Effectiveness of COVID-19 Vaccination on Reduction of Hospitalizations and Deaths in Elderly Patients in Rio Grande do Norte, Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Caseload and Data Acquisition
2.2. Data Dictionary, Processing of Information, and Interpretation of Results
2.3. Statistical Analysis
3. Results
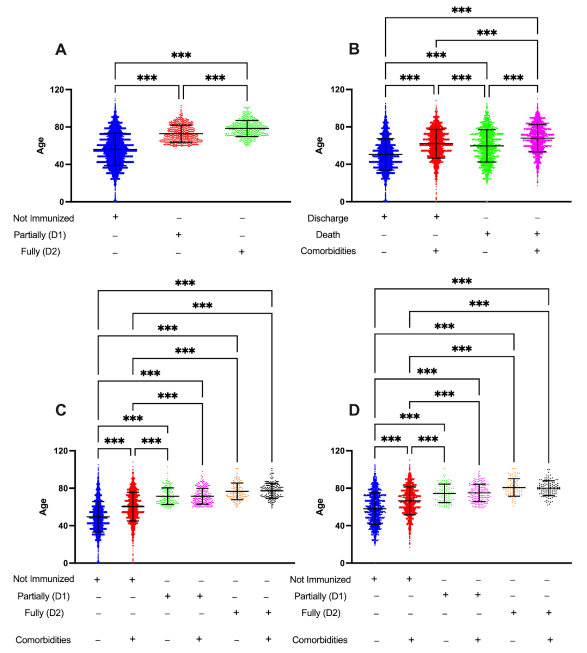
3.1. Age and Immunization Status, but Not Comorbidities, Are Associated with the Development of Moderate and Severe COVID-19 Cases
3.2. Vaccination Changes the Profile of Hospitalizations and Deaths Related to COVID-19
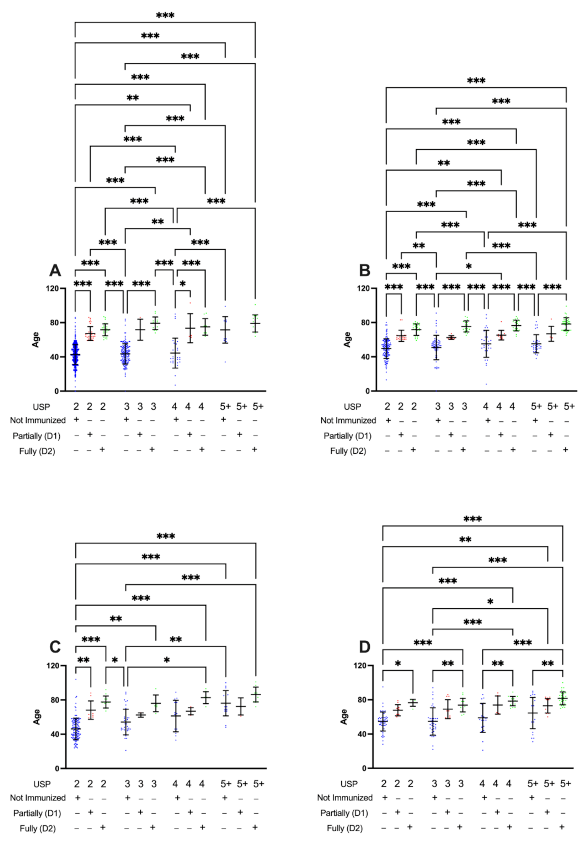
3.3. Partially (D1) or Fully Immunized (D2) Patients Present Lower Levels of Unified Score for Prioritization (USP) When Developing Moderate or Severe COVID-19 Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19---11-march-2020 (accessed on 22 April 2022).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19): The Epidemic and the Challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 Vaccines: The Status and Perspectives in Delivery Points of View. Adv. Drug Deliv. Rev. 2021, 170, 1–25. [Google Scholar] [CrossRef] [PubMed]
- NIH. ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=Vaccine&cntry=&state=&city=&dist=&Search=Search (accessed on 22 April 2022).
- U.S. Food & Drug Administration Comirnaty and Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine (accessed on 22 April 2022).
- Lemos, D.R.Q.; D’Angelo, S.M.; Farias, L.A.B.G.; Almeida, M.M.; Gomes, R.G.; Pinto, G.P.; Cavalcante Filho, J.N.; Feijão, L.X.; Cardoso, A.R.P.; Lima, T.B.R.; et al. Health System Collapse 45 Days after the Detection of COVID-19 in Ceará, Northeast Brazil: A Preliminary Analysis. Rev. Soc. Bras. Med. Trop. 2020, 53. [Google Scholar] [CrossRef]
- Naveca, F.G.; Nascimento, V.; de Souza, V.C.; de Corado, A.L.; Nascimento, F.; Silva, G.; Costa, Á.; Duarte, D.; Pessoa, K.; Mejía, M.; et al. COVID-19 in Amazonas, Brazil, Was Driven by the Persistence of Endemic Lineages and P.1 Emergence. Nat. Med. 2021, 27, 1230–1238. [Google Scholar] [CrossRef]
- Imai, M.; Halfmann, P.J.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Chiba, S.; Watanabe, T.; Nakajima, N.; Ito, M.; Kuroda, M.; Kiso, M.; et al. Characterization of a New SARS-CoV-2 Variant That Emerged in Brazil. Proc. Natl. Acad. Sci. USA 2021, 118, e2106535118. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.J.R.; Pena, L. Collapse of the Public Health System and the Emergence of New Variants during the Second Wave of the COVID-19 Pandemic in Brazil. One Health 2021, 13, 100287. [Google Scholar] [CrossRef]
- Victora, C.G.; Castro, M.C.; Gurzenda, S.; Medeiros, A.C.; França, G.V.A.; Barros, A.J.D. Estimating the Early Impact of Vaccination against COVID-19 on Deaths among Elderly People in Brazil: Analyses of Routinely-Collected Data on Vaccine Coverage and Mortality. eClinicalMedicine 2021, 38, 101036. [Google Scholar] [CrossRef]
- Hitchings, M.D.T.; Ranzani, O.T.; Torres, M.S.S.; de Oliveira, S.B.; Almiron, M.; Said, R.; Borg, R.; Schulz, W.L.; de Oliveira, R.D.; da Silva, P.V.; et al. Effectiveness of CoronaVac among Healthcare Workers in the Setting of High SARS-CoV-2 Gamma Variant Transmission in Manaus, Brazil: A Test-Negative Case-Control Study. Lancet Reg. Health-Am. 2021, 1, 100025. [Google Scholar] [CrossRef]
- Clemens, S.A.C.; Folegatti, P.M.; Emary, K.R.W.; Weckx, L.Y.; Ratcliff, J.; Bibi, S.; de Almeida Mendes, A.V.; Milan, E.P.; Pittella, A.; Schwarzbold, A.v.; et al. Efficacy of ChAdOx1 NCoV-19 (AZD1222) Vaccine against SARS-CoV-2 Lineages Circulating in Brazil. Nat. Commun. 2021, 12, 5861. [Google Scholar] [CrossRef]
- Hitchings, M.D.T.; Ranzani, O.T.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; de Moura Villela, E.F.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of ChAdOx1 Vaccine in Older Adults during SARS-CoV-2 Gamma Variant Circulation in São Paulo. Nat. Commun. 2021, 12, 6220. [Google Scholar] [CrossRef]
- Banho, C.A.; Sacchetto, L.; Campos, G.R.F.; Bittar, C.; Possebon, F.S.; Ullmann, L.S.; de Marques, B.C.; da Silva, G.C.D.; Moraes, M.M.; Parra, M.C.P.; et al. Impact of SARS-CoV-2 Gamma Lineage Introduction and COVID-19 Vaccination on the Epidemiological Landscape of a Brazilian City. Commun. Med. 2022, 2, 41. [Google Scholar] [CrossRef]
- World Health Organization. Brazil-Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/region/amro/country/br (accessed on 31 August 2022).
- Spencer, A.J.; McKay, P.F.; Belij-Rammerstorfer, S.; Ulaszewska, M.; Bissett, C.D.; Hu, K.; Samnuan, K.; Blakney, A.K.; Wright, D.; Sharpe, H.R.; et al. Heterologous Vaccination Regimens with Self-Amplifying RNA and Adenoviral COVID Vaccines Induce Robust Immune Responses in Mice. Nat. Commun. 2021, 12, 2893. [Google Scholar] [CrossRef]
- Shaw, R.H.; Stuart, A.; Greenland, M.; Liu, X.; Nguyen Van-Tam, J.S.; Snape, M.D. Heterologous Prime-Boost COVID-19 Vaccination: Initial Reactogenicity Data. Lancet 2021, 397, 2043–2046. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and Reactogenicity of Heterologous ChAdOx1 NCoV-19/MRNA Vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 Vaccine Effort: Viruses, Vaccines and Variants versus Efficacy, Effectiveness and Escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Cerqueira-Silva, T.; Katikireddi, S.V.; de Araujo Oliveira, V.; Flores-Ortiz, R.; Júnior, J.B.; Paixão, E.S.; Robertson, C.; Penna, G.O.; Werneck, G.L.; Barreto, M.L.; et al. Vaccine Effectiveness of Heterologous CoronaVac plus BNT162b2 in Brazil. Nat. Med. 2022, 28, 838–843. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; González, C.; Pizarro, A.; Acevedo, J.; Leo, K.; Paredes, F.; Bralic, T.; Vergara, V.; et al. Effectiveness of Homologous and Heterologous Booster Doses for an Inactivated SARS-CoV-2 Vaccine: A Large-Scale Prospective Cohort Study. Lancet Glob. Health 2022, 10, e798–e806. [Google Scholar] [CrossRef]
- Duarte-Salles, T.; Prieto-Alhambra, D. Heterologous Vaccine Regimens against COVID-19. Lancet 2021, 398, 94–95. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; el Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous Covid-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Alex Rodrigues Brazil Stges Massive Vaccination Campaign Agaist COVID-19. Available online: https://agenciabrasil.ebc.com.br/en/saude/noticia/2021-11/brazil-stages-massive-vaccination-campaign-against-covid-19 (accessed on 24 April 2022).
- CREMEPE. Recomendação CREMEPE No. 05/2020. Available online: https://sistemas.cfm.org.br/normas/visualizar/recomendacoes/PE/2020/5 (accessed on 22 April 2022).
- CREMEPE Calculadora Do Escore Unificado Para Priorização em Unidades de Terapia Intensiva—EUP-UTI. Available online: https://www.cremepe.org.br/portal2015/calculadoraeup/ (accessed on 22 April 2022).
- de Valentim, R.A.M.; Lima, T.S.; Cortez, L.R.; da Barros, D.M.S.; da Silva, R.D.; de Paiva, J.C.; Coutinho, K.D.; de Morais, P.S.G.; de Lacerda, J.S.; de André, F.R. The Relevance a Technology Ecosystem in the Brazilian National Health Service’s Covid-19 Response: The Case of Rio Grande Do Norte, Brazil. Ciência Saúde Coletiva 2021, 26, 2035–2052. [Google Scholar] [CrossRef]
- LAIS/UFRN Plataforma RegulaRN—Sala de Situação Pública. Available online: https://regulacao.saude.rn.gov.br/sala-situacao/sala_publica/ (accessed on 19 April 2022).
- Amodio, D.; Ruggiero, A.; Sgrulletti, M.; Pighi, C.; Cotugno, N.; Medri, C.; Morrocchi, E.; Colagrossi, L.; Russo, C.; Zaffina, S.; et al. Humoral and Cellular Response Following Vaccination with the BNT162b2 MRNA COVID-19 Vaccine in Patients Affected by Primary Immunodeficiencies. Front. Immunol. 2021, 12, 3947. [Google Scholar] [CrossRef] [PubMed]
- Marot, S.; Malet, I.; Leducq, V.; Zafilaza, K.; Sterlin, D.; Planas, D.; Gothland, A.; Jary, A.; Dorgham, K.; Bruel, T.; et al. Rapid Decline of Neutralizing Antibodies against SARS-CoV-2 among Infected Healthcare Workers. Nat. Commun. 2021, 12, 844. [Google Scholar] [CrossRef]
- Healy, K.; Pin, E.; Chen, P.; Söderdahl, G.; Nowak, P.; Mielke, S.; Hansson, L.; Bergman, P.; Smith, C.I.E.; Ljungman, P.; et al. Salivary IgG to SARS-CoV-2 Indicates Seroconversion and Correlates to Serum Neutralization in MRNA-Vaccinated Immunocompromised Individuals. Med 2022, 3, 137–153.e3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, Q.; Deng, C.; Li, M.; Li, L.; Liu, D.; Liu, M.; Ruan, X.; Mei, J.; Mo, R.; et al. Robust Induction of B Cell and T Cell Responses by a Third Dose of Inactivated SARS-CoV-2 Vaccine. Cell Discov. 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Cristaldo, H.; Brandão, M. Vacinação Contra a COVID-19 Começa em Todo País. Available online: https://agenciabrasil.ebc.com.br/saude/noticia/2021-01/vacinacao-contra-covid-19-começa-em-todo-o-pais (accessed on 22 April 2022).
- IBGE Censo Do Rio Grande Do Norte, Brasil. Available online: https://cidades.ibge.gov.br/brasil/rn/panorama (accessed on 5 May 2022).
- Martins-Filho, P.R.; Barberia, L.G. The Unjustified and Politicized Battle against Vaccination of Children and Adolescents in Brazil. Lancet Reg. Health-Am. 2022, 8, 100206. [Google Scholar] [CrossRef]
- Fernandez, M.; Matta, G.; Paiva, E. COVID-19, Vaccine Hesitancy and Child Vaccination: Challenges from Brazil. Lancet Reg. Health-Am. 2022, 8, 100246. [Google Scholar] [CrossRef] [PubMed]
- Lotta, G.; Fernandez, M.; Kuhlmann, E.; Wenham, C. COVID-19 Vaccination Challenge: What Have We Learned from the Brazilian Process? Lancet Glob. Health 2022, 10, e613–e614. [Google Scholar] [CrossRef]
- Cerqueira-Silva, T.; Andrews, J.R.; Boaventura, V.S.; Ranzani, O.T.; de Araújo Oliveira, V.; Paixão, E.S.; Júnior, J.B.; Machado, T.M.; Hitchings, M.D.T.; Dorion, M.; et al. Effectiveness of CoronaVac, ChAdOx1 NCoV-19, BNT162b2, and Ad26.COV2.S among Individuals with Previous SARS-CoV-2 Infection in Brazil: A Test-Negative, Case-Control Study. Lancet Infect. Dis. 2022, 22, 791–801. [Google Scholar] [CrossRef]
- Brizzi, A.; Whittaker, C.; Servo, L.M.S.; Hawryluk, I.; Prete, C.A.; de Souza, W.M.; Aguiar, R.S.; Araujo, L.J.T.; Bastos, L.S.; Blenkinsop, A.; et al. Spatial and Temporal Fluctuations in COVID-19 Fatality Rates in Brazilian Hospitals. Nat. Med. 2022, 28, 1476–1485. [Google Scholar] [CrossRef] [PubMed]

| Vaccinal Status | Discharged Patients without Comorbidities | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unified Score of Prioritization (USP) | ||||||||||
| 2 | 3 | 4 | 5+ | Total | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Not Iummunized | 759 | 70.47 | 157 | 14.58 | 35 | 3.25 | 16 | 1.49 | 967 | 89.79 |
| Partially (D1) | 31 | 2.88 | 4 | 0.37 | 6 | 0.56 | 0 | 0.00 | 41 | 3.81 |
| Fully (D2) | 29 | 2.69 | 15 | 1.39 | 10 | 0.93 | 15 | 1.39 | 69 | 6.41 |
| Total | 819 | 76.04 | 176 | 16.34 | 51 | 4.74 | 31 | 2.88 | 1077 | 100 |
| Vaccinal Status | Discharged Patients with Comorbidities | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unified Score of Prioritization (USP) | ||||||||||
| 2 | 3 | 4 | 5+ | Total | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Not Iummunized | 141 | 32.87 | 63 | 14.69 | 36 | 8.39 | 33 | 7.69 | 273 | 63.64 |
| Partially (D1) | 21 | 4.90 | 7 | 1.63 | 11 | 2.56 | 7 | 1.63 | 46 | 10.72 |
| Fully (D2) | 27 | 6.29 | 20 | 4.66 | 21 | 4.90 | 42 | 9.79 | 110 | 25.64 |
| Total | 189 | 44.06 | 90 | 20.98 | 68 | 15.85 | 82 | 19.11 | 429 | 100 |
| Vaccinal Status | Lethal Outcome Patients without Comorbidities | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unified Score of Prioritization (USP) | ||||||||||
| 2 | 3 | 4 | 5+ | Total | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Not Iummunized | 136 | 50.75 | 38 | 14.18 | 14 | 5.22 | 18 | 6.72 | 206 | 76.87 |
| Partially (D1) | 10 | 3.73 | 3 | 1.12 | 5 | 1.87 | 3 | 1.12 | 21 | 7.84 |
| Fully (D2) | 12 | 4.48 | 6 | 2.24 | 8 | 2.99 | 15 | 5.60 | 41 | 15.30 |
| Total | 158 | 58.96 | 47 | 17.54 | 27 | 10.07 | 36 | 13.43 | 268 | 100 |
| Vaccinal Status | Lethal Outcome Patients with Comorbidities | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unified Score of Prioritization (USP) | ||||||||||
| 2 | 3 | 4 | 5+ | Total | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Not Iummunized | 56 | 21.96 | 38 | 14.90 | 20 | 7.84 | 27 | 10.59 | 141 | 55.29 |
| Partially (D1) | 10 | 3.92 | 10 | 3.92 | 7 | 2.75 | 13 | 5.10 | 40 | 15.69 |
| Fully (D2) | 6 | 2.35 | 16 | 6.27 | 19 | 7.45 | 33 | 12.94 | 74 | 29.02 |
| Total | 72 | 28.24 | 64 | 25.10 | 46 | 18.04 | 73 | 28.63 | 255 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales-Moioli, A.I.L.; Galvão-Lima, L.J.; Pinto, T.K.B.; Cardoso, P.H.; Silva, R.D.; Fernandes, F.; Barbalho, I.M.P.; Farias, F.L.O.; Veras, N.V.R.; Souza, G.F.; et al. Effectiveness of COVID-19 Vaccination on Reduction of Hospitalizations and Deaths in Elderly Patients in Rio Grande do Norte, Brazil. Int. J. Environ. Res. Public Health 2022, 19, 13902. https://doi.org/10.3390/ijerph192113902
Sales-Moioli AIL, Galvão-Lima LJ, Pinto TKB, Cardoso PH, Silva RD, Fernandes F, Barbalho IMP, Farias FLO, Veras NVR, Souza GF, et al. Effectiveness of COVID-19 Vaccination on Reduction of Hospitalizations and Deaths in Elderly Patients in Rio Grande do Norte, Brazil. International Journal of Environmental Research and Public Health. 2022; 19(21):13902. https://doi.org/10.3390/ijerph192113902
Chicago/Turabian StyleSales-Moioli, Ana Isabela L., Leonardo J. Galvão-Lima, Talita K. B. Pinto, Pablo H. Cardoso, Rodrigo D. Silva, Felipe Fernandes, Ingridy M. P. Barbalho, Fernando L. O. Farias, Nicolas V. R. Veras, Gustavo F. Souza, and et al. 2022. "Effectiveness of COVID-19 Vaccination on Reduction of Hospitalizations and Deaths in Elderly Patients in Rio Grande do Norte, Brazil" International Journal of Environmental Research and Public Health 19, no. 21: 13902. https://doi.org/10.3390/ijerph192113902
APA StyleSales-Moioli, A. I. L., Galvão-Lima, L. J., Pinto, T. K. B., Cardoso, P. H., Silva, R. D., Fernandes, F., Barbalho, I. M. P., Farias, F. L. O., Veras, N. V. R., Souza, G. F., Cruz, A. S., Andrade, I. G. M., Gama, L., & Valentim, R. A. M. (2022). Effectiveness of COVID-19 Vaccination on Reduction of Hospitalizations and Deaths in Elderly Patients in Rio Grande do Norte, Brazil. International Journal of Environmental Research and Public Health, 19(21), 13902. https://doi.org/10.3390/ijerph192113902













