Changes in Microeukaryotic Communities in the Grand Canal of China in Response to Floods
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Pretreatment
2.3. Physiochemical Properties Analysis
2.4. DNA Extraction and PCR Amplification
2.5. Statistical Analysis
3. Results
3.1. Physiochemical Properties
3.2. Species Richness, Evenness, and Diversity
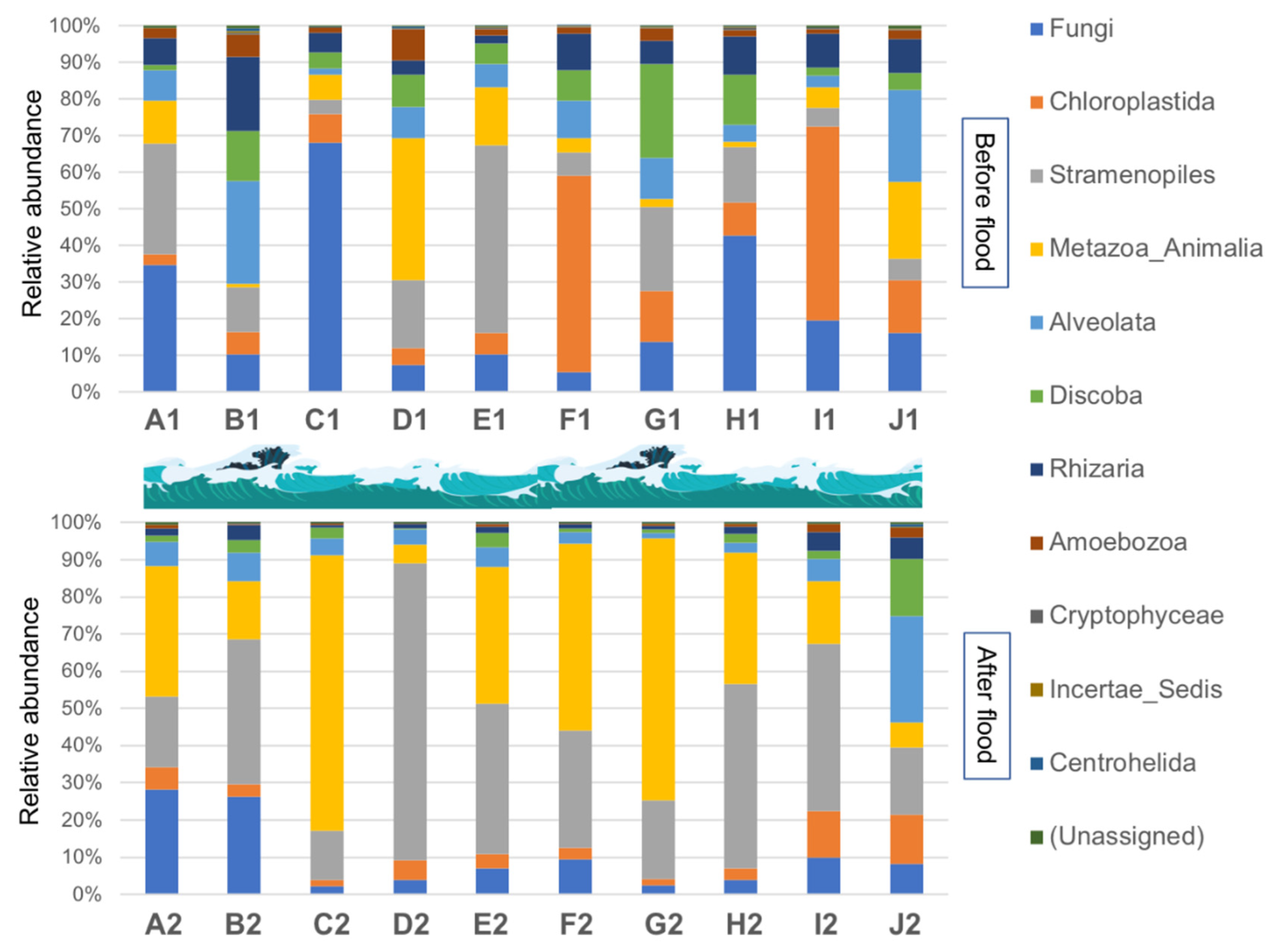
3.3. Composition and Distribution Characteristics of Eukaryotic Communities
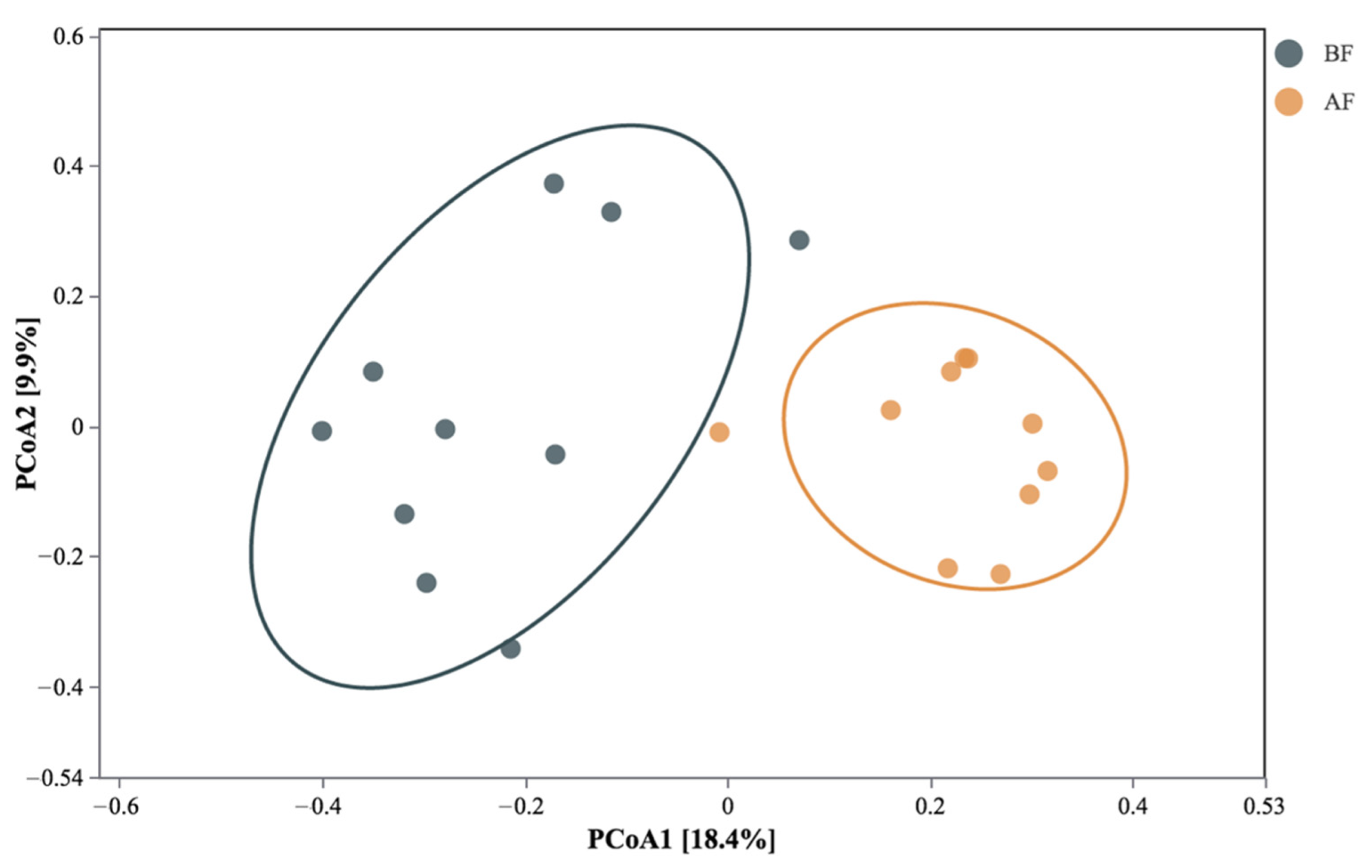
3.4. Multivariate Analysis of Eukaryotic Communities
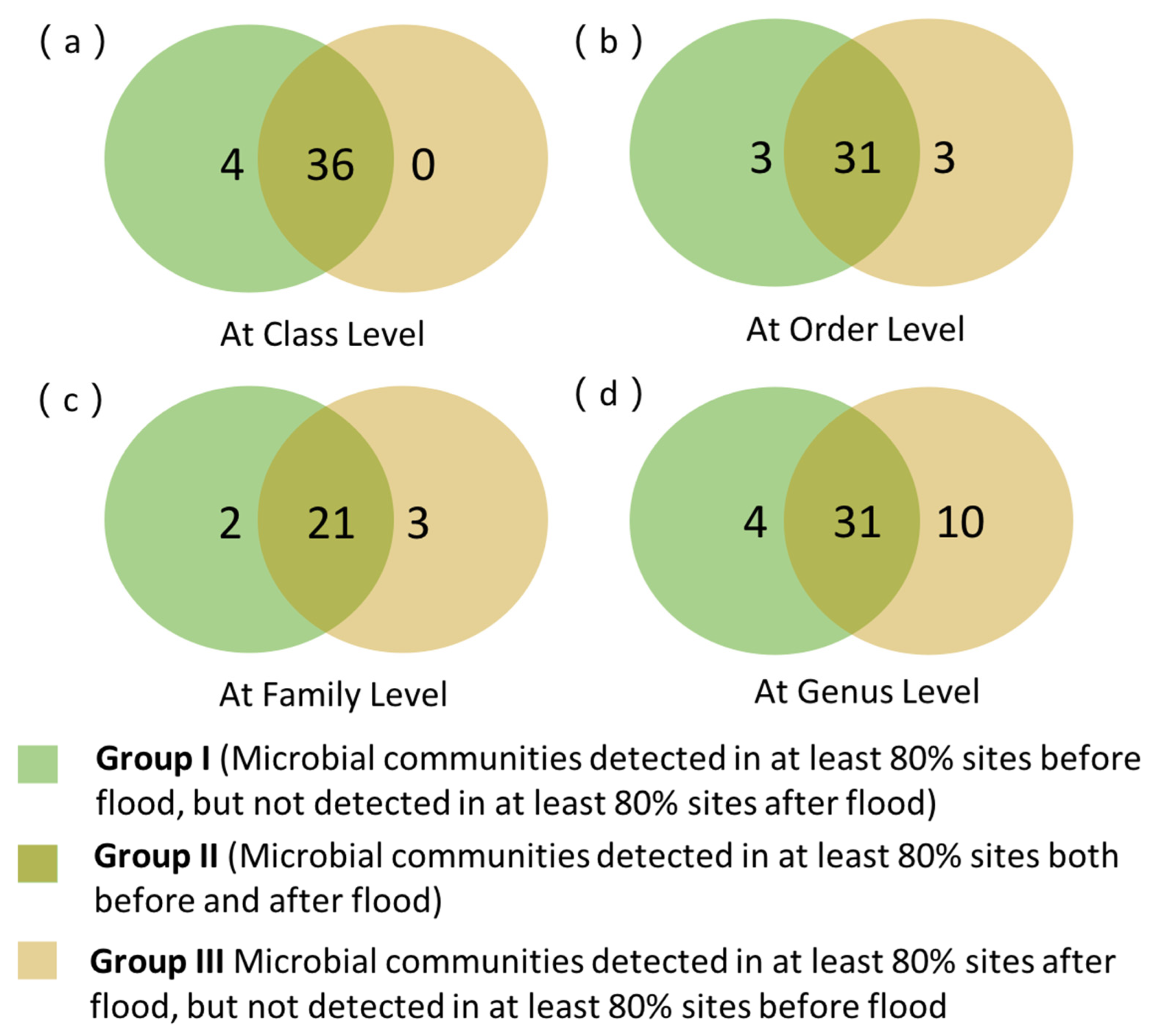
3.4.1. PCoA Analysis and Venn Analysis
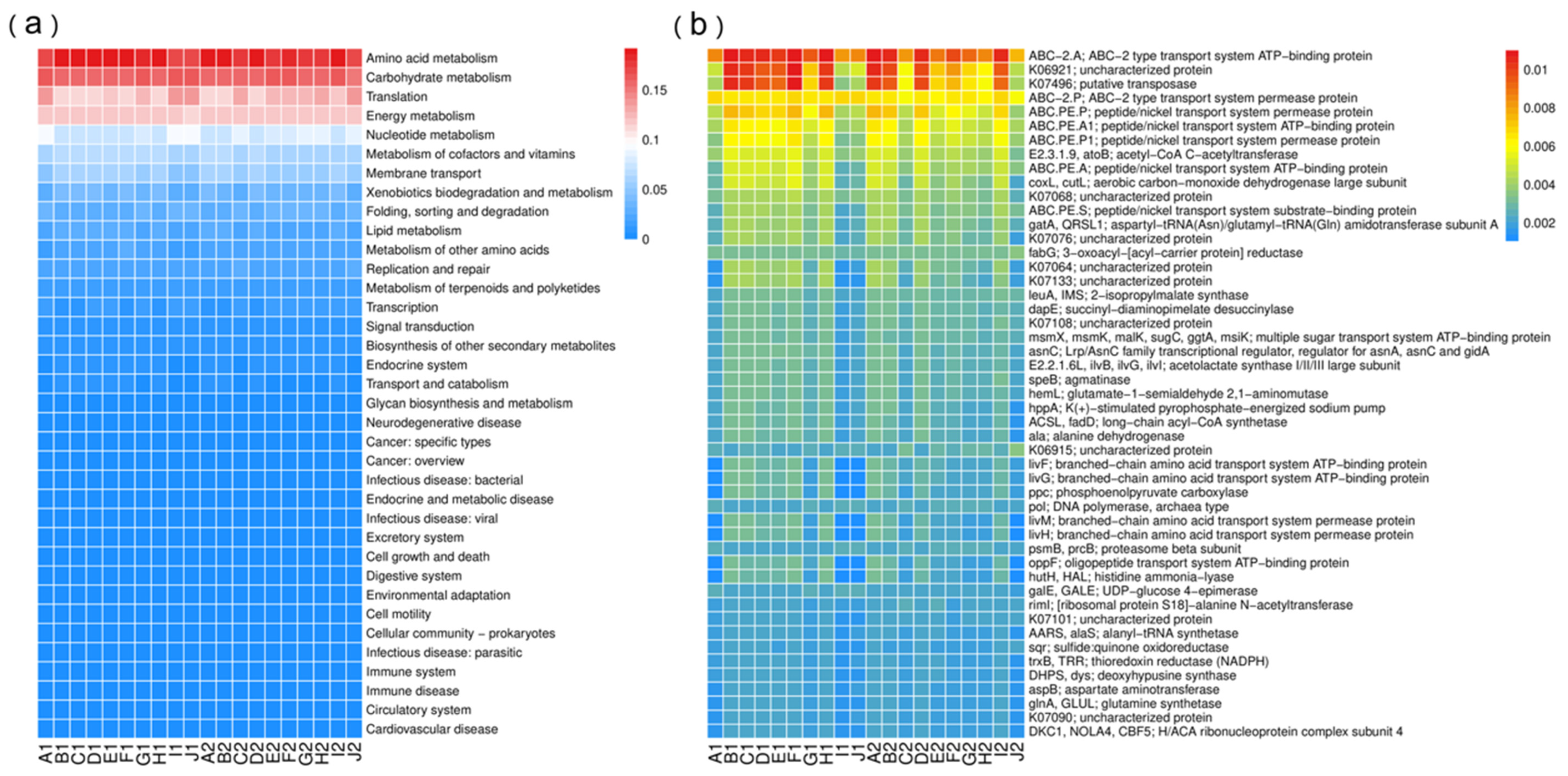
3.4.2. Functional Analysis from PICRUSt2
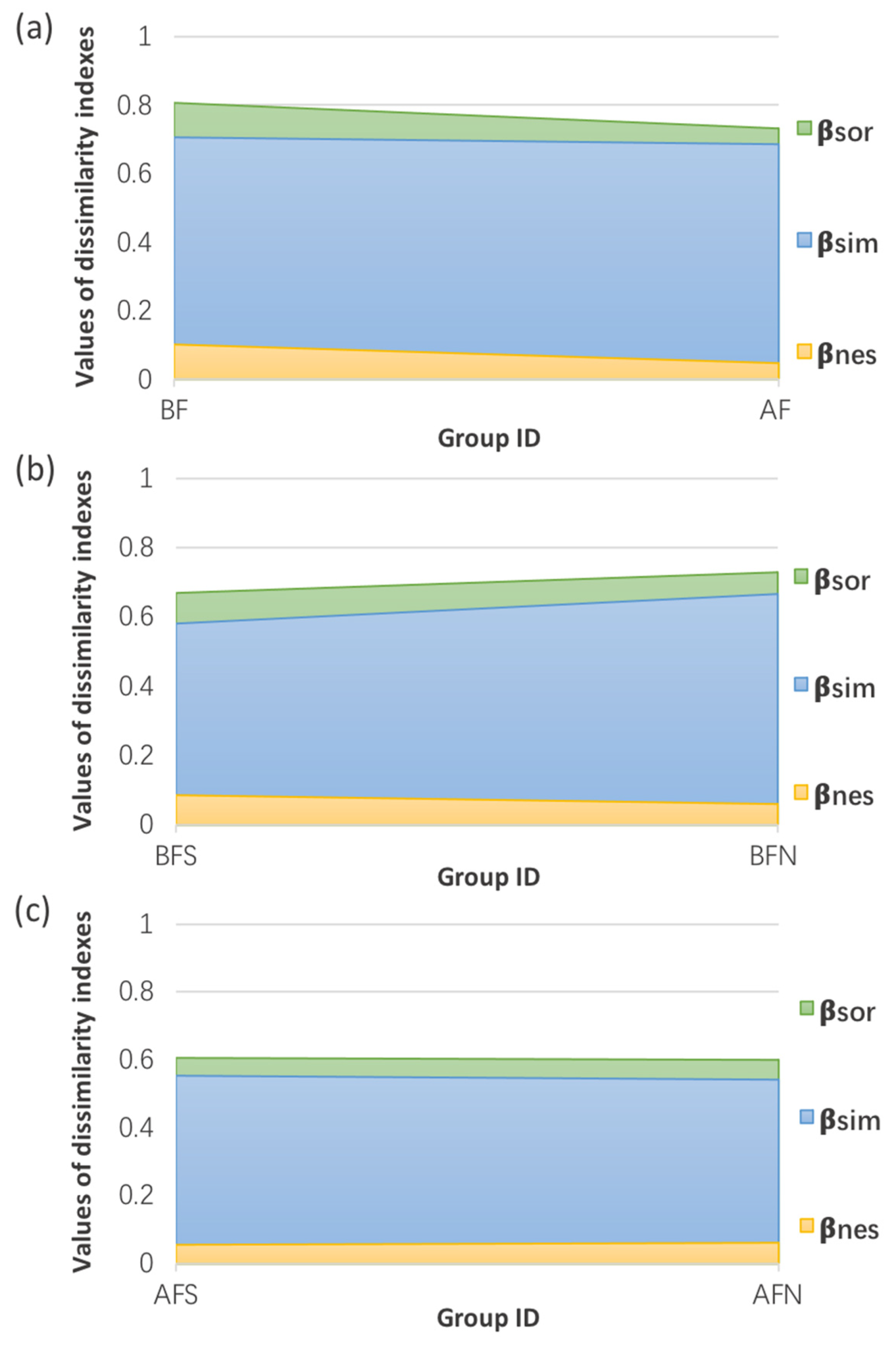
3.5. β-Diversity Measurement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, F.; Wang, L.; Guo, Y.; Fu, M.; Huang, N.; Duan, W.; Luo, M.; Zhang, J.; Li, W.; Song, W. Spatio-temporal variation and coupling coordination relationship between urbanisation and habitat quality in the grand canal, China. Land Use Policy 2022, 117, 106119. [Google Scholar] [CrossRef]
- Li, X.; Rao, Z.; Yang, Z.; Guo, X.; Huang, Y.; Zhang, J.; Guo, F.; Liu, C. A Survey of 42 Semi-Volatile Organic Contaminants in Groundwater along the Grand Canal from Hangzhou to Beijing, East China. Int. J. Environ. Res. Public Health 2015, 12, 16070–16081. [Google Scholar] [CrossRef] [PubMed]
- Carles, L.; Artigas, J. Interaction between glyphosate and dissolved phosphorus on bacterial and eukaryotic communities from river biofilms. Sci. Total Environ. 2020, 719, 137463. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-J.; Shao, Y.; Li, Y.-J.; Lin, L.-A.; Chen, Y.; Tian, W.; Li, B.-L.; Li, Y.-Y. Rhizosphere Bacterial Community Structure and Predicted Functional Analysis in the Water-Level Fluctuation Zone of the Danjiangkou Reservoir in China During the Dry Period. Int. J. Environ. Res. Public Health 2020, 17, 1266. [Google Scholar] [CrossRef]
- Yan, M.; Chen, S.; Huang, T.; Li, B.; Li, N.; Liu, K.; Zong, R.; Miao, Y.; Huang, X. Community Compositions of Phytoplankton and Eukaryotes during the Mixing Periods of a Drinking Water Reservoir: Dynamics and Interactions. Int. J. Environ. Res. Public Health 2020, 17, 1128. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, M.J.; Álvarez-Manzaneda, M.I.; León-Palmero, E.; Guerrero-Jiménez, G.; Domis, L.N.D.S.; Teurlincx, S.; González-Olalla, J.M. Warming and co2 effects under oligotrophication on temperate phytoplankton communities. Water Res. 2020, 173, 115579. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, J. Coupled effects of land use pattern and hydrological regime on composition and diversity of riverine eukaryotic community in a coastal watershed of southeast China. Sci. Total Environ. 2019, 660, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Volant, A.; Hery, M.; Desoeuvre, A.; Casiot, C.; Bertin, P.N.; Bruneel, O. Spatial distribution of eukaryotic communities using high-throughput sequencing along a pollution gradient in the arsenic-rich creek sediments of carnoulss mine, france. Microb. Ecol. Int. J. 2016, 72, 608–620. [Google Scholar] [CrossRef]
- Dottori, F.; Szewczyk, W.; Ciscar, J.-C.; Zhao, F.; Alfieri, L.; Hirabayashi, Y.; Bianchi, A.; Mongelli, I.; Frieler, K.; Betts, R.A.; et al. Increased human and economic losses from river flooding with anthropogenic warming. Nat. Clim. Chang. 2018, 8, 781–786. [Google Scholar] [CrossRef]
- Carney, R.L.; Mitrovic, S.M.; Jeffries, T.; Westhorpe, D.; Seymour, J.R. River bacterioplankton community responses to a high inflow event. Aquat. Microb. Ecol. 2015, 75, 187–205. [Google Scholar] [CrossRef]
- Chung, C.-C.; Gong, G.-C.; Huang, C.-Y.; Lin, J.-Y.; Lin, Y.-C. Changes in the synechococcus assemblage composition at the surface of the east China sea due to flooding of the Changjiang River. Microb. Ecol. 2015, 70, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Zoppini, A.; Ademollo, N.; Bensi, M.; Berto, D.; Bongiorni, L.; Campanelli, A.; Casentini, B.; Patrolecco, L.; Amalfitano, S. Impact of a river flood on marine water quality and planktonic microbial communities. Estuar. Coast. Shelf Sci. 2019, 224, 62–72. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, Y.; Wen, B.; Huang, W.; Li, S. Floods in China, COVID-19, and climate change. Lancet Planet. Health 2020, 4, 443–444. [Google Scholar] [CrossRef]
- Qian, R.; Sun, M.; Tan, L. Analysis on characteristics of Yangzhou transit flood in 2020. J. Jiangsu Water Resour. 2021, 8, 70–72. [Google Scholar]
- Sun, Z. Analysis of Flood Discharge Capacity in Control Channel of Huaihe River into Yangtze River. J. China Hydrol. 2019, 39, 52–58. [Google Scholar]
- Tang, W.; Fu, Y.; Wang, X.; Lu, Y.; Xu, M.; Xie, W.; Song, A. Decreasing spring persistent rainfall over the yangtzehuai river valley of China during 1960–2019 and its possible causes. Int. J. Climatol. 2021, 42, 3809–3819. [Google Scholar] [CrossRef]
- Tang, V.T.; Rene, E.R.; Fu, D.F.; Singh, R.P.; Behera, S.K.; Pugazhendhi, A. Effect of mixed microbial culture addition on enhanced river water quality: Pollutant removal and microbial community characteristics. Environ. Technol. Innov. 2020, 18, 100707. [Google Scholar] [CrossRef]
- Zhang, N.; Xiao, X.; Pei, M.; Liu, X.; Liang, Y. Discordant temporal turnovers of sediment bacterial and eukaryotic communities in response to dredging: Nonresilience and functional changes. Appl. Environ. Microbiol. 2017, 83, e02526-16. [Google Scholar] [CrossRef]
- Ji, B.; Wang, S.; Guo, D.; Pang, H. Comparative and comprehensive analysis on bacterial communities of two full-scale wastewater treatment plants by second and third-generation sequencing. Bioresour. Technol. Rep. 2020, 11, 100450. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. Picrust2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A. Partitioning the turnover and nestedness com- ponents of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Mulligan, M.; Rubiano, J.; Hyman, G.; White, D.; Garcia, J.; Saravia, M.; Leon, J.G.; Selvaraj, J.J.; Guttierez, T.; Saenz-Cruz, L.L.; et al. The andes basins: Biophysical and developmental diversity in a climate of change. Water Int. 2010, 35, 472–492. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Wang, X.; Xu, S. Changes in microbial community structure under reclaimed water replenishment conditions. Int. J. Environ. Res. Public Health 2020, 17, 1174. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, W.; Li, Y. Microbial community coalescence: Does it matter in the three gorges reservoir? Water Res. 2021, 205, 117638. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, L.; Wamelink, W.; Schaepman-Strub, G.; Schaepman, M.; Han, V.D.; Aduaka, U.; Batelaan, O. Assessing and predicting biodiversity in a floodplain ecosystem: Assimilation of net primary production derived from imaging spectrometer data into a dynamic vegetation model. Remote Sens. Environ. 2008, 112, 2118–2130. [Google Scholar] [CrossRef]
- Downs, P.W.; Thorne, C.R. Rehabilitation of a lowland river: Reconciling flood defence with habitat diversity and geomorphological sustainability. J. Environ. Manag. 2000, 58, 249–268. [Google Scholar] [CrossRef]
- Reis, M.C.D.; Bagatini, I.L.; de Oliveira Vidal, L.; Bonnet, M.-P.; da Motta Marques, D.; Sarmento, H. Spatial heterogeneity and hydrological fluctuations drive bacterioplankton community composition in an amazon floodplain system. PLoS ONE 2019, 14, 0220695. [Google Scholar]
- Aguilera, A.; Zettler, E.; Gómez, F.; Amaral-Zettler, L.; Rodríguez, N.; Amils, R. Distribution and seasonal variability in the benthic eukaryotic community of río tinto (sw, spain), an acidic, high metal extreme environment. Syst. Appl. Microbiol. 2007, 30, 531–546. [Google Scholar] [CrossRef]
- Hirano, S.I.; Ito, Y.; Tanaka, S.; Nagaoka, T.; Oyama, T. Microbial community composition in iron deposits and manganese crusts formed in riverine environments around the aso area in japan. Res. Microbiol. 2020, 171, 271–280. [Google Scholar] [CrossRef]
- Karanis, P.; Chronis, I.; Zakas, G.; Kourenti, C.; Sotiriadou, I.; Papadopoulou, C. A preliminary survey of the level of microbiological pollution of major rivers in northern greece. Acta Hydrochim. Hydrobiol. 2005, 33, 346–354. [Google Scholar] [CrossRef]
- Thomas, M.C.; Selinger, L.B.; Inglis, G.D. Seasonal diversity of planktonic protists in southwestern alberta rivers over a 1-year period as revealed by terminal restriction fragment length polymorphism and 18s rrna gene library analyses. Appl. Environ. Microbiol. 2012, 78, 5653–5660. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yao, J.; Fu, G.; Guo, H.; Duan, D. Isolation, expression, and characterization of blue light receptor aureochrome gene from saccharina japonica (laminariales, phaeophyceae). Mar. Biotechnol. 2014, 16, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Firsova, A.D.; Bessudova, A.Y.; Sorokovikova, L.M.; Tomberg, I.V.; Likhoshway, Y.V. The diversity of chrysophycean algae in an arctic zone of river and sea water mixing, russia. Am. J. Plant Sci. 2015, 6, 2439–2452. [Google Scholar] [CrossRef]
- Ueda, H.; Kimura, H. Plankton of spring waters in the shigenobu river basin. Jpn. J. Limnol. 2001, 62, 219–227. [Google Scholar] [CrossRef]
- Zoppini, A.; Amalfitano, S.; Fazi, S.; Puddu, A. Dynamics of a benthic microbial community in a riverine environment subject to hydrological fluctuations (Mulargia River, Italy). Hydrobiologia 2010, 657, 37–51. [Google Scholar] [CrossRef]
- Dumestre, J.; Casamayor, E.; Massana, R.; Pedrós-Alió, C. Changes in bacterial and archaeal assemblages in an equatorial river induced by the water eutrophication of petit saut dam reservoir (French Guiana). Aquat. Microb. Ecol. 2002, 26, 209–221. [Google Scholar] [CrossRef]
- Hansen, K.; Perry, B.A.; Dranginis, A.W.; Pfister, D.H. A phylogeny of the highly diverse cup-fungus family pyronemataceae (pezizomycetes, ascomycota) clarifies relationships and evolution of selected life history traits. Mol. Phylogenet. Evol. 2013, 67, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.H.; Healy, R. Pezizomycetes. In Encyclopedia of Mycology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 295–309. [Google Scholar]
- Coutinho, E.S.; Barbosa, M.; Beiroz, W.; Mescolotti, D.L.C.; Bonfim, J.A.; Louro Berbara, R.L.; Fernandes, G.W. Soil constraints for arbuscular mycorrhizal fungi spore community in degraded sites of rupestrian grassland: Implications for restoration. Eur. J. Soil Biol. 2018, 90, 51–57. [Google Scholar] [CrossRef]
- Bergfeld, T.; Scherwass, A.; Ackermann, B.; Arndt, H.; Schoel, A. Comparison of the components of the planktonic food web in three large rivers (Rhine, Moselle and Saar). River Res. Appl. 2010, 25, 1232–1250. [Google Scholar] [CrossRef]
- Korgina, E.M. The turbellaria fauna of a small water reservoir in central russia. Inland Water Biol. 2011, 4, 419–424. [Google Scholar] [CrossRef]
- Ligęza, S.; Wilk-Woźniak, E. The occurrence of a euglena pascheri and lepocinclis ovum bloom in an oxbow lake in southern poland under extreme environmental conditions. Ecol. Indic. 2011, 11, 925–929. [Google Scholar] [CrossRef]
- Klaveness, D. Hydrurus foetidus (Chrysophyceae)—An inland macroalga with potential. J. Appl. Phycol. 2017, 29, 1485–1491. [Google Scholar] [CrossRef]
- Urabe, O.; Gurung, T.B.; Yoshida, T. Effects of phosphorus supply on phagotrophy by the mixotrophic alga uroglena americana (Chrysophyceae). Aquat. Microb. Ecol. 1999, 18, 77–83. [Google Scholar] [CrossRef]
- Sahu, G.; Panigrahi, S.; Mohanty, A.K.; Satpathy, K.K.; Dovgal, I. New record of a protozoan ciliate epibiont, acineta karamani hadi 1940 on copepod host labidocera acuta from the indian ocean. Indian J. Geo-Mar. Sci. 2016, 46, 1802–1805. [Google Scholar]
- Pilichowski, S.; Giertych, M.J. Does hartigiola annulipes (diptera: Cecidomyiidae) distribute its galls randomly? Eur. J. Entomol. 2018, 115, 504–511. [Google Scholar] [CrossRef]
- Chubareva, L.A.; Iankovski, A.V. a new blackfly species Cnetha itelmenica sp. n. (diptera: Simuliidae) from Kamchatka. Parazitologiia 2016, 40, 94–101. [Google Scholar]
- Fredricks, D.N.; Jolley, J.A.; Lepp, P.W.; Kosek, J.C.; Relman, D.A. Rhinosporidium seeberi: A human pathogen from a novel group of aquatic protistan parasites. Emerg. Infect. Dis. 2000, 6, 273–282. [Google Scholar] [CrossRef]
- Fg, A.; Smh, A.; Aab, C. Influence of river cross-section data resolution on flood inundation modeling: Case study of kashkan river basin in western iran-sciencedirect. J. Hydrol. 2020, 584, 124743. [Google Scholar]
- Ron, R.; Fragman-Sapir, O.; Kadmon, R. Dispersal increases ecological selection by increasing effective community size. Proc. Natl. Acad. Sci. USA 2018, 115, 11280–11285. [Google Scholar] [CrossRef]


| Sample ID | Reads | OTUs | ACE | Chao1 | Shannon | Simpson | Pielou |
|---|---|---|---|---|---|---|---|
| A1 | 72,281 | 1198 | 1366.2 | 1345.0 | 4.15 | 0.941 | 0.58 |
| B1 | 44,532 | 828 | 926.7 | 961.4 | 5.02 | 0.983 | 0.75 |
| C1 | 80,321 | 1122 | 1260.4 | 1262.7 | 2.90 | 0.732 | 0.41 |
| D1 | 48,964 | 1070 | 1168.4 | 1180.5 | 4.30 | 0.896 | 0.62 |
| E1 | 15,962 | 567 | 676.9 | 698.1 | 4.09 | 0.931 | 0.64 |
| F1 | 5917 | 330 | 511.6 | 488.3 | 2.77 | 0.728 | 0.48 |
| G1 | 7975 | 526 | 651.2 | 671.3 | 5.00 | 0.982 | 0.80 |
| H1 | 11,450 | 560 | 705.2 | 691.7 | 4.49 | 0.955 | 0.71 |
| I1 | 5149 | 364 | 477.9 | 472.2 | 3.56 | 0.882 | 0.60 |
| J1 | 12,170 | 587 | 733.3 | 783.1 | 4.37 | 0.955 | 0.69 |
| Mean | 30,472 | 715 | 855.4 | 847.8 | 4.07 | 0.899 | 0.63 |
| A2 | 25,226 | 798 | 990.6 | 1017.0 | 3.67 | 0.882 | 0.55 |
| B2 | 41,408 | 864 | 1092.2 | 1129.3 | 4.04 | 0.946 | 0.60 |
| C2 | 65,131 | 1090 | 1441.1 | 1479.3 | 3.42 | 0.895 | 0.49 |
| D2 | 53,131 | 797 | 1114.5 | 1188.2 | 2.75 | 0.772 | 0.41 |
| E2 | 29,441 | 1008 | 1315.5 | 1321.0 | 4.27 | 0.954 | 0.62 |
| F2 | 73,023 | 978 | 1260.6 | 1281.1 | 3.58 | 0.926 | 0.52 |
| G2 | 70,893 | 1013 | 1314.1 | 1300.2 | 3.30 | 0.875 | 0.48 |
| H2 | 59,445 | 1090 | 1352.2 | 1339.2 | 3.68 | 0.904 | 0.53 |
| I2 | 51,684 | 1122 | 1288.6 | 1279.8 | 4.56 | 0.966 | 0.65 |
| J2 | 6975 | 714 | 925.0 | 911.0 | 4.80 | 0.964 | 0.73 |
| Mean | 47,636 | 947 | 1224.6 | 1209.4 | 3.81 | 0.908 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.; Li, H.; Wen, X.; Huang, H.; Chen, G.; Cheng, H.; Wu, H.; Piao, Z. Changes in Microeukaryotic Communities in the Grand Canal of China in Response to Floods. Int. J. Environ. Res. Public Health 2022, 19, 13948. https://doi.org/10.3390/ijerph192113948
Cai W, Li H, Wen X, Huang H, Chen G, Cheng H, Wu H, Piao Z. Changes in Microeukaryotic Communities in the Grand Canal of China in Response to Floods. International Journal of Environmental Research and Public Health. 2022; 19(21):13948. https://doi.org/10.3390/ijerph192113948
Chicago/Turabian StyleCai, Wei, Huiyu Li, Xin Wen, Huang Huang, Guwang Chen, Haomiao Cheng, Hainan Wu, and Zhe Piao. 2022. "Changes in Microeukaryotic Communities in the Grand Canal of China in Response to Floods" International Journal of Environmental Research and Public Health 19, no. 21: 13948. https://doi.org/10.3390/ijerph192113948
APA StyleCai, W., Li, H., Wen, X., Huang, H., Chen, G., Cheng, H., Wu, H., & Piao, Z. (2022). Changes in Microeukaryotic Communities in the Grand Canal of China in Response to Floods. International Journal of Environmental Research and Public Health, 19(21), 13948. https://doi.org/10.3390/ijerph192113948








