | Resistance Training | Detraining Duration | Muscle Hypertrophy Assessment |
|---|---|---|---|---|
| Correa et al. (2013) [11] | 10 sedentary elderly women (67 ± 5 years) | 12 weeks, 2 times per week; 2–4 sets of 8–20 repetitions using 60–80% of 1 RM | 12 weeks | Quadriceps muscle volume using B-mode ultrasound |
| Ivey et al. (2000) [12] | 11 older men and 11 older women (65–75 years) | 9 weeks, 3 times per week; 5 sets of 5–20 repetitions using 50–80% of 1 RM | 31 weeks | Quadriceps muscle volume using MRI |
| Snijders et al. (2019) [13] | 19 older men and women (65+ years) | 24 weeks, 3 times per week; 2–4 sets of 8 repetitions using 80% of 1 RM | 1 year | Quadriceps muscle cross-sectional area using CT |
| Taaffe et al. (1997) [14] | 11 older men (65–77 years) | 24 weeks, 3 times per week; 3 sets of 8 repetitions using 75% of 1 RM | 12 weeks | Biopsies of the vastus lateralis muscle |
| Taaffe et al. (2009) [15] | 13 older men and women (65–83 years) | 24 weeks, 2 times per week; 3 sets of 8 repetitions using 65–75% of 1 RM | 24 weeks | Quadriceps muscle volume using CT |
| Trappe et al. (2002) [16] | 5 older men (70 ± 4 years) | 12 weeks, 3 times per week; 3 sets of 10 repetitions using 80% 1 RM | 24 weeks | Quadriceps muscle cross-sectional area using CT |
| Study | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correa et al. (2013) [11] | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 5 |
| Ivey et al. (2000) [12] | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Snijders et al. (2019) [13] | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 5 |
| Taaffe et al. (1997) [14] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 |
| Taaffe et al. (2009) [15] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 |
| Trappe et al. (2002) [16] | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grgic, J. Use It or Lose It? A Meta-Analysis on the Effects of Resistance Training Cessation (Detraining) on Muscle Size in Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 14048. https://doi.org/10.3390/ijerph192114048
Grgic J. Use It or Lose It? A Meta-Analysis on the Effects of Resistance Training Cessation (Detraining) on Muscle Size in Older Adults. International Journal of Environmental Research and Public Health. 2022; 19(21):14048. https://doi.org/10.3390/ijerph192114048
Chicago/Turabian StyleGrgic, Jozo. 2022. "Use It or Lose It? A Meta-Analysis on the Effects of Resistance Training Cessation (Detraining) on Muscle Size in Older Adults" International Journal of Environmental Research and Public Health 19, no. 21: 14048. https://doi.org/10.3390/ijerph192114048
APA StyleGrgic, J. (2022). Use It or Lose It? A Meta-Analysis on the Effects of Resistance Training Cessation (Detraining) on Muscle Size in Older Adults. International Journal of Environmental Research and Public Health, 19(21), 14048. https://doi.org/10.3390/ijerph192114048






