Effectiveness and Cost-Effectiveness of Case Management in Advanced Heart Failure Patients Attended in Primary Care: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Inclusion Criteria
2.1.1. Types of Design
2.1.2. Types of Participants
2.1.3. Types of Interventions
- Studies where the nurse-led CM model effect was measured.
- Community interventions including those commencing in hospital.
- Nurse-led CM interventions developed only in hospitals.
- Cardiac rehabilitation programs, unless providing elements of nurse-led CM.
- Community interventions from specialized HF clinics directed by cardiologists.
- Only one educational session, without follow-up phone calls/patient interaction.
2.1.4. Type of Comparator/Control
2.1.5. Outcomes
Primary Outcome
Secondary Outcomes
Types of Outcome Measures
- QoL measured by EuroQol-5D, SF-8, SF-36, and the Kansas City Cardiomyopathy Questionnaire (KCCQ) scales, etc.
- All-cause and HF mortality.
- Number of HF hospitalizations or for any other cause during follow-up.
- Self-care measured by the Appraisal of Self-care Agency (ASA) Scale, European Heart Failure Self-care Behavior Scale, and Self-care of Heart Failure Index.
- Costs associated with health resources.
- Cost per QALY (quality-adjusted life year), cost per year of life gained.
2.2. Search Methods
2.2.1. Electronic Searches
2.2.2. Other Resources
2.3. Data Collection and Analysis
2.3.1. Study Selection
2.3.2. Data Extraction/Management
2.3.3. Risk-of-Bias Assessment
2.3.4. Intervention Characteristics
2.3.5. Data Synthesis and Registry
3. Results
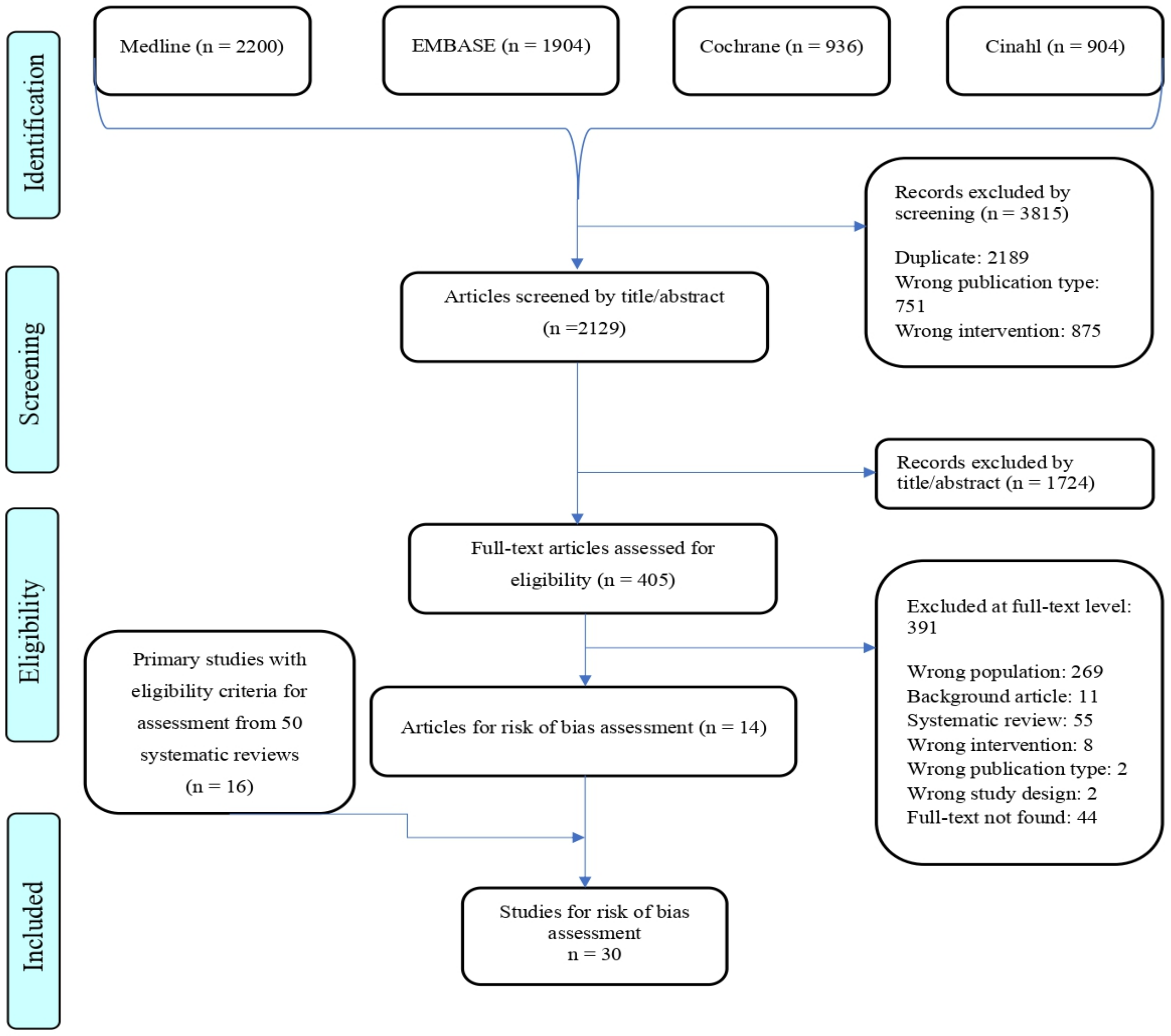
3.1. Selection of Primary Studies
3.2. Study Characteristics
3.2.1. Evidence of Effects (Benefits and Risks)
3.2.2. Cost-Effectiveness Studies
3.3. Quality of Included Studies
3.3.1. Randomized Control Trials
3.3.2. Nonrandomized Trials
3.3.3. Economic Evaluations
3.4. Evidence of Effects
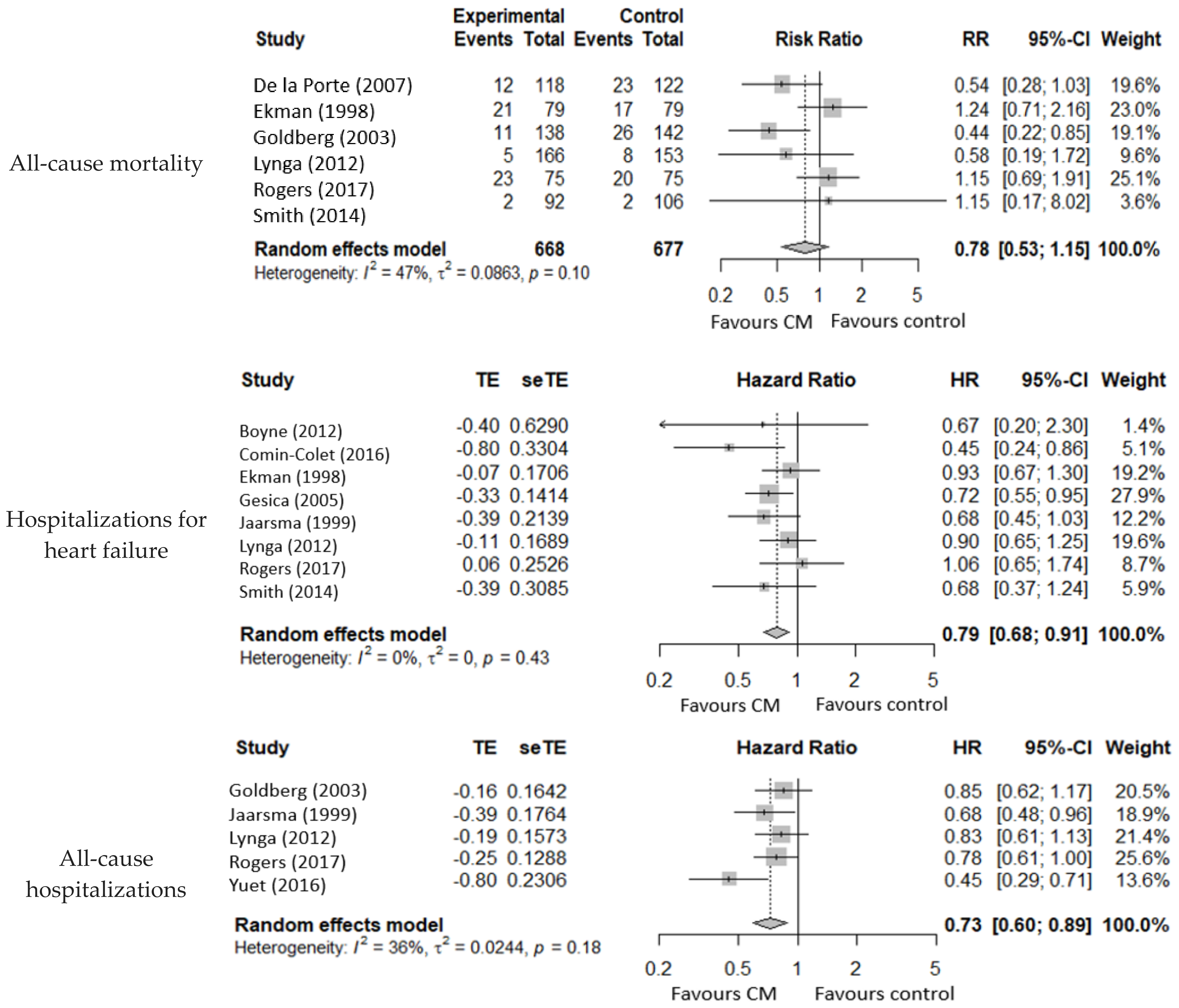
3.4.1. All-Cause Mortality
3.4.2. Mortality for Heart Failure
3.4.3. Hospitalizations for Heart Failure
3.4.4. All-Cause Hospitalizations
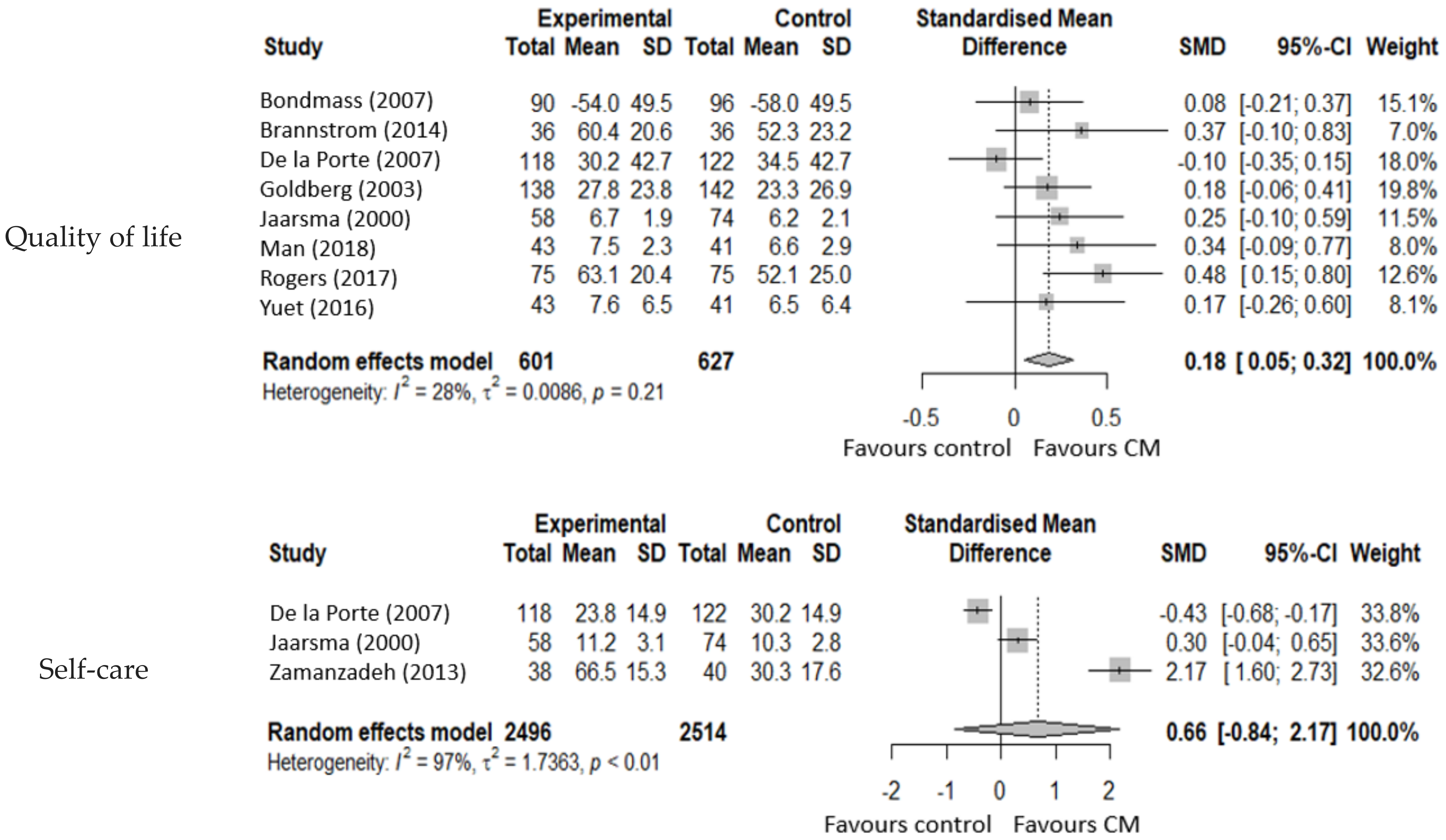
3.4.5. Quality of Life
3.4.6. Self-Care
3.5. Costs and Cost-Effectiveness of Nurse-Led CM
3.5.1. Cost of the Intervention
3.5.2. Cost-Effectiveness (Cost per QALY)
3.5.3. Cost-Benefit Studies
4. Discussion
Limitations
- The limitations of this systematic review are mostly derived from those of the primary included studies. We found seven, five, and four studies corresponding to all-cause hospitalizations, HF hospitalizations, and QoL, respectively, with concerns of a high risk of bias leading to their exclusion from the pooled analysis. We did, however, narratively summarize these data and found similar results in most cases.
- Nurse-led CM interventions may have varying characteristics according to their settings which could result in heterogeneity. For clarification, we created a descriptive table with all the characteristics of each intervention.
- The CM overall effect can be affected over time. We observed a short-term beneficial effect that was depleted on the medium/long term. We therefore carried out the meta-analysis with different follow-up time groups to analyze this factor.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Electronic Database Strategy
| Medline, Via Pubmed | |
| Heart Failure | |
| #1 | Search “Heart Failure”[Mesh] |
| #2 | Search “Heart Arrest”[Mesh] |
| #3 | Search “Shock, Cardiogenic”[Mesh] |
| #4 | Search (heart[Title/Abstract] OR cardi *[Title/Abstract] OR myocard *[Title/Abstract]) AND (failur *[Title/Abstract] OR decompensat *[Title/Abstract] OR insufficien *[Title/Abstract] OR incompet *[Title/Abstract] OR arrest[Title/Abstract] OR shock[Title/Abstract]) |
| #5 | Search #1 OR #2 OR #3 OR #4 344766 |
| Nurses | |
| #6 | Search “Nurses”[Mesh] |
| #7 | Search “Nurses, Community Health”[Mesh] |
| #8 | Search “Nursing”[Mesh] |
| #9 | Search “Primary Nursing”[Mesh] |
| #10 | Search “Nursing Care”[Mesh] |
| #11 | Search “Primary Care Nursing”[Mesh] |
| #12 | Search “Practice Patterns, Nurses”[Mesh] |
| #13 | Search nurs *[Title/Abstract] |
| #14 | Search “Patient Care Team”[Mesh] |
| #15 | Search (multidisciplinar *[Title/Abstract] OR multi-disciplinar *[Title/Abstract] OR interdisciplinar *[Title/Abstract]) OR ((care[Title/Abstract] OR healthcare[Title/Abstract] OR “health care”)[Title/Abstract] AND (team[Title/Abstract] OR teams[Title/Abstract])) |
| #16 | Search #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 754499 |
| Case Management | |
| #17 | Search “Patient Care Management”[Mesh] |
| #18 | Search “Patient Care Planning”[Mesh] |
| #19 | Search “Case Management”[Mesh] |
| #20 | Search “Case Managers”[Mesh] |
| #21 | Search “Disease Management”[Mesh] |
| #22 | Search manag *[Title/Abstract] |
| #23 | Search #17 OR #18 OR #19 OR #20 OR #21 OR 1893972 |
| Primary Care | |
| #24 | Search “Primary Health Care”[Mesh] |
| #25 | Search “General Practice”[Mesh] |
| #26 | Search “Family Practice”[Mesh] |
| #27 | Search “Community Medicine”[Mesh] |
| #28 | Search “Community Health Services”[Mesh] |
| #29 | Search “Community Health Nursing”[Mesh] |
| #30 | Search “Home Care Services”[Mesh] |
| #31 | Search “Home Health Nursing”[Mesh] |
| #32 | Search “Community Health Planning”[Mesh] |
| #33 | Search “Community Health Centers”[Mesh] |
| #34 | Search “Ambulatory Care”[Mesh] |
| #35 | Search “Ambulatory Care Facilities”[Mesh] |
| #36 | Search (primary[Title/Abstract]) AND (care[Title/Abstract] OR healthcare[Title/Abstract] OR “health care”[Title/Abstract]) |
| #37 | Search ((general[Title/Abstract] OR family[Title/Abstract])) AND (practic *[Title/Abstract] OR medicine[Title/Abstract]) |
| #38 | Search (community[Title/Abstract]) AND (medicine[Title/Abstract] OR care[Title/Abstract] OR healthcare[Title/Abstract] OR service[Title/Abstract] OR services[Title/Abstract] OR health[Title/Abstract]) |
| #39 | Search ((ambulatory[Title/Abstract] OR outpatient[Title/Abstract])) AND care[Title/Abstract] |
| #40 | Search ((community[Title/Abstract] OR neighbo*[Title/Abstract] OR outpatient[Title/Abstract] OR walk-in[Title/Abstract] OR “walk in”[Title/Abstract])) AND (center[Title/Abstract] OR centers[Title/Abstract] OR centre[Title/Abstract] OR centres[Title/Abstract] OR clinic[Title/Abstract] OR clinics[Title/Abstract]) |
| #41 | Search (home[Title/Abstract]) AND (care[Title/Abstract] OR health[Title/Abstract]) |
| #42 | Search #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 1133580 |
| TOTAL | |
| #43 | Search #5 AND #16 AND #23 AND #42 2055 |
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.C.; Ewald, G.A.; Allen, L.A.; Butler, J.; Canary, C.A.; Colvin-Adams, M.; Dickinson, M.G.; Levy, P.; Stough, W.G.; Sweitzer, N.K.; et al. Advanced (stage D) heart failure: A statement from the Heart Failure Society of America Guidelines Committee. J. Card. Fail. 2015, 21, 519–534. [Google Scholar] [CrossRef]
- Bjork, J.B.; Alton, K.K.; Georgiopoulou, V.V.; Butler, J.; Kalogeropoulos, A.P. Defining advanced heart failure: A systematic review of criteria used in clinical trials. J. Card. Fail. 2016, 22, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.P.; Parissis, J.; Laroche, C.; Piepoli, M.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Lesyuk, W.; Kriza, C.; Kolominsky-Rabas, P. Cost-of-illness studies in heart failure: A systematic review 2004–2016. BMC Cardiovasc. Disord. 2018, 18, 74. [Google Scholar] [CrossRef]
- Moradi, M.; Daneshi, F.; Behzadmehr, R.; Rafiemanesh, H.; Bouya, S.; Raeisi, M. Quality of life of chronic heart failure patients: A systematic review and meta-analysis. Heart Fail. Rev. 2020, 25, 993–1006. [Google Scholar] [CrossRef]
- Truby, L.K.; O’Connor, C.; Fiuzat, M.; Stebbins, A.; Coles, A.; Patel, C.B.; Granger, B.; Pagidipati, N.; Agarwal, R.; Rymer, J.; et al. Sex Differences in Quality of Life and Clinical Outcomes in Patients With Advanced Heart Failure: Insights From the PAL-HF Trial. Circ. Heart Fail. 2020, 13, e006134. [Google Scholar] [CrossRef]
- Ahmed, O.I. Disease management, case management, care management, and care coordination: A framework and a brief manual for care programs and staff. Prof. Case Manag. 2016, 21, 137–146. [Google Scholar] [CrossRef]
- What Is A Case Manager-Case Management Society of America. Available online: https://www.cmsa.org/who-we-are/what-is-a-case-manager (accessed on 4 January 2021).
- Takeda, A.; Martin, N.; Taylor, R.S.; Taylor, S.J. Disease management interventions for heart failure. Cochrane Database Syst. Rev. 2019, 1, CD002752. [Google Scholar] [CrossRef]
- Rogers, J.G.; Patel, C.B.; Mentz, R.J.; Granger, B.B.; Steinhauser, K.E.; Fiuzat, M.; Adams, P.A.; Speck, A.; Johnson, K.S.; Krishnamoorthy, A.; et al. Palliative Care in Heart Failure: The PAL-HF Randomized, Controlled Clinical Trial. J. Am. Coll. Cardiol. 2017, 70, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Lyngå, P.; Persson, H.; Hägg-Martinell, A.; Hägglund, E.; Hagerman, I.; Langius-Eklöf, A.; Rosenqvist, M. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. Eur. J. Heart Fail. 2012, 14, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Mourad, O.; Hossam, H.; Zbys, F.; Ahmed, E. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Evers, S.; Goossens, M.; De Vet, H.; Van Tulder, M.; Ament, A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int. J. Technol. Assess. Health Care 2005, 21, 240–245. [Google Scholar] [CrossRef]
- Aiken, L.S.; Butner, J.; Lockhart, C.A.; Volk-Craft, B.E.; Hamilton, G.; Williams, F.G. Outcome evaluation of a randomized trial of the PhoenixCare intervention: Program of case management and coordinated care for the seriously chronically ill. J. Palliat. Med. 2006, 9, 111–126. [Google Scholar] [CrossRef]
- Bondmass, M.D. Improving outcomes for African Americans with Chronic Heart Failure: A comparison of two home care management delivery methods. Home Health Care Manag. Pract. 2007, 20, 8–20. [Google Scholar] [CrossRef]
- Boyne, J.J.; Vrijhoef, H.J.; Crijns, H.J.; De Weerd, G.; Kragten, J.; Gorgels, A.P. TEHAF investigators. Tailored telemonitoring in patients with heart failure: Results of a multicentre randomized controlled trial. Eur. J. Heart Fail. 2012, 14, 791–801. [Google Scholar] [CrossRef]
- Brännström, M.; Boman, K. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: A randomized controlled study. Eur. J. Heart Fail. 2014, 16, 1142–1151. [Google Scholar] [CrossRef]
- Comín-Colet, J.; Enjuanes, C.; Verdú-Rotellar, J.M.; Linas, A.; Ruiz-Rodriguez, P.; González-Robledo, G.; Farre, N.; Moliner-Borja, P.; Ruiz-Bustillo, S.; Bruguera, J. Impact on clinical events and healthcare costs of adding telemedicine to multidisciplinary disease management programmes for heart failure: Results of a randomized controlled trial. J. Telemed. Telecare 2016, 22, 282–295. [Google Scholar] [CrossRef] [PubMed]
- De la Porte, P.W.; Lok, D.J.; van Veldhuisen, D.J.; van Wijngaarden, J.; Cornel, J.H.; Zuithoff, N.P.; Badings, E.; Hoes, A.W. Added value of a physician-and-nurse-directed heart failure clinic: Results from the Deventer-Alkmaar heart failure study. Heart 2007, 93, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.; Apostolidis, B. Pilot testing of a multicomponent home care intervention for older adults with heart failure: An academic clinical partnership. J. Cardiovasc. Nurs. 2010, 25, E27–E40. [Google Scholar] [CrossRef] [PubMed]
- Ekman, I.; Andersson, B.; Ehnfors, M.; Matejka, G.; Persson, B.; Fagerberg, B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur. Heart J. 1998, 19, 1254–1260. [Google Scholar] [CrossRef][Green Version]
- Fonarow, G.C.; Stevenson, L.W.; Walden, J.A.; Livingston, N.A.; Steimle, A.E.; Hamilton, M.A.; Moriguchi, J.; Tillisch, J.H.; Woo, M.A. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J. Am. Coll. Cardiol. 1997, 30, 725–732. [Google Scholar] [CrossRef]
- GESICA Investigators. Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ 2005, 331, 425. [Google Scholar] [CrossRef]
- Goldberg, L.R.; Piette, J.D.; Walsh, M.N.; Frank, T.A.; Jaski, B.E.; Smith, A.L.; Rodriguez, R.; Mancini, D.M.; Hopton, L.A.; Orav, E.J.; et al. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: The Weight Monitoring in Heart Failure (WHARF) trial. Am. Heart J. 2003, 146, 705–712. [Google Scholar] [CrossRef]
- Holst, D.P.; Kaye, D.; Richardson, M.; Krum, H.; Prior, D.; Aggarwal, A.; Wolfe, R.; Bergin, P. Improved outcomes from a comprehensive management system for heart failure. Eur. J. Heart Fail. 2001, 3, 619–625. [Google Scholar] [CrossRef]
- Jaarsma, T.; Halfens, R.; Huijer, H.; Dracup, K.; Gorgels, T.; van Ree, J.; Stappers, J. Effects of education and support on self-care and resource utilization in patients with heart failure. Eur. Heart J. 1999, 20, 673–682. [Google Scholar] [CrossRef]
- Jaarsma, T.; Halfens, R.; Tan, F.; Abu-Saad, H.H.; Dracup, K.; Diederiks, J. Self-care and quality of life in patients with advanced heart failure: The effect of a supportive educational intervention. Heart Lung 2000, 29, 319–330. [Google Scholar] [CrossRef]
- Man, N.G.; Wong, F.K.Y. Effects of a Home-Based Palliative Heart Failure Program on Quality of Life, Symptom Burden, Satisfaction and Caregiver Burden: A Randomized Controlled Trial. J. Pain Symptom Manag. 2018, 55, 1–11. [Google Scholar] [CrossRef]
- McDonald, K.; Ledwidge, M.; Cahill, J.; Kelly, J.; Quigley, P.; Maurer, B.; Begley, F.; Ryder, M.; Travers, B.; Timmons, L.; et al. Elimination of early rehospitalization in a randomized, controlled trial of multidisciplinary care in a high-risk, elderly heart failure population: The potential contributions of specialist care, clinical stability and optimal angiotensin-converting enzyme inhibitor dose at discharge. Eur. J. Heart Fail. 2001, 3, 209–215. [Google Scholar] [CrossRef]
- Ong, M.K.; Romano, P.S.; Edgington, S.; Aronow, H.U.; Auerbach, A.D.; Black, J.T.; De Marco, T.; Escarce, J.J.; Evangelista, L.S.; Hanna, B.; et al. Better Effectiveness After Transition–Heart Failure (BEAT-HF) Research Group. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients With Heart Failure: The Better Effectiveness After Transition—Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 310–318. [Google Scholar] [CrossRef]
- Schellinger, S.; Sidebottom, A.; Briggs, L. Disease specific advance care planning for heart failure patients: Implementation in a large health system. J. Palliat. Med. 2011, 14, 1224–1230. [Google Scholar] [CrossRef]
- Shah, N.B.; Der, E.; Ruggerio, C.; Heidenreich, P.A.; Massie, B.M. Prevention of hospitalizations for heart failure with an interactive home monitoring program. Am. Heart J. 1998, 135, 373–378. [Google Scholar] [CrossRef]
- Smith, C.E.; Piamjariyakul, U.; Wick, J.A.; Spertus, J.A.; Russell, C.; Dalton, K.M.; Elyachar, A.; Vacek, J.L.; Reeder, K.M.; Nazir, N.; et al. Multidisciplinary group clinic appointments: The Self-Management and Care of Heart Failure (SMAC-HF) trial. Circ. Heart Fail. 2014, 7, 888–894. [Google Scholar] [CrossRef]
- Vavouranakis, I.; Lambrogiannakis, E.; Markakis, G.; Dermitzakis, A.; Haroniti, Z.; Ninidaki, C.; Borbantonaki, A.; Tsoutsoumanou, K. Effect of home-based intervention on hospital readmission and quality of life in middle-aged patients with severe congestive heart failure: A 12-month follow up study. Eur. J. Cardiovasc. Nurs. 2003, 2, 105–111. [Google Scholar] [CrossRef]
- Yuet-Wong, F.K.; Ng, A.Y.; Lee, P.H.; Lam, P.T.; Ng, J.S.; Ng, N.H.; Knong-Sham, M.M. Effects of a transitional palliative care model on patients with end-stage heart failure: A randomized controlled trial. Heart 2016, 102, 1100–1108. [Google Scholar] [CrossRef]
- Zamanzadeh, V.; Valizadeh, L.; Howard, A.F.; Jamshidi, F. A supportive-educational intervention for heart failure patients in Iran: The effect on self-care behaviours. Nurs. Res. Pract. 2013, 2013, 492729. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G.; Carpenter, J.R.; Schumacher, M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med. Res. Methodol. 2008, 8, 79. [Google Scholar] [CrossRef]
- Gomersall, J.S.; MCom, B.A.; Jadotte, Y.T.; Xue, Y.; Lockwood, S.; Riddle, D. Conducting systematic reviews of economic evaluations. Int. J. Evid. Based Healthc. 2015, 3, 170–178. [Google Scholar] [CrossRef]
- Gregory, D.; Kimmelstiel, C.; Perry, K.; Parikh, A.; Konstam, V.; Konstam, M.A. Hospital cost effect of a heart failure disease management program: The Specialized Primary and Networked Care in Heart Failure (SPAN-CHF) trial. Am. Heart J. 2006, 151, 1013–1018. [Google Scholar] [CrossRef]
- Ledwidge, M.; Barry, M.; Cahill, J.; Ryan, E.; Maurer, B.; Ryder, M.; Travers, B.; Timmons, L.; McDonald, K. Is multidisciplinary care of heart failure cost-beneficial when combined with optimal medical care? Eur. J. Heart Fail. 2003, 5, 381–389. [Google Scholar] [CrossRef]
- Postmus, D.; Pari, A.A.; Jaarsma, T.; Luttik, M.L.; van Veldhuisen, D.J.; Hillege, H.L.; Buskens, E. A trial-based economic evaluation of 2 nurse-led disease management programs in heart failure. Am. Heart J. 2011, 162, 1096–1104. [Google Scholar] [CrossRef][Green Version]
- Grustam, A.S.; Severens, J.L.; De Massari, D.; Buyukkaramikli, N.; Koymans, R.; Vrijhoef, H.J.M. Cost-Effectiveness Analysis in Telehealth: A Comparison between Home Telemonitoring, Nurse Telephone Support, and Usual Care in Chronic Heart Failure Management. Value Health 2018, 21, 772–782. [Google Scholar] [CrossRef]
- Sahlen, K.G.; Boman, K.; Brännström, M. A cost-effectiveness study of person-centered integrated heart failure and palliative home care: Based on a randomized controlled trial. Palliat. Med. 2016, 30, 296–302. [Google Scholar] [CrossRef]
- Van Spall, H.G.C.; Rahman, T.; Mytton, O.; Ramasundarahettige, C.; Ibrahim, Q.; Kabali, C.; Coppens, M.; Haynes, R.B.; Connolly, S. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: A systematic review and network meta-analysis. Eur. J. Heart Fail. 2017, 19, 1427–1443. [Google Scholar] [CrossRef]
- Bryant-Lukosius, D.; Carter, N.; Reid, K.; Donald, F.; Martin-Misener, R.; Kilpatrick, K.; Harbman, P.; Kaasalainen, S.; Marshall, D.; Charbonneau-Smith, R.; et al. The clinical effectiveness and cost-effectiveness of clinical nurse specialist-led hospital to home transitional care: A systematic review. J. Eval. Clin. Pract. 2015, 21, 763–781. [Google Scholar] [CrossRef]
- Bashi, N.; Karunanithi, M.; Fatehi, F.; Ding, H.; Walters, D. Remote Monitoring of Patients With Heart Failure: An Overview of Systematic Reviews. J. Med. Internet Res. 2017, 19, e18. [Google Scholar] [CrossRef]
- Flodgren, G.; Rachas, A.; Farmer, A.J.; Inzitari, M.; Shepperd, S. Interactive telemedicine: Effects on professional practice and health care outcomes. Cochrane Database Syst. Rev. 2015, 2015, CD002098. [Google Scholar] [CrossRef]
- Desai, A.S.; Stevenson, L.W. Rehospitalization for heart failure: Predict or prevent? Circulation 2012, 126, 501–506. [Google Scholar] [CrossRef]
- Setoguchi, S.; Stevenson, L.W.; Schneeweiss, S. Repeated hospitalizations predict mortality in the community population with heart failure. Am. Heart J. 2007, 154, 260–266. [Google Scholar] [CrossRef]
- Rice, H.; Say, R.; Betihavas, V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ. Couns. 2018, 101, 363–374. [Google Scholar] [CrossRef]
- Bauce, K.; Fahs, D.B.; Batten, J.; Whittemore, R. Videoconferencing for Management of Heart Failure: An Integrative Review. J. Gerontol. Nurs. 2018, 44, 45–52. [Google Scholar] [CrossRef]
- Inglis, S.C.; Clark, R.A.; Dierckx, R.; Prieto-Merino, D.; Cleland, J.G. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst. Rev. 2015, 10, CD007228. [Google Scholar] [CrossRef]
- Boyde, M.; Peters, R.; New, N.; Hwang, R.; Ha, T.; Korczyk, D. Self-care educational intervention to reduce hospitalisations in heart failure: A randomised controlled trial. Eur. J. Cardiovasc. Nurs. 2018, 17, 178–185. [Google Scholar] [CrossRef]
- Fergenbaum, J.; Bermingham, S.; Krahn, M.; Alter, D.; Demers, C. Care in the Home for the Management of Chronic Heart Failure: Systematic Review and Cost-Effectiveness Analysis. J. Cardiovasc. Nurs. 2015, 30, S44–S51. [Google Scholar] [CrossRef]
| Studies Evaluating Clinical Efficacy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, Year Country | Study Design (Number of Subjects Included) | Mean Age (Standard Deviation) Case Management vs. Control | Gender (% Women) Case Management vs. Control | Population | Intervention Characteristics and Main Component * | Control | Maximum Follow-Up Time (Days) | Outcome Measures |
| Aiken, 2006 [18] USA | RCT (N = 129) | 68 (14) vs. 70 (13) β | 58 vs. 70 | Patients from community or hospitalized with chronic heart failure in NYHA III or IV | Intensive intervention b,f Home visits | Usual care | 270 | Quality of life |
| Bondmass, 2007 [19] USA | RCT (N = 186) | 62.1 (13.9) vs. 62.8 (12.4) | 63.3 vs. 60.4 | Patients hospitalized with HF in NYHA III or IV | Intensive intervention c,f Telemedicine | NHV: Nurse home visits | 90 | Treatment adherence, quality of life |
| Boyne, 2012 [20] Netherlands | RCT Total: (N = 382) Subgroup NYHA III: 153 Subgroup NYHA IV: 10 | Total: 71.0 (11.9) vs. 71.9 (10.5) Subgroup in NYHA III and IV: not reported | Total: 42 vs. 40 Subgroup in NYHA III and IV: not reported | Patients in the community diagnosed with HF >18 years and being treated by an HF nurse and a cardiologist in an HF clinic Subgroup analysis in NYHA III and IV | Intensive intervention a,c,e Telemedicine | Usual Care | 365 | Hospitalizations for HF |
| Brännström, 2014 [21] Sweden | RCT (N = 72) | 81.9 (7.2) vs. 76.6 (10.2) | 27.8 vs. 30.6 | Patients in the community diagnosed with HF in NYHA III or IV | Intensive intervention b,f Home visits | Usual Care | 180 | All-cause hospitalizations, quality of life, self-efficacy |
| Comin-Colet, 2016 [22] Spain | RCT (N = 178) | Total: 74 (11) vs. 75 (11) Subgroup in NYHA III and IV: not reported | Total: 43 vs. 39 Subgroup in NYHA III and IV: not reported | Patients hospitalized with HF Subgroup analysis in NYHA III and IV | Intensive intervention c,d,f Telemedicine | HF program | 180 | Hospitalizations for HF |
| De la Porte, 2007 [23] Netherlands | RCT (N = 340) | 70 (10) vs. 71 (10) | 34 vs. 21 | Patients in the community or hospitalized with NYHA III or IV | Intensive intervention a,f,g Clinical consultations | Usual Care | 365 | All-cause mortality, hospitalizations for HF, self-care, and quality of life |
| Delaney, 2010 [24] USA | Quasi-experimental studio with control without randomization (N = 24) | Overall: 79.04 (11.8) π | 58.3 vs. 58.3 | Patients with a primary diagnosis of HF in NYHA III or IV | Intensive intervention b,c Telemedicine | Usual care | 90 | Hospitalizations for HF, quality of life |
| Ekman, 1998 [25] Sweden | RCT (N = 158) | Overall: 80.3 (6.8) π | 42 π | Patients hospitalized with HF in NYHA III or IV | Basic intervention a,f,g (office hours) Clinical consultations | Usual care | 180 | All-cause mortality, hospitalizations for HF, all-cause hospitalizations |
| Fonarow, 1997 [26] USA | Quasi-experimental pre-post (N = 214) | 52.6 (10) | 19 | Patients in the community diagnosed with HF in NYHA III or IV and potential candidates for transplantation | Basic intervention a,f Clinical consultations | Usual care | 180 pre and 180 post | All-cause mortality, hospitalizations for HF |
| GESICA, 2005 [27] Argentina | RCT Total: (N = 1518) Subgroup NYHA III or IV: (N = 750) | Total: 64.8 (13.9) vs. 65.2 (12.7) Subgroup: not reported | Total: 27.4 vs. 31.1 Subgroup in NYHA III and IV: not reported | Patients in the community diagnosed with HF and >18 years Subgroup analysis in NYHA III and IV | Basic intervention f Phone calls | Usual Care | From 180 to 365 | Hospitalizations for HF |
| Goldberg, 2003 [28] USA | RCT (N = 280) | 57.9 (15.7) vs. 60.2 (14.9) | 30.4 vs. 34.5 | Patients hospitalized with HF in NYHA III or IV | Intensive intervention c Telemedicine | Usual Care | 180 | All-cause mortality, hospitalizations for HF, all cause hospitalizations and quality of life |
| Holst, 2001 [29] Australia | Quasi-experimental (N = 42) | 54 (13) | 16.6 | Patients with NYHA III or IV | Basic intervention a Clinic consultations | Usual care | 180 | All-cause hospitalizations, quality of life |
| Jaarsma, 1999 [30] Netherlands | RCT (N = 179) | 73 (9) vs. 73 (9) | 44 vs. 41 | Patients hospitalized for HF with NYHA III or IV | Basic intervention b,g Home visits | Usual care | 270 | Hospitalizations for HF, All-cause hospitalizations, treatment adherence |
| Jaarsma, 2000 [31] Netherlands | RCT (N = 132) | 72 (9) vs. 72 (10) | 45 vs. 36 | Patients admitted in cardiology unit for HF with NYHA III or IV | Basic intervention a,b,f,g Home visits | Usual care | 270 | Self-care, quality of life |
| Lynga, 2012 [13] Sweden | RCT (N = 319) | 73.7 (9.9) vs. 73.5 (10.4) | 24.1 vs. 26.1 | Patients hospitalized for HF with NYHA III or IV | Basic intervention a,c,g Telemedicine | Usual Care | Up to cardiac hospitalization or 365 days | All-cause mortality, hospitalizations for HF, all-cause hospitalizations |
| Man, 2018 [32] China | RCT (N = 84) | 78.3 (16.8) vs. 78.4 (10) | 56.1 vs. 39 | Patients hospitalized for HF with NYHA III or IV | Intensive intervention b,f Home visits | Usual care | 90 | Quality of life |
| McDonald, 2001 [33] Ireland | RCT (N = 70) | 69.9 (11.3) vs. 67.9 (12) | 14.3 vs. 18.6 | Patients hospitalized with HF and NYHA III or IV | Basic intervention a,f,g Clinical consultations | Usual care | 30 | Hospitalizations for HF, all-cause mortality |
| Ong, 2016 [34] USA | RCT (N = 1437) | Median (interquartile range) Total: 73 (62–84) vs. 74 (63–82) Subgroup in NYHA III and IV: not reported | Total: 50.2 vs. 50.5 Subgroup in NYHA III and IV: not reported | Patients admitted to hospital for decompensated HF and >50 years old Subgroup analysis in NYHA III and IV | Intensive intervention c,f,g Telemedicine | Usual care | 180 | All-cause hospitalizations |
| Rogers, 2017 [12] USA | RCT (N = 150) | 71.9 (12.4) vs. 69.8 (13.4) | 44 vs. 50.7 | Patients hospitalized for HF or within 2 weeks of discharge and dyspnea at rest or minimal exertion | Intensive intervention b Not clearly reported | Usual Care | 180 | All-cause mortality, hospitalizations for HF, all-cause hospitalizations, quality of life |
| Schellinger, 2011 [35] USA | Cohort study (N = 1894) | 75.63 vs. 73.84 ∞ | 52 vs. 48.4 ∞ | Patients with a primary or secondary HF diagnosis in community setting | Basic intervention a Clinical consultations | Usual care | 60 | All-cause hospitalizations |
| Shah, 1998 [36] USA | Quasi-experimental (N = 27) Subgroup NYHA III and IV (N = 17) | 62 (range 42–81) | 0 | Patients hospitalized for HF | Basic intervention f,g Phone calls | Usual care | 365 | All-cause hospitalizations |
| Smith, 2014 [37] USA | RCT (N = 198) | 62.6 (14.1) vs. 62.1 (12.5) | 44 vs. 34 | Patients hospitalized with HF in NYHA III or IV | Basic intervention a Clinical consultations | Usual Care | 365 | All-cause mortality, hospitalizations for HF |
| Vavouranakis, 2003 [38] Greece | Quasi-experimental (N = 33) | 65.4 (6.7) | 12.1 | Patients in the community with HF and NYHA III or IV | Basic intervention b,f,g Home visits | Usual care | 365 | All-cause hospitalizations, quality of life |
| Yuet, 2016 [39] China | RCT (N = 84) | 78.3 (16.8) vs. 78.4 (10.0) | 55.1 vs. 39 | Patients hospitalized with HF in NYHA III or IV | Intensive intervention b,f Home visits | Two placebo calls from assistant unrelated to clinical issues | 90 | All-cause hospitalizations, quality of life |
| Zamanzadeh, 2013 [40] Iran | RCT (N = 78) | 65.82 (9.87) vs. 61.63 (12.47) | 42.1 vs. 52.5 | Patients diagnosed with HF in NYHA III or IV and an ejection fraction <40% | Basic intervention a,f,g Clinical consultations | Usual Care | 90 | Self-care (treatment adherence) |
| Studies Evaluating the Economic Evidence for Nurse Case Management | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Study Design, Country | Population | Intervention Characteristics * | Control | Time Horizon, Perspective | Difference in Cost (Year Value) | Difference in Outcome | ICER | Risk of Bias (CHEC) Score) | ||
| Gregory, 2006 [43] | Cost-benefit, USA | Patients admitted to hospital with primary diagnosis of HF in NYHA III or IV | Intensive intervention: 2, 6, 7 (24 h availability) | SC | 90 days, healthcare perspective | Reference SC: USD 3979 (2003) USA Dollars Intensive vs. SC: USD 996 additional cost per patient | USD −759 due to reduction in hospitalization costs per patient | USD +237 not considered cost saving | 11.5/19 | ||
| Ledwidge, 2003 [44] | Cost-benefit, Ireland | Patients admitted to hospital with a diagnosis of HF in NYHA IV | Basic intervention: 1, 6, 7 (working hours) | SC | 3 months, healthcare perspective | Reference SC: no cost Basic vs. SC: EUR 113 (1999) additional cost per patient | EUR −43,955 due to reduction in hospitalization costs per patient | Net saving EUR −379.75 per patient | 12/19 | ||
| Postmus, 2011 [45] | Cost-effectiveness, Sweden | Patients admitted to hospital with primary diagnosis of HF in NYHA III or IV | Basic intervention: 1, 7 (working hours) Intensive intervention: 1, 2, 6, 7 (24 h availability) | SC | 18 months, healthcare perspective | Reference SC: EUR 10,692 per patient (2009) | QALY | LY | QALY (cost/QALY) | LY (cost/LY) | 14/19 |
| Basic vs. SC: EUR 1101 additional cost per patient (2009) | Basic vs. SC: 0.014 | Basic vs. SC: 0.042 | Basic vs. SC: EUR 77,335 | Basic vs. SC: EUR 25,923 | |||||||
| Intensive vs: SC: EUR 1770 additional cost per patient (2009) | Intensive vs. SC: 0.029 | Intensive vs. SC: 0.057 | Intensive vs. SC: EUR 59,289 | Intensive vs. SC: EUR 30,933 | |||||||
| Intensive vs. basic: EUR 669 additional cost per patient (2009) | Intensive vs. basic: 0.015 | Intensive vs. basic: 0.014 | Intensive vs. basic: EUR 42,839 | Intensive vs. basic: EUR 45,219 | |||||||
| Grustam, 2018 [46] | Cost-effectiveness Markov model, Netherlands | Patients > 70 years admitted to hospital with a diagnosis of HF in NYHA IV | Basic intervention (nurse telephone support): 1, 6, 7 (working hours) Intensive intervention (home telemonitoring): 3 | SC | Lifetime (20 years), health system perspective | Reference SC: EUR 15,407 per patient (2015) | QALY | LY | QALY (cost/QALY) | LY (cost/LY) | 16/19 |
| Basic vs. SC: EUR 7042 additional cost per patient (2015) | Basic vs. SC: 0.75 | Basic vs. SC: 0.96 | Basic vs. SC: EUR 9398 | Basic vs. SC: EUR 7364 | |||||||
| Intensive vs. SC: EUR 12,131 additional cost per patient (2015) | Intensive vs. SC: 0.86 | Intensive vs. SC: 1.14 | Intensive vs. SC: EUR 14,027 | Intensive vs. SC: EUR 10,644 | |||||||
| Intensive vs. basic: EUR 5090 additional cost per patient (2015) | Intensive vs. basic: 0.12 | Intensive vs. basic: 0.18 | Intensive vs. basic: EUR 44,040 | Intensive vs. basic: EUR 27,733 | |||||||
| Sahlen, 2016 [47] | Cost-effectiveness, Sweden | Patients diagnosed with HF in NYHA III or IV and attended in the community | Intensive intervention: 2, 6 | SC | 6 months, healthcare perspective | Reference SC: EUR 5727 per patient (2012) Intensive vs. SC: EUR −1649 saving cost per patient | 0.25 QALY | Dominant | 13/19 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checa, C.; Canelo-Aybar, C.; Suclupe, S.; Ginesta-López, D.; Berenguera, A.; Castells, X.; Brotons, C.; Posso, M. Effectiveness and Cost-Effectiveness of Case Management in Advanced Heart Failure Patients Attended in Primary Care: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 13823. https://doi.org/10.3390/ijerph192113823
Checa C, Canelo-Aybar C, Suclupe S, Ginesta-López D, Berenguera A, Castells X, Brotons C, Posso M. Effectiveness and Cost-Effectiveness of Case Management in Advanced Heart Failure Patients Attended in Primary Care: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(21):13823. https://doi.org/10.3390/ijerph192113823
Chicago/Turabian StyleCheca, Caterina, Carlos Canelo-Aybar, Stefanie Suclupe, David Ginesta-López, Anna Berenguera, Xavier Castells, Carlos Brotons, and Margarita Posso. 2022. "Effectiveness and Cost-Effectiveness of Case Management in Advanced Heart Failure Patients Attended in Primary Care: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 21: 13823. https://doi.org/10.3390/ijerph192113823
APA StyleCheca, C., Canelo-Aybar, C., Suclupe, S., Ginesta-López, D., Berenguera, A., Castells, X., Brotons, C., & Posso, M. (2022). Effectiveness and Cost-Effectiveness of Case Management in Advanced Heart Failure Patients Attended in Primary Care: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(21), 13823. https://doi.org/10.3390/ijerph192113823


_Basu.png)




