Abstract
Breast cancer (BC) is the leading cause of cancer in women, and has implications for sexual function (SF). In this study, we used an evidence map to identify, describe, and organise the current available evidence regarding SF in women with BC. We searched the MEDLINE, PsycINFO, and CINAHL databases for observational studies assessing SF in women with BC published in English, Spanish, Portuguese, and French between 2000 and 2021 (sample ≥ 50 women). Of the 64 included studies (13,257 women with BC), 58 were published since 2010. Women who were married, partnered, or in relationships represented 74.1% of the entire sample. Only a single study was conducted on women representing a sexual minority. We identified 22 assessment instruments and 40 sexual dysfunction (SdF) domains. The number of publications on SF in women with BC has increased in the last 10 years, but still remains low. Some groups of women are underrepresented, and some SdF domains are underdiagnosed, with the assessment instrument used affecting which domains are studied. Women with BC need to be better screened, as their quality of life (QoL) is affected by SdF.
1. Introduction
Female breast cancer (BC) is the fifth leading cause of cancer mortality worldwide; it accounts for one in four cancer cases and one in six cancer deaths. In 2020, BC represented 11.7% of all new cancer cases (an estimated 2.3 million new cases). The incidence rates are 88% higher in emerging countries than in transitioned countries, and countries with lower human-development-index (HDI) scores have a 17% higher mortality rate than countries with higher HDI scores [1].
In recent years, BC survival has improved due to early detection strategies and access to timely, effective, and affordable care [2]. However, health providers and health systems need to address BC survivor issues and concerns regarding the treatment and disease course.
In a recent systematic review on adverse mental health outcomes, the researchers revealed that BC survivors have an increased risk of anxiety, depression, neurocognitive dysfunction, sexual dysfunction (SdF), and suicide compared with noncancer groups. The prevalence of SdF, in particular, is reported to range between 20% and 60% [3]. In another study, the researchers reported that women with BC have a high prevalence of SdF (73.4%) and lower average sexual function (SF) scores [4]; the main problems include penetration pain, desire, lubrication, dysfunctional excitement, and reproductive concerns [5,6]. In a study conducted in our hospital to evaluate the social, economic, and professional impacts of BC on women, the researchers found that SF was the most affected quality-of-life (QoL) dimension, and especially during treatment, with a score of 20.7% in the European Organisation for Research and Treatment of Cancer Quality of Life 23-item breast cancer questionnaire [7].
According to the fifth edition of the Diagnostic and Statistical Manual of Mental Health Disorders (DSM-5) [8], SdF covers a heterogeneous group of disorders that are typically characterised by a clinically substantial impairment in a person’s ability to sexually respond or experience sexual pleasure. Female SF, for which researchers have characterised several models in the literature, comprises more than just arousal and orgasm [9].
We need to organise the substantial evidence reported in the scientific literature assessing SF in women who have had BC through a standardised methodology.
The Global Evidence Mapping (GEM) initiative was established in 2007 to provide a general overview of the existing research on traumatic brain and spinal cord injuries [10]. Such evidence maps, which are based on systematic and wide-ranging searches to identify knowledge gaps and future research needs, present results in a user-friendly format (often a visual, graph, or searchable database) [11]. They are both a useful first step in the systematic review process and support for the decision-making process for policy and practice [12,13].
The purpose of our evidence map was to identify, describe, and organise the available evidence regarding SF in women who have BC in order to aid a better understanding of the existing studies, identify the evidence gaps in the literature, and make recommendations for future research.
2. Materials and Methods
2.1. Study Design
We based this mapping review, which we carried out in accordance with the GEM initiative, on a methodology that involved three core tasks: setting the map boundaries and context; searching for and selecting relevant studies; reporting on the yield and study characteristics [10]. We also followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extension for Scoping Reviews (PRISMA-ScR) recommendations [14]. We designed a protocol and published it in the Open Science Framework (https://osf.io/4qbgu/).
2.2. Eligibility Criteria
We considered observational studies with samples of ≥50 women with BC, published between January 2000 and March 2021 in English, Spanish, Portuguese, or French, and evaluating SF using a specific structured measure, as eligible studies. We did not include older published studies, as sexuality is a controversial subject that is influenced by multiple factors and is variable over the years.
2.3. Search Strategy
We performed the systematic literature searches for original articles in the following databases: MEDLINE (via PubMed), PsycINFO, and CINAHL. We conducted the last search on 7 March 2021. We used comprehensive controlled vocabulary and free-text terms. The full electronic search strategy for MEDLINE (via PubMed) was as follows: ((“breast neoplasms” [mesh] OR “breast cancer” [ti]) AND (“sexuality” [majr] OR “sexual dysfunction, physiological” [mesh] OR “sexual activit*” [tiab] OR “sexual dysfunction” [tiab] OR “sexual function*” [tiab] OR “sexual interest*” [tiab] OR “sexual desire*” [tiab] OR “sexuality” [tiab] OR “sexual*” [ti])). We adapted the search strategy to the requirements of each database.
2.4. Study Selection
We imported the retrieved studies into COVIDENCE reference manager software. We first eliminated duplicate articles, after which two independent researchers screened the titles and abstracts and applied the predefined eligibility criteria to remove ineligible studies. We next eliminated the full texts of the eligible studies to make a final decision regarding the eligibility. We resolved the disagreements in a final online meeting. We clearly justified the reasons for the study exclusions.
2.5. Data Extraction
To manage the data and records, we designed a template form in Microsoft Word to individually record the data for each included study. We recorded data on the authors, publication years, journals, study designs, countries, objectives, participants (marital status, sexual orientation, and age recorded as mean/median or range), instruments used to assess sexual function, and domains.
3. Results
3.1. Selected Studies
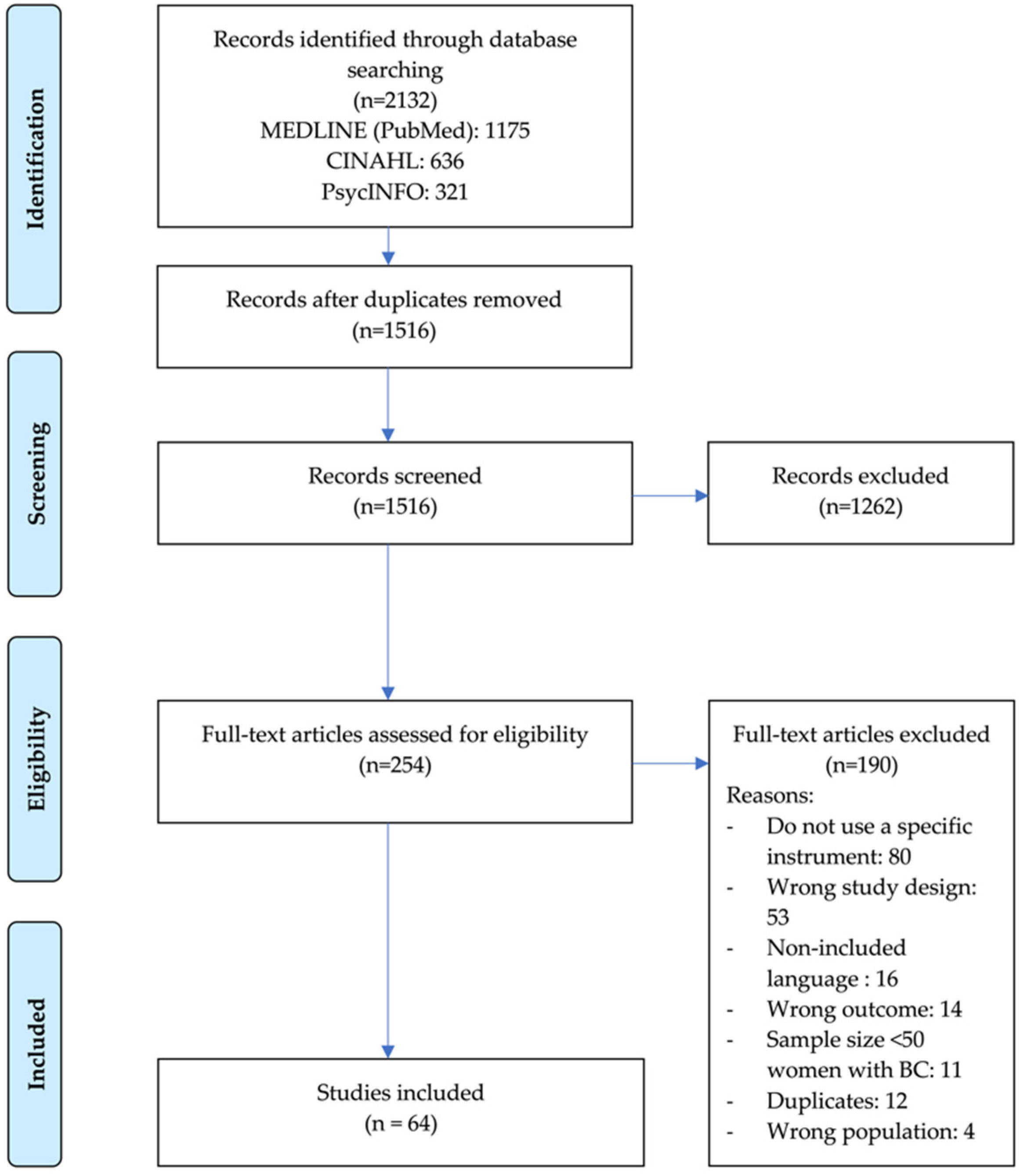
After we removed the duplicates, we screened a total of 1516 titles and abstracts for eligibility. Of the 254 articles selected for full-text screening, we included 64 that met the eligibility criteria in this review. We present the details on the study inclusion and reasons for exclusion in the flowchart in Figure 1.
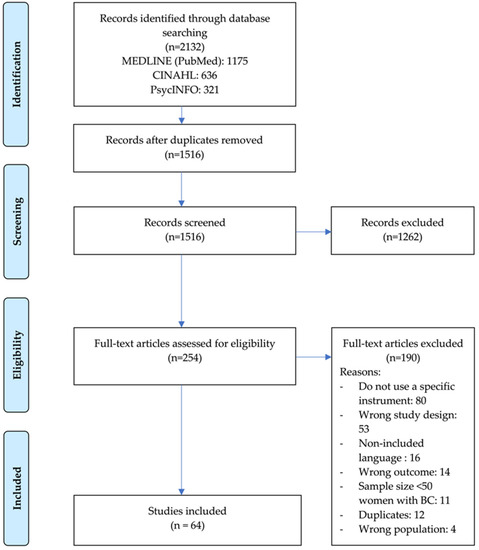
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow diagram of study selection process.
3.2. Study Characteristics
We present the details of the authors, years of publication, designs, countries, objectives, and participants for the 64 studies in Table 1. The 64 studies, with minimum and maximum sample sizes of 55 and 1957, respectively, included 13,257 women with BC. One study was conducted with 85 lesbian or bisexual women with a female partner, and 51 studies provided data on the mean age, which ranged from 31.4 to 63.4 years. Of the 59 studies (9825 women, 74.1%) that included women who were married, partnered, or in a relationship, in 19 of them, the researchers exclusively focused on women with this profile.

Table 1.
Characteristics of studies included in evidence map.
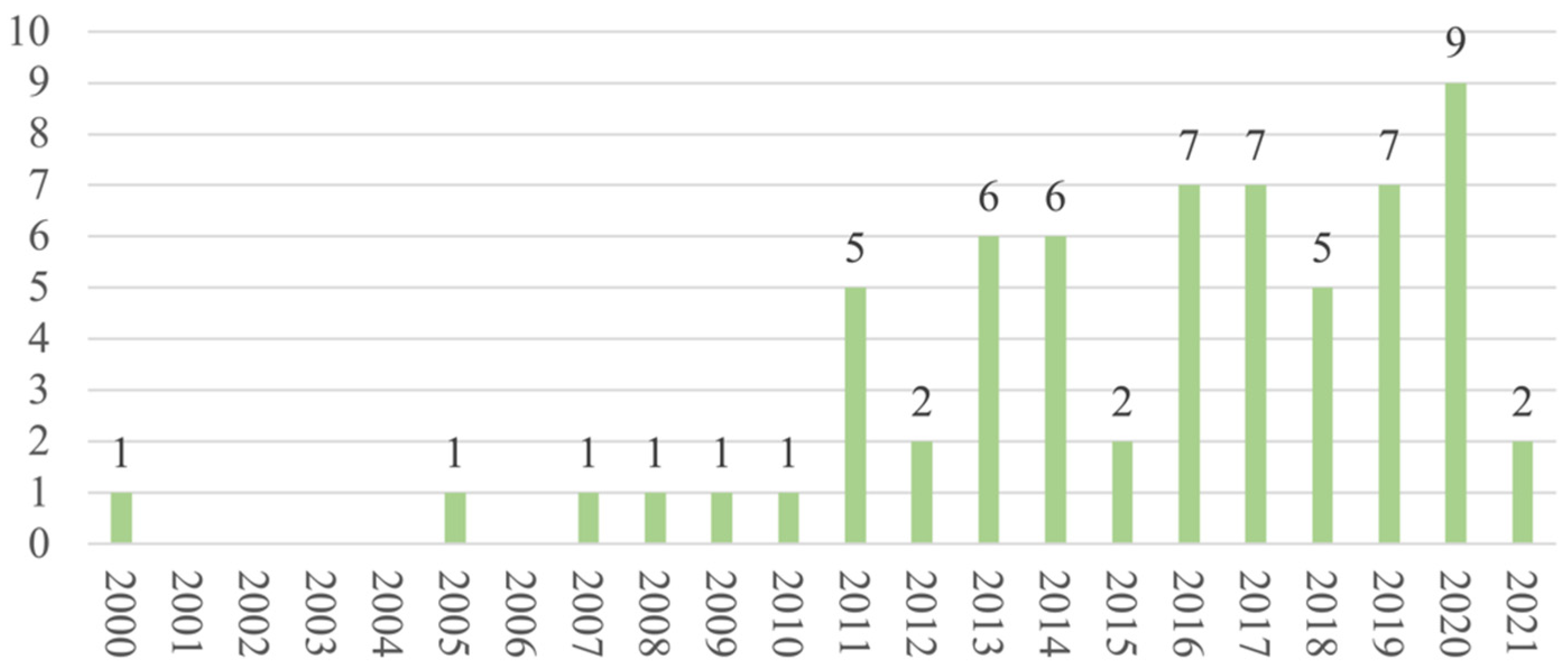
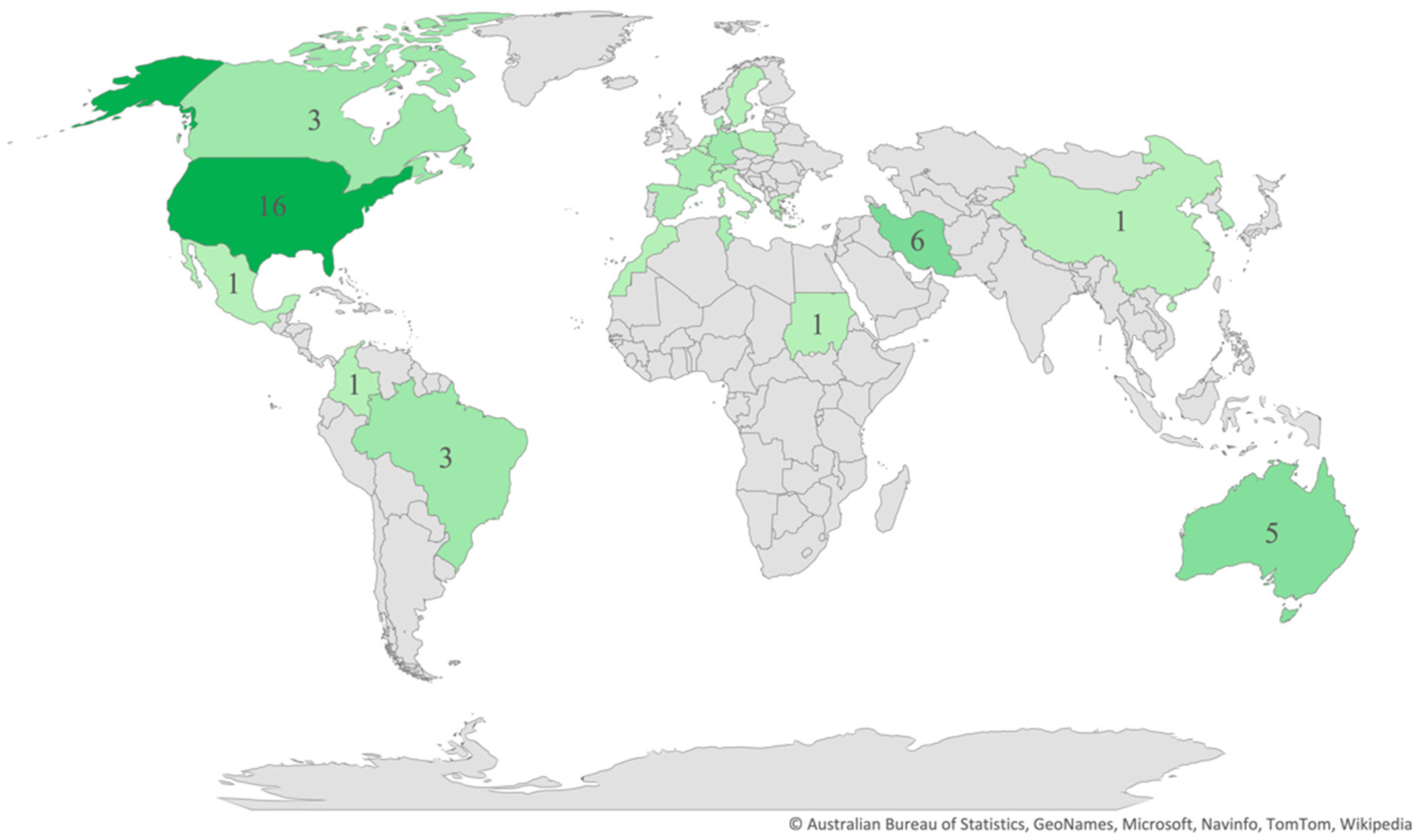
Regarding the publication years, most of the studies (n = 58) were published between 2011 and 2021 (Figure 2), and most (n = 42) were cross-sectional in design. As for the regions and countries, 20 studies were conducted in North America, 16 in Europe, 11 in the Middle East, 5 in Australia, 4 in South America, 4 in Africa, 3 in East Asia, and 1 in South Asia (Figure 3). The USA and Iran were the most represented countries (n = 16 and n = 6, respectively), followed by Australia and Turkey (n = 5 each).
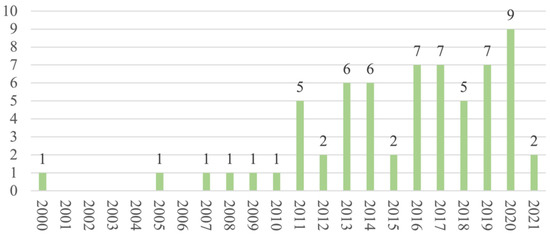
Figure 2.
Distribution of articles by year.
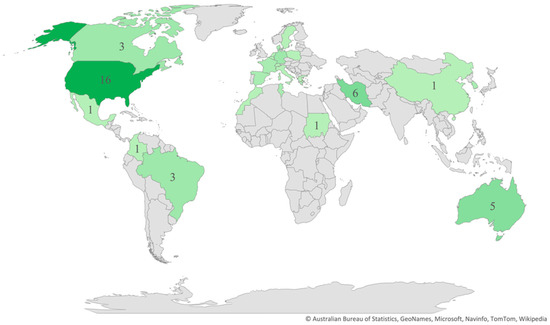
Figure 3.
Distribution of articles by country.
3.3. SF Assessment
In most of the studies, the researchers used a single instrument to assess the SF, although six studies used two instruments. We present the summaries of the 22 different instruments that we identified in Table S1. The most frequently used instrument was the Female Sexual Function Index (n = 35), followed by the Sexual Activity Questionnaire (n = 8), PROMIS Sexual Function and Satisfaction Measures Brief Profile (SexFS) (n = 4), and Watts Sexual Function Questionnaire and Cancer Rehabilitation Evaluation System Short Form (CARES-SF) sexual subscale (n = 3 each). The instruments used in just a single study were as follows: the Short Sexual Function Scale; Specific Sexual Problems Questionnaire; Golombock–Rust Inventory of Sexual Satisfaction; Questionnaire on Women’s Sexual Function; MacCoy Female Sexuality Questionnaire; Sexual Complaint Screener for Women; Sexual Function Questionnaire; Arizona Sexual Experience Scale; Short Form of the Questionnaire for Screening Sexual Dysfunction; Changes in Sexual Function Questionnaire; Sexual Quotient–Female Version; Sexual Interest and Desire Inventory–Female; Sexual Functioning Questionnaire–Women; Short Form of the Personal Experience Questionnaire; 10-item Menopausal Sexual Interest Questionnaire; 28-item Sexual Function Questionnaire; Relationship and Sexuality Questionnaire.
We identified a total of 40 independently assessed domains, as follows: desire and satisfaction (n = 41 each); orgasm (n = 40); lubrication (n = 39); arousal (n = 37); pain (n = 36); pleasure (n = 8); discomfort and interest (n = 6 each); frequency (n = 5); vaginal discomfort (n = 4); relationship (n = 3); vaginismus, excitation, habit, vulvar discomfort–labial, and vulvar discomfort–clitoral (n = 2 each). Other domains were evaluated only in a single study (swelling of the labia; reduced length of the vagina; reduced elasticity of the vagina; communication; avoidance; touch; anticipatory anxiety; sexual initiative; masturbation problems; activity; sexual drive; sexual aversion; importance; tiredness; sexual attractiveness; feeling; responsibility; libido; partner problems; responsiveness; sexual enjoyment; distress).
In 32 studies, researchers report data on the relationship between SdF and age, which is associated with older age groups (n = 14), younger age groups (n = 3), and non-associated with age (n = 15). The type of treatment was negatively related to SdF, whether it was mastectomy (n = 15/29), chemotherapy (n = 11/28), or hormone therapy (n = 11/30).
4. Discussion
On this evidence map, we included 64 studies published in the last 20 years, comprising a total of 13,257 women with BC for whom SdF was assessed. We observed an evident increase in the number of publications over time (58 of the 64 included studies were published in the last 10 years), which suggests a growing interest in understanding how BC impacts women’s SF.
However, given the importance of the issue, we consider the number of publications to be too low. SdF is a common side effect of cancer treatments, and so it is imperative for health providers to routinely include a comprehensive evaluation of sexual health in the workups for such patients from the outset [77]. Female sexuality is a complex issue, as physiological, psychological, and sociocultural factors and interpersonal relationships all play a part, not to mention the fact that the delayed study of this issue may be due to the historical stigmatisation of women and their sexuality [78].
Interestingly, most of the included women were married, partnered, or in relationships, with a substantial number of researchers selecting this status as an inclusion criterion, which suggests that women without partners, who may experience their sexuality in a different way (e.g., masturbation), are underrepresented.
Only one study was conducted with lesbian or bisexual women having a female partner [21]. Researchers have found that SF is independent of sexual orientation, and the fact that sexual minorities appear to have different social attitudes and sexual practices may imply specific consequences of the physical effects of cancer and its treatment for lesbian, gay, and bisexual populations [79,80,81]. The limited literature on SF in women with BC from sexual minorities remains a key aspect of survivor health outcomes that requires further study [82,83].
On our evidence map, we identified 22 validated instruments used by the authors to assess SF in women with BC. Authors used the Female Sexual Function Index in over 50% of the studies (n = 35). This brief 19-item multidimensional self-report instrument for assessing the key dimensions of SF in women, which incorporates the criteria of SdF as defined and recognised by international diagnostic systems [8], was designed and validated for use in clinical trials and epidemiological studies [84]. Women with BC found it to be the right length, easy to complete, and relevant to their experience, and it also demonstrates excellent psychometric properties (high internal consistency, test–retest reliability, and evidence of construct validity) [85]. The second most-used instrument was the Sexual Activity Questionnaire, which is rapidly and easily administered and an acceptably reliable measure. The questionnaire describes SF in terms of the levels of sexual activity, pleasure, and discomfort [86], has been demonstrated to be a useful instrument for measuring sexual activity in women with cancer [87], has been validated in different countries, and has good psychometric properties [88,89,90]. The PROMIS Sexual Function and Satisfaction Measures Brief Profile (SexFS) is also widely used, which is another reliable and valid tool for measuring self-reported SF and satisfaction among men and women with cancer. The instrument is comprehensive in scope, covering both physical and psychological constructs, and provides a comprehensive assessment of satisfaction and key SF domains [91]. Although researchers have previously validated all the instruments and they have good psychometric properties, their scopes differ, and they do not evaluate the same domains. Furthermore, some authors present results as a total SF score, overlooking the fact that an individual may have several simultaneous SdF disorders and that diagnosis should be individual [8].
The researchers assessed a total of 40 domains with the 22 assessment instruments. The most studied domains were desire, satisfaction, orgasm, lubrication, arousal, and pain. Some of the domains reflect internationally accepted classifications as reviewed by the Fourth International Consultation on Sexual Medicine [92], with the following considered to reflect SdF: hypoactive sexual desire dysfunction; female sexual arousal dysfunction; female orgasmic dysfunction; female genital–pelvic pain dysfunction; persistent genital arousal disorder; postcoital syndrome; hypohedonic orgasm; painful orgasm. However, several of the domains reflected in our review are not included in international classifications, which indicates a lack of agreement and the need for further work regarding the definitions and diagnostic criteria, given the need to avoid underestimating SdF [93]. No single assessment instrument measured all the reported domains, which is a fact that adds further complexity to the concept of SdF and leads to the underestimation and underdiagnosis of potential SdF in women with BC.
Finally, in some studies, researchers report measures of the relationships between SdF and age, mastectomy, chemotherapy, and hormone therapy. While Park et al. found that older women were more likely to have a poorer QoL than younger women, especially in terms of the items directly related to sexuality [94], other researchers found that younger women experienced more SF problems [95,96]. Some of the heterogeneity in the data may be explained by the age cut-off for younger versus older women, as well as certain sociocultural factors. In relation to the type of treatment, in a systematic review, the authors report that surgical options other than mastectomy have a better impact on the SF of women with BC [97]. In another study, the authors report that hormone therapy has significant side effects for the QoL of women, including SdF, specifically in terms of the loss of sexual interest, vaginal dryness, and pain during sex [98]. The loss of ovarian function and subsequent early menopause, as side effects due to chemotherapy, often cause SdF symptoms [99]. Nonetheless, certain individual features (e.g., culture, religion, and psychological and physical status) may also influence the way women experience BC.
4.1. Study Limitations
This evidence map has some limitations. The search was last updated in March 2021, which means that we may have missed studies published after that date. Furthermore, we only searched three databases; however, these databases are comprehensive, and so we consider that this limitation did not substantially affect our main findings. Another limitation was that we only selected specific SF assessment instruments and excluded studies based on general instruments (e.g., QoL instruments) that only partially evaluate certain aspects of SF. Although we recognise the importance of general instruments, we consider that specific measures may be more sensitive for the detection of small changes in this specific concept.
As for the strengths, as far as we are aware, ours may be the first evidence map that provides a comprehensive synthesis of the available evidence on SF in women with BC reported in the last 20 years. Furthermore, we based our evidence map on a standardised methodology that requires a systematic search and enables the results to be presented for easy and user-friendly interpretation.
4.2. Clinical Implications
The evidence map methodology used in this study highlights the important knowledge gaps and identifies future research needs. Our review suggests that SF in women with BC is a broad and complex concept. We suggest that health providers, researchers, and policymakers may need to rethink the concept of SF in a more inclusive way, and they should furthermore consider improving the assessment instruments used to date by including every domain, thereby avoiding underdiagnosis. A valid, reliable, interesting, and easy-to-use measurement instrument allows us to more precisely evaluate SF. Although every health system depends on its own principles, culture, and resources, we strongly recommend that clinicians use/introduce the most suitable instrument in their clinical practice to support decision-making and improve the QoL of woman with BC.
5. Conclusions
This evidence map provides a broad vision on how the research on SF in women with BC has been conducted so far.
While studies of SF in women with BC have substantially increased in number over the last 10 years, the importance and frequency of this health problem indicate that the number is still too low. Most of the studies include only women who are married, partnered, or in relationships, which leaves single, lesbian, and bisexual women underrepresented, which can be solved with new studies conducted on these specific groups of women.
Although the studies include a significant number of SF-related domains, this very much depended on the specific assessment instrument used, leading to the underestimation and underdiagnosis of some dysfunctions. Therefore, SdF should not be undervalued, as it can cause suffering in these women and might delay recovery. Future research should focus on ways to better screen for SdF in women with BC and improve their QoL.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192113976/s1, Table S1: Assessment instruments used.
Author Contributions
Conceptualisation, N.R.-M., M.J.Q. and X.B.-C.; methodology, N.R.-M., M.J.Q. and X.B.-C.; software, N.R.-M., M.J.Q. and X.B.-C.; formal analysis, N.R.-M., M.J.Q. and X.B.-C.; investigation, N.R.-M.; data curation, N.R.-M., M.J.Q., R.G.-G. and X.B.-C.; writing—original draft preparation, N.R.-M.; writing—review and editing, N.R.-M., M.J.Q. and X.B.-C.; supervision, M.J.Q. and X.B.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated or analysed during this study are included in the published review article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Es-timates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, O.; Yip, C.; Brooks, A.; Cabanes, A.; Caleffi, M.; Yataco, J.A.D.; Gyawali, B.; McCormack, V.; de Anderson, M.M.; Mehrotra, R.; et al. Breast cancer early detection: A phased approach to implementation. Cancer 2020, 126, 2379–2393. [Google Scholar] [CrossRef] [PubMed]
- Carreira, H.; Williams, R.; Müller, M.; Harewood, R.; Stanway, S.; Bhaskaran, K. Associations Between Breast Cancer Survivorship and Adverse Mental Health Outcomes: A Systematic Review. J. Natl. Cancer Inst. 2018, 110, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Zhang, C.; Li, W.; Jin, F.; Wang, A. Incidence and severity of sexual dysfunction among women with breast cancer: A me-ta-analysis based on female sexual function index. Support. Care Cancer 2019, 27, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Cuenca, A.I.; Espínosa, N.M.M.; Sampietro-Crespo, A.; Rodríguez-Borrego, M.A.; Carmona-Torres, J.M. Sexual dysfunction in Spanish women with breast cancer. PLoS ONE 2018, 13, e0203151. [Google Scholar] [CrossRef]
- Ljungman, L.; Ahlgren, J.; Petersson, L.-M.; Flynn, K.; Weinfurt, K.; Gorman, J.R.; Wettergren, L.; Lampic, C. Sexual dysfunction and reproductive concerns in young women with breast cancer: Type, prevalence, and predictors of problems. Psycho-Oncology 2018, 27, 2770–2777. [Google Scholar] [CrossRef]
- Masià, J.; Merchán-Galvis, Á.; Salas, K.; Requeijo, C.; Cánovas, E.; Quintana, M.J.; Bonfill, X. Socio-economic impact on women diagnosed and treated for breast cancer: A cross-sectional study. Clin. Transl. Oncol. 2019, 21, 1736–1745. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Boswell, E.N.; Dizon, D.S. Breast cancer and sexual function. Transl. Androl. Urol. 2015, 4, 160–168. [Google Scholar] [CrossRef]
- Bragge, P.; Clavisi, O.; Turner, T.; Tavender, E.; Collie, A.; Gruen, R.L. The Global Evidence Mapping Initiative: Scoping research in broad topic areas. BMC Med. Res. Methodol. 2011, 11, 92. [Google Scholar] [CrossRef]
- Miake-Lye, I.M.; Hempel, S.; Shanman, R.; Shekelle, P.G. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst. Rev. 2016, 5, 1–21. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Bernes, C.; Jonsson, B.-G.; Hedlund, K. The benefits of systematic mapping to evidence-based environmental management. Ambio 2016, 45, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Snilstveit, B.; Vojtkova, M.; Bhavsar, A.; Stevenson, J.; Gaarder, M. Evidence & Gasp Maps: A tool for promoting evidence informed policy and strategic research agendas. J. Clin. Epidemiol. 2016, 79, 120–129. [Google Scholar] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Abasher, S.M. Sexual health issues in Sudanese women before and during hormonal treatment for breast cancer. Psycho-Oncology 2009, 18, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Christiaens, M.; Enzlin, P.; Neven, P.; Amant, F. Sexual functioning in women after mastectomy versus breast conserving therapy for early-stage breast cancer: A prospective controlled study. Breast 2014, 23, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Alacacioglu, A.; Ulger, E.; Varol, U.; Yildiz, I.; Salman, T.; Bayoglu, V.; Dirican, A.; Demir, L.; Akyol, M.; Yildiz, Y.; et al. Depression, Anxiety and Sexual Satisfaction in Breast Cancer Patients and their Partners-Izmir Oncology Group Study. Asian Pac. J. Cancer Prev. 2014, 15, 10631–10636. [Google Scholar] [CrossRef]
- Archangelo S de, C.V.; Sabino, M.; Veiga, D.F.; Garcia, E.B.; Ferreira, L.M. Sexuality, depression and body image after breast recon-struction. Clinics 2019, 74, e883. [Google Scholar] [CrossRef]
- Assogba, E.L.; Mamguem Kamga, A.; Costaz, H.; Jankowski, C.; Dumas, A.; Roignot, P.; Jolimoy, G.; Coutant, C.; Arveux, P.; Dabakuyo-Yonli, T.S. What Are Young Women Living Conditions after Breast Cancer? Health-Related Quality of Life, Sexual and Fertility Issues, Professional Reinsertion. Cancers 2020, 12, 1564. [Google Scholar] [CrossRef]
- Bober, S.L.; Giobbie-Hurder, A.; Emmons, K.M.; Winer, E.; Partridge, A. Psychosexual Functioning and Body Image Following a Diagnosis of Ductal Carcinoma In Situ. J. Sex. Med. 2013, 10, 370–377. [Google Scholar] [CrossRef]
- Boehmer, U.; Ozonoff, A.; Timm, A.; Winter, M.; Potter, J. After Breast Cancer: Sexual Functioning of Sexual Minority Survivors. J. Sex Res. 2014, 51, 681–689. [Google Scholar] [CrossRef]
- Brédart, A.; Dolbeault, S.; Savignoni, A.; Besancenet, C.; This, P.; Giami, A.; Michaels, S.; Flahault, C.; Falcou, M.-C.; Asselain, B.; et al. Prevalence and associated factors of sexual problems after early-stage breast cancer treatment: Results of a French exploratory survey. Psycho-Oncology 2011, 20, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Robles, L.S.B.; Soto-Lesmes, V.I. Salud sexual y alteraciones emocionales en mujeres colombianas con cáncer de mama. Psicooncol. Investig. Clín. Biopsicosoc. Oncol. 2015, 12, 405–416. [Google Scholar] [CrossRef][Green Version]
- Córdoba-de Juan, C.; Arranz-Martín, B.; Torres-Lacomba, M. Disfunción sexual en mujeres diagnosticadas y tratadas de cáncer de mama. Estudio descriptivo longitudinal. Fisioterapia 2019, 41, 73–82. [Google Scholar] [CrossRef]
- Cornell, L.F.; Mussallem, D.M.; Gibson, T.C.; Diehl, N.N.; Bagaria, S.P.; McLaughlin, S.A. Trends in Sexual Function After Breast Cancer Surgery. Ann. Surg. Oncol. 2017, 24, 2526–2538. [Google Scholar] [CrossRef]
- Cortés-Flores, A.O.; Vargas-Meza, A.; Morgan-Villela, G.; Jiménez-Tornero, J.; Del Valle, C.J.Z.-F.; Solano-Genesta, M.; Miranda-Ackerman, R.C.; Vázquez-Reyna, I.; García-González, L.A.; Cervantes-Cardona, G.A.; et al. Sexuality Among Women Treated for Breast Cancer: A Survey of Three Surgical Procedures. Aesthetic Plast. Surg. 2017, 41, 1275–1279. [Google Scholar] [CrossRef]
- Davis, S.C.; Meneses, K.; Messias, D.K.H. Exploring sexuality & quality of life in women after breast cancer surgery. Nurse Pract. 2010, 35, 25–31. [Google Scholar] [CrossRef]
- Ellouz, F.; Marrakchi, N.; Raies, H.; Masmoudi, S.; Mezlini, A.; M’Rad, M. Dysfonction sexuelle chez 100 femmes tunisiennes atteintes d’un cancer du sein. Sexologies 2019, 28, 43–48. [Google Scholar] [CrossRef]
- Elmas, Ö.; ÇAkmak, G.K.; Bakkal, B.H. A Comparison between Breast-Conserving Surgery and Modified Radical Mastectomy Concerning the Female Sexual Function in Breast Cancer Patients under 50 Years of Age. Turk. J. Oncol. 2020, 35, 26–30. [Google Scholar]
- Farthmann, J.; Hanjalicbeck, A.; Veit, J.J.; Rautenberg, B.; Stickeler, E.; Erbes, T.; Földi, M.; Hasenburg, A. The impact of chemotherapy for breast cancer on sexual function and health-related quality of life. Support. Care Cancer 2016, 24, 2603–2609. [Google Scholar] [CrossRef]
- Fogh, M.; Højgaard, A.; Rotbøl, C.B.; Jensen, A.B. The majority of Danish breast cancer survivors on adjuvant endocrine therapy have clinically relevant sexual dysfunction: A cross-sectional study. Acta Oncol. 2021, 60, 61–68. [Google Scholar] [CrossRef]
- Fouladi, N.; Feizi, I.; Mehriar, N.; Mehrara, E.; Adldoosti, R.; Alimohammadi, S. The Predictors of Sexual Satisfaction among Iranian Women with Breast Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 391–396. [Google Scholar] [CrossRef]
- Frechette, D.; Paquet, L.; Verma, S.; Clemons, M.; Wheatley-Price, P.; Gertler, S.Z.; Song, X.; Graham, N.; Dent, S. The impact of endocrine therapy on sexual dysfunction in postmenopausal women with early stage breast cancer: Encouraging results from a prospective study. Breast Cancer Res. Treat. 2013, 141, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, A.; Esposito, D.; Accardo, G.; Taddeo, M.; Letizia, A.; Tagliafierro, R.; Esposito, K.; Pasquali, D. Sexual function and sex hormones in breast cancer patients. Endocrine 2018, 60, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, C.; Butler, E.; Pesek, S.; Kwait, R.; Edmonson, D.; Raker, C.; Clark, M.A.; Stuckey, A.; Gass, J. Sexual Dysfunction in Breast Cancer Survivors. Am. J. Clin. Oncol. 2019, 42, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Harirchi, I.; Montazeri, A.; Bidokhti, F.Z.; Mamishi, N.; Zendehdel, K. Sexual function in breast cancer patients: A prospective study from Iran. J. Exp. Clin. Cancer Res. 2012, 31, 20–26. [Google Scholar] [CrossRef][Green Version]
- Herbenick, D.; Reece, M.; Hollub, A.; Satinsky, S.; Dodge, B. Young Female Breast Cancer Survivors. Cancer Nurs. 2008, 31, 417–425. [Google Scholar] [CrossRef]
- İzci, F.; Özdem, G.; İlgün, A.S.; Ağaçayak, F.; Duymaz, T.; Erdoğan, Z.; Alço, G.; Elbüken, F.; Öztürk, A.; Ordu, Ç.; et al. Pre-Treatment and Post-Treatment Anxiety, Depression, Sleep and Sexual Function Levels in Patients with Breast Cancer. Eur. J. Breast Health 2020, 16, 219–225. [Google Scholar]
- Kedde, H.; Van De Wiel, H.B.M.; Schultz, W.C.M.W.; Wijsen, C. Sexual dysfunction in young women with breast cancer. Support. Care Cancer 2013, 21, 271–280. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Nowosielski, K.; Cedrych, I.; Krzystanek, M.; Glogowska, I.; Streb, J.; Kucharz, J.; Lew-Starowicz, Z. Factors Affecting Sexual Function and Body Image of Early-Stage Breast Cancer Survivors in Poland: A Short-Term Observation. Clin. Breast Cancer 2019, 19, e30–e39. [Google Scholar] [CrossRef]
- Landi, S.N.; Doll, K.M.; Bensen, J.T.; Hendrix, L.; Anders, C.K.; Wu, J.M.; Nichols, H.B. Endocrine therapy and urogenital outcomes among women with a breast cancer diagnosis. Cancer Causes Control 2016, 27, 1325–1332. [Google Scholar] [CrossRef]
- Lashani, F.; Rohani, C.; Estebsari, F.; Nasiri, M. Exploring the relationship between sexual function, sense of coherence, and well-being in a sample of Iranian breast cancer survivors. Support. Care Cancer 2020, 29, 3191–3199. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, Y.H.; Jeon, M.J. Risk factors for negative impacts on sexual activity and function in younger breast cancer survivors. Psycho-Oncology 2015, 24, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Manganiello, A.; Hoga, L.A.K.; Reberte, L.M.; Miranda, C.M.; Rocha, C.A.M. Sexuality and quality of life of breast cancer patients post mastectomy. Eur. J. Oncol. Nurs. 2011, 15, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Iborra, S.; Grimm, D.; Steinsiek, L.; Mahner, S.; Bossart, M.; Woelber, L.; Voss, P.J.; Gitsch, G.; Hasenburg, A. Sexual activity and quality of life in patients after treatment for breast and ovarian cancer. Arch. Gynecol. Obstet. 2019, 299, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.A.; Semple, J.; Quan, M.-L.; Vadaparampil, S.T.; Holloway, C.; Brown, M.; Bower, B.; Sun, P.; Narod, S.A. Changes in Psychosocial Functioning 1 Year After Mastectomy Alone, Delayed Breast Reconstruction, or Immediate Breast Reconstruction. Ann. Surg. Oncol. 2012, 19, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Notari, S.C.; Favez, N.; Notari, L.; Panes-Ruedin, B.; Antonini, T.; Delaloye, J.-F. Women’s experiences of sexual functioning in the early weeks of breast cancer treatment. Eur. J. Cancer Care 2018, 27, e12607. [Google Scholar] [CrossRef]
- Oberguggenberger, A.; Martini, C.; Huber, N.; Fallowfield, L.; Hubalek, M.; Daniaux, M.; Sperner-Unterweger, B.; Holzner, B.; Sztankay, M.; Gamper, E.; et al. Self-reported sexual health: Breast cancer survivors compared to women from the general population—An observational study. BMC Cancer 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Ztürk, D.; Akyolcu, N. Assessing sexual function and dysfunction in Turkish women undergoing surgical breast cancer treatment. Jpn. J. Nurs. Sci. 2016, 13, 220–228. [Google Scholar] [CrossRef]
- Paiva, C.E.; Rezende, F.F.; Paiva, B.S.R.; Mauad, E.C.; Zucca-Matthes, G.; Carneseca, E.C.; Syrjänen, K.J.; Schover, L.R. Associations of Body Mass Index and Physical Activity with Sexual Dysfunction in Breast Cancer Survivors. Arch. Sex. Behav. 2016, 45, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yoon, H.G. Menopausal symptoms, sexual function, depression, and quality of life in Korean patients with breast cancer receiving chemotherapy. Support. Care Cancer 2013, 21, 2499–2507. [Google Scholar] [CrossRef]
- Parker, P.A.; Youssef, A.; Walker, S.; Basen-Engquist, K.; Cohen, L.; Gritz, E.R.; Wei, Q.X.; Robb, G.L. Short-Term and Long-Term Psychosocial Ad-justment and Quality of Life in Women Undergoing Different Surgical Procedures for Breast Cancer. Ann. Surg. Oncol. 2007, 14, 3078–3089. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Sharma, K.; Thornton, B.M.; Myckatyn, T.M.; Tenenbaum, M.M. Vaginal Laxity, Sexual Distress, and Sexual Dysfunction: A Cross-Sectional Study in a Plastic Surgery Practice. Aesthetic Surg. J. 2018, 38, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Raggio, G.A.; Butryn, M.L.; Arigo, D.; Mikorski, R.; Palmer, S.C. Prevalence and correlates of sexual morbidity in long-term breast cancer survivors. Psychol. Health 2014, 29, 632–650. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.B.; Sorice, K.A.; Pollard, W.; Zimmaro, L.A.; Beach, M.C.; Handorf, E.; Lepore, S.J. Understanding Sexual Help-Seeking for Women with Breast Cancer: What Distinguishes Women Who Seek Help From Those Who Do Not? J. Sex. Med. 2020, 17, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.J.; Bell, R.J.; Christakis, M.K.; Ivezic, S.R.; Davis, S.R. Aromatase Inhibitors are Associated with Low Sexual Desire Causing Distress and Fecal Incontinence in Women: An Observational Study. J. Sex. Med. 2017, 14, 1566–1574. [Google Scholar] [CrossRef]
- Rojas, K.; Onstad, M.; Raker, C.; Clark, M.; Stuckey, A.; Gass, J. The impact of mastectomy type on the Female Sexual Function Index (FSFI), satisfaction with appearance, and the reconstructed breast’s role in intimacy. Breast Cancer Res. Treat. 2017, 163, 273–279. [Google Scholar] [CrossRef]
- Rosenberg, S.M.; Dominici, L.S.; Gelber, S.; Poorvu, P.D.; Ruddy, K.J.; Wong, J.S.; Tamimi, R.M.; Schapira, L.; Come, S.; Peppercorn, J.M.; et al. Association of Breast Cancer Surgery with Quality of Life and Psychosocial Well-being in Young Breast Cancer Survivors. JAMA Surg. 2020, 155, 1035. [Google Scholar] [CrossRef]
- Rottmann, N.; Hansen, D.G.; Christensen, R.D.; Hagedoorn, M.; Frisch, M.; Nicolaisen, A.; Kroman, N.; Flyger, H.; Johansen, C. Satisfaction with sex life in sexually active heterosexual couples dealing with breast cancer: A nationwide longitudinal study. Acta Oncol. 2017, 56, 212–219. [Google Scholar] [CrossRef]
- Rowland, J.H.; Desmond, K.A.; Meyerowitz, B.E.; Belin, T.R.; Wyatt, G.E.; Ganz, P.A. Role of Breast Reconstructive Surgery in Physical and Emotional Outcomes Among Breast Cancer Survivors. J. Natl. Cancer Inst. 2000, 92, 1422–1429. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S. Quality of life and sexual functioning in young women with early-stage breast cancer 1 year after lumpectomy. Psycho-Oncology 2013, 22, 1242–1248. [Google Scholar] [CrossRef]
- Sayakhot, P.; Vincent, A.; Deeks, A.; Teede, H. Potential adverse impact of ovariectomy on physical and psychological function of younger women with breast cancer. Menopause 2011, 18, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Sbitti, Y.; Kadiri, H.; Essaidi, I.; Fadoukhair, Z.; Kharmoun, S.; Slimani, K.; Ismaili, N.; Ichou, M.; Errihani, H. Breast cancer treatment and sexual dysfunction: Moroccan women’s perception. BMC Women’s Health 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Schover, L.R.; Baum, G.P.; Fuson, L.A.; Brewster, A.; Melhem-Bertrandt, A. Sexual Problems During the First 2 Years of Adjuvant Treatment with Aromatase Inhibitors. J. Sex. Med. 2014, 11, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Shandiz, F.H.; Karimi, F.Z.; Rahimi, N.; Abdolahi, M.; Anbaran, Z.K.; Ghasemi, M.; Mazlom, S.R.; Kheirabadi, A.N. Investigating Sexual Function and Affecting Factors in Women with Breast Cancer in Iran. Asian Pac. J. Cancer Prev. 2016, 17, 3583–3586. [Google Scholar]
- Soldera, S.V.; Ennis, M.; Lohmann, A.E.; Goodwin, P.J. Sexual health in long-term breast cancer survivors. Breast Cancer Res Treat. 2018, 172, 159–166. [Google Scholar] [CrossRef]
- Sorouri, F.; Yaghubi, H. Comparing the Negative Emotions, Body Image, Sexual Schemas and Sexual Function in Women with Breast Cancer and Healthy Women. Arch. Psychiatry Res. 2019, 55, 49–60. [Google Scholar] [CrossRef]
- Speer, J.J.; Hillenberg, B.; Sugrue, D.P.; Blacker, C.; Kresge, C.L.; Decker, V.B.; Zakalik, D.; Decker, D.A. Study of Sexual Functioning Determinants in Breast Cancer Survivors. Breast J. 2005, 11, 440–447. [Google Scholar] [CrossRef]
- Tahir, K.; Khan, N. Mediating role of body image between sexual functioning and marital intimacy in Pakistani women with breast cancer. Psycho-Oncology 2020, 30, 260–266. [Google Scholar] [CrossRef]
- Tucker, P.; Cohen, P.A.; Bulsara, M.K.; Jeffares, S.; Saunders, C. The impact of bilateral salpingo-oophorectomy on sexuality and quality of life in women with breast cancer. Support. Care Cancer 2020, 29, 369–375. [Google Scholar] [CrossRef]
- Tucker, P.E.; Saunders, C.; Bulsara, M.K.; Tan, J.J.-S.; Salfinger, S.G.; Green, H.; Cohen, P.A. Sexuality and quality of life in women with a prior diagnosis of breast cancer after risk-reducing salpingo-oophorectomy. Breast 2016, 30, 26–31. [Google Scholar] [CrossRef]
- Usta, O.Y.; Gokcol, D. Sexual Dysfunction in Women with Breast Cancer Receiving Chemotherapy. Int. J. Caring Sci. 2017, 10, 1439–1446. [Google Scholar]
- Vaidakis, D.; Panoskaltsis, T.; Poulakaki, N.; Kouloura, A.; Kassanos, D.; Papadimitriou, G.; Salamalekis, E. Female sexuality after female cancer treatment: A clinical issue. Eur. J. Gynaecol. Oncol. 2014, 35, 635–640. [Google Scholar] [PubMed]
- Webber, K.; Mok, K.; Bennett, B.; Lloyd, A.R.; Friedlander, M.; Juraskova, I.; Goldstein, D. FolCan study group If I Am in the Mood, I Enjoy It: An Exploration of Cancer-Related Fatigue and Sexual Functioning in Women with Breast Cancer. Oncologist 2011, 16, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Bender, C.M.; Zhang, N.; Yuan, C. Patterns of sexual health in patients with breast cancer in China: A latent class analysis. Support. Care Cancer 2020, 28, 5147–5156. [Google Scholar] [CrossRef]
- Zaied, S.; Fatma, L.B.; Laadhari, A.; Boudegga, M.Z.; Hochlef, M.; Chabchoub, I.; Ezzairi, F.; Gharbi, O.; Gaha, L.; Ahmed, S.B. Étude de la sexualité chez les femmes tuni-siennes en rémission complète d’un cancer du sein non métastatique, à propos de 100 femmes. Bull. Cancer 2013, 100, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Del Pup, L.; Villa, P.; Amar, I.D.; Bottoni, C.; Scambia, G. Approach to sexual dysfunction in women with cancer. Int. J. Gynecol. Cancer 2019, 29, 630–634. [Google Scholar] [CrossRef]
- Studd, J.; Schwenkhagen, A. The historical response to female sexuality. Maturitas 2009, 63, 107–111. [Google Scholar] [CrossRef]
- Hill, G.; Holborn, C. Sexual minority experiences of cancer care: A systematic review. J. Cancer Policy 2015, 6, 11–22. [Google Scholar] [CrossRef]
- Kamen, C.S.; Smith-Stoner, M.; Heckler, C.E.; Flannery, M.; Margolies, L. Social Support, Self-Rated Health, and Lesbian, Gay, Bisexual, and Transgender Identity Disclosure to Cancer Care Providers. Oncol. Nurs. Forum 2015, 42, 44–51. [Google Scholar] [CrossRef]
- Wandrey, R.L.; Qualls, W.D.; Mosack, K.E. Rejection of Breast Reconstruction Among Lesbian Breast Cancer Patients. LGBT Health 2016, 3, 74–78. [Google Scholar] [CrossRef]
- Mattingly, A.E.; Kiluk, J.V.; Lee, M.C. Clinical Considerations of Risk, Incidence, and Outcomes of Breast Cancer in Sexual Mi-norities. Cancer Control 2016, 23, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Pratt-Chapman, M.L.; Alpert, A.B.; Castillo, D.A. Health outcomes of sexual and gender minorities after cancer: A systematic review. Syst. Rev. 2021, 10, 1–30. [Google Scholar] [CrossRef]
- Rosen, C.; Brown, J.; Heiman, S.; Leiblum, C.; Meston, R.; Shabsigh, D.; Ferguson, R.; D’Agostino, R. The Female Sexual Function Index (FSFI): A Multidi-mensional Self-Report Instrument for the Assessment of Female Sexual Function. J. Sex Marital Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Bartula, I.; Sherman, K.A. The Female Sexual Functioning Index (FSFI): Evaluation of acceptability, reliability, and validity in women with breast cancer. Support. Care Cancer 2015, 23, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Thirlaway, K.; Fallowfield, L.; Cuzick, J. The Sexual Activity Questionnaire: A measure of women’s sexual functioning. Qual. Life Res. 1996, 5, 81–90. [Google Scholar] [CrossRef]
- Atkins, L.; Fallowfield, L. Fallowfield’s Sexual Activity Questionnaire in women with without and at risk of cancer. Menopause Int. 2007, 13, 103–109. [Google Scholar] [CrossRef]
- da Costa, F.A.; Ribeiro, M.C.; Braga, S.; Carvalho, E.; Francisco, F.; da Costa Miranda, A.; Moreira, A.; Fallowfield, L. Sexual Dysfunction in Breast Cancer Sur-vivors: Cross-Cultural Adaptation of the Sexual Activity Questionnaire for Use in Portugal. Acta Med. Port. 2016, 29, 533. [Google Scholar] [CrossRef]
- Oppenheimer, A.; Panel, P.; Rouquette, A.; du Cheyron, J.; Deffieux, X.; Fauconnier, A. Validation of the Sexual Activity Question-naire in women with endometriosis. Hum. Reprod. 2019, 34, 824–833. [Google Scholar] [CrossRef]
- Vistad, I.; Fosså, S.D.; Kristensen, G.B.; Mykletun, A.; Dahl, A.A. The Sexual Activity Questionnaire: Pychometric Properties and Normative Data in A Norwegian Population Sample. J. Women’s Health 2007, 16, 139–148. [Google Scholar] [CrossRef]
- Flynn, K.E.; Lin, L.; Cyranowski, J.M.; Reeve, B.B.; Reese, J.B.; Jeffery, D.D.; Smith, A.W.; Porter, L.S.; Dombeck, C.B.; Bruner, D.W.; et al. Development of the NIH PROMIS® Sexual Function and Satisfaction Measures in Patients with Cancer. J. Sex. Med. 2013, 10, 43–52. [Google Scholar] [CrossRef]
- McCabe, M.P.; Sharlip, I.D.; Atalla, E.; Balon, R.; Fisher, A.D.; Laumann, E.; Lee, S.W.; Lewis, R.; Segraves, R.T. Definitions of Sexual Dysfunctions in Women and Men: A Consensus Statement From the Fourth International Consultation on Sexual Medicine 2015. J. Sex. Med. 2016, 13, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lin, Y.-C.; Chiu, L.-H.; Chu, Y.-H.; Ruan, F.-F.; Liu, W.-M.; Wang, P.-H. Female sexual dysfunction: Definition, classification, and debates. Taiwan. J. Obstet. Gynecol. 2013, 52, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-W.; Lee, S.; Lee, A.R.; Lee, K.-H.; Hwang, S.Y. Quality of Life Differences between Younger and Older Breast Cancer Patients. J. Breast Cancer 2011, 14, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chang, S.-R.; Chiu, S.-C. Sexual Problems of Patients with Breast Cancer after Treatment. Cancer Nurs. 2019, 42, 418–425. [Google Scholar] [CrossRef]
- Mokhtari-Hessari, P.; Montazeri, A. Health-related quality of life in breast cancer patients: Review of reviews from 2008 to 2018. Health Qual. Life Outcomes 2020, 18, 338. [Google Scholar] [CrossRef]
- Zehra, S.; Doyle, F.; Barry, M.; Walsh, S.; Kell, M.R. Health-related quality of life following breast reconstruction compared to total mastectomy and breast-conserving surgery among breast cancer survivors: A systematic review and meta-analysis. Breast Cancer 2020, 27, 534–566. [Google Scholar] [CrossRef]
- Peddie, N.; Agnew, S.; Crawford, M.; Dixon, D.; MacPherson, I.; Fleming, L. The impact of medication side effects on adherence and persistence to hormone therapy in breast cancer survivors: A qualitative systematic review and thematic synthesis. Breast 2021, 58, 147–159. [Google Scholar] [CrossRef]
- Valpey, R.; Kucherer, S.; Nguyen, J. Sexual dysfunction in female cancer survivors: A narrative review. Gen. Hosp. Psychiatry 2019, 60, 141–147. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).