Fentanyl Induces Novel Conditioned Place Preference in Adult Zebrafish, Disrupts Neurotransmitter Homeostasis, and Triggers Behavioral Changes
Abstract
1. Introduction
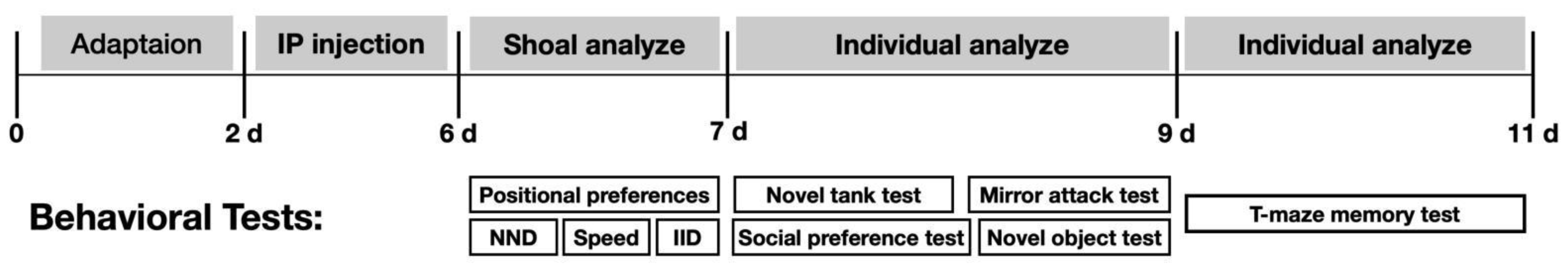
2. Materials and Methods
2.1. Zebrafish Maintenance
2.2. Chemicals
2.3. Zebrafish Intraperitoneal Injection Protocol
2.4. Behavioral Test
2.4.1. Conditional Place Preference Test
2.4.2. Shoaling Test
2.4.3. Novel Tank Test
2.4.4. Novel Object Exploration Test
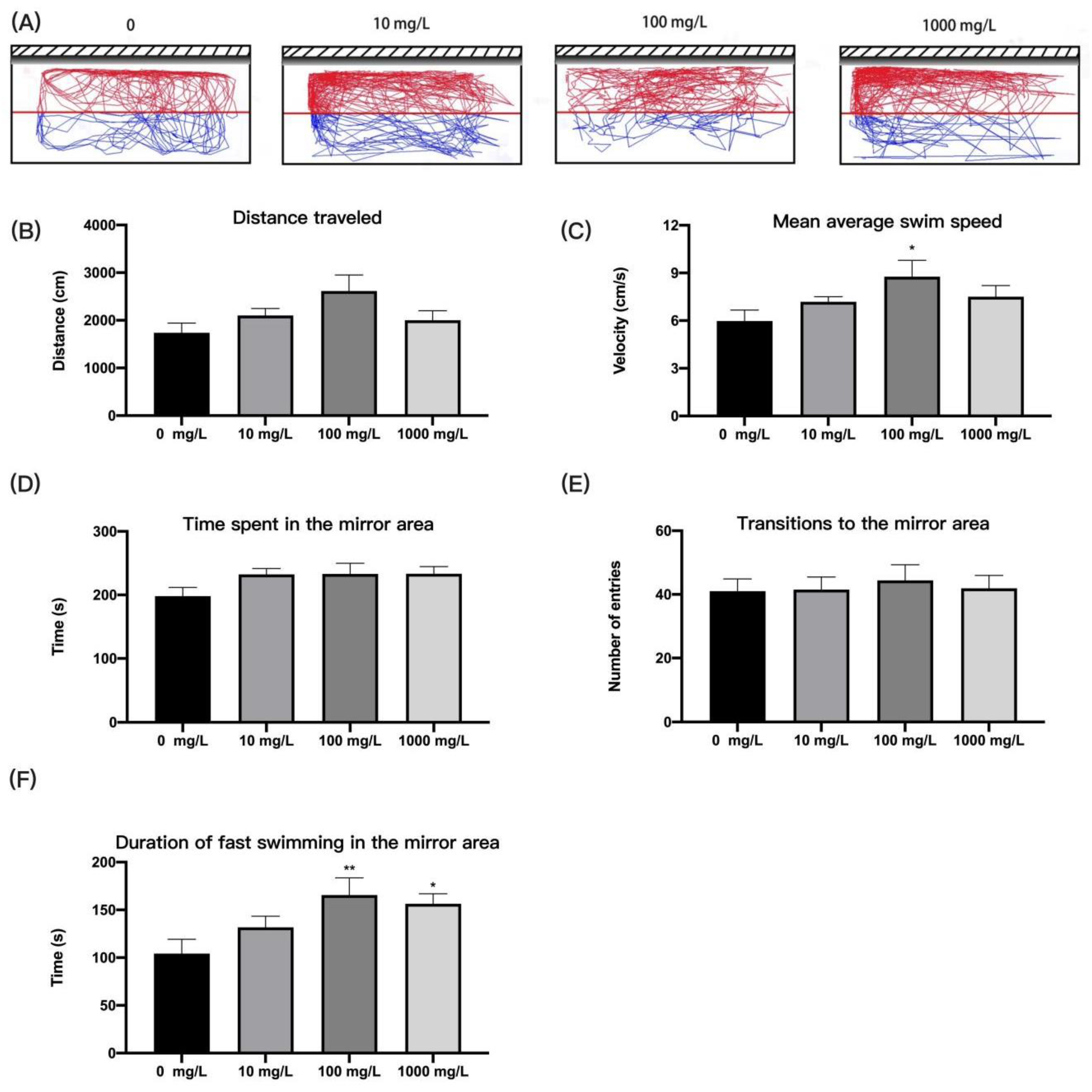
2.4.5. Mirror Attack Test
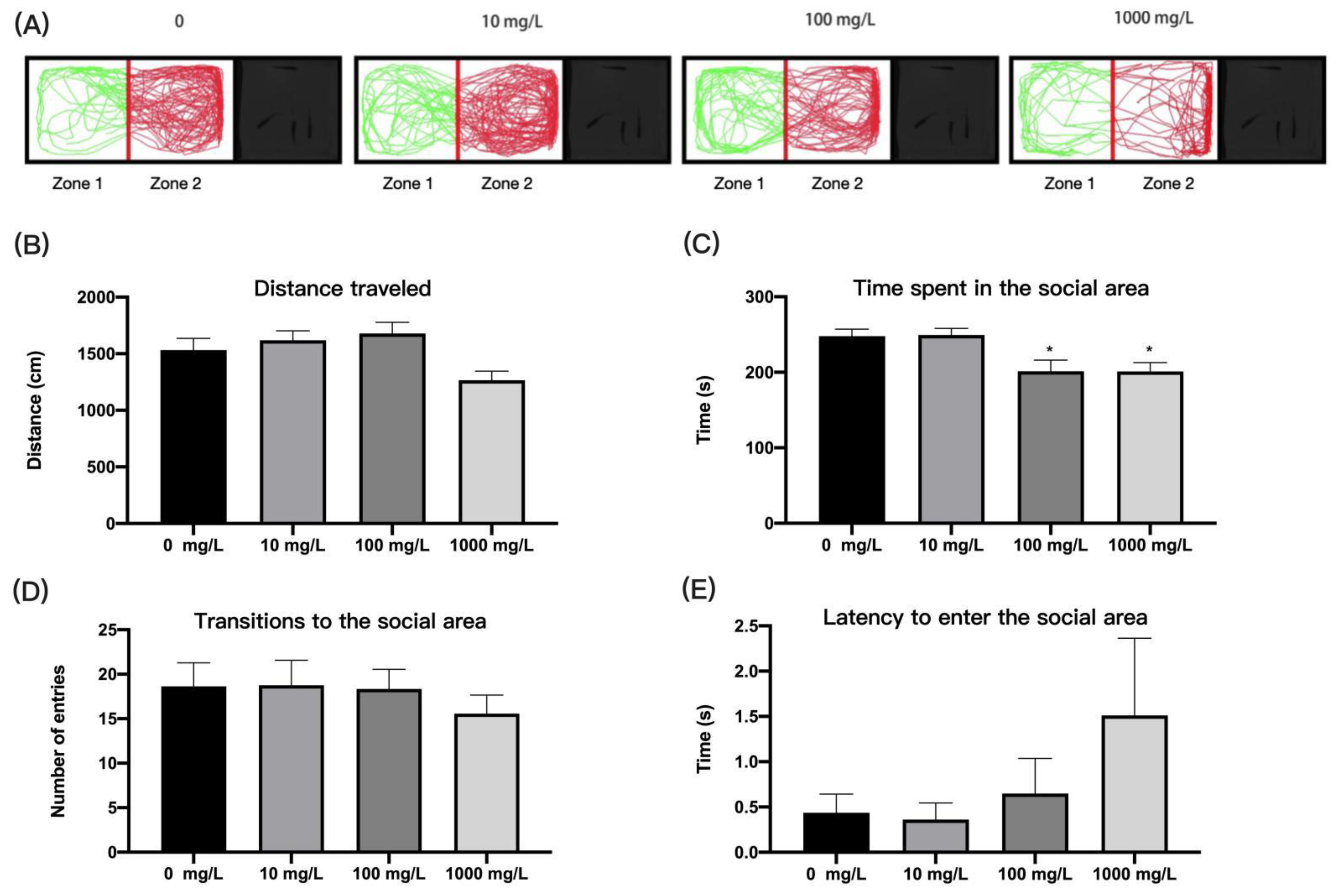
2.4.6. Social Preference Test
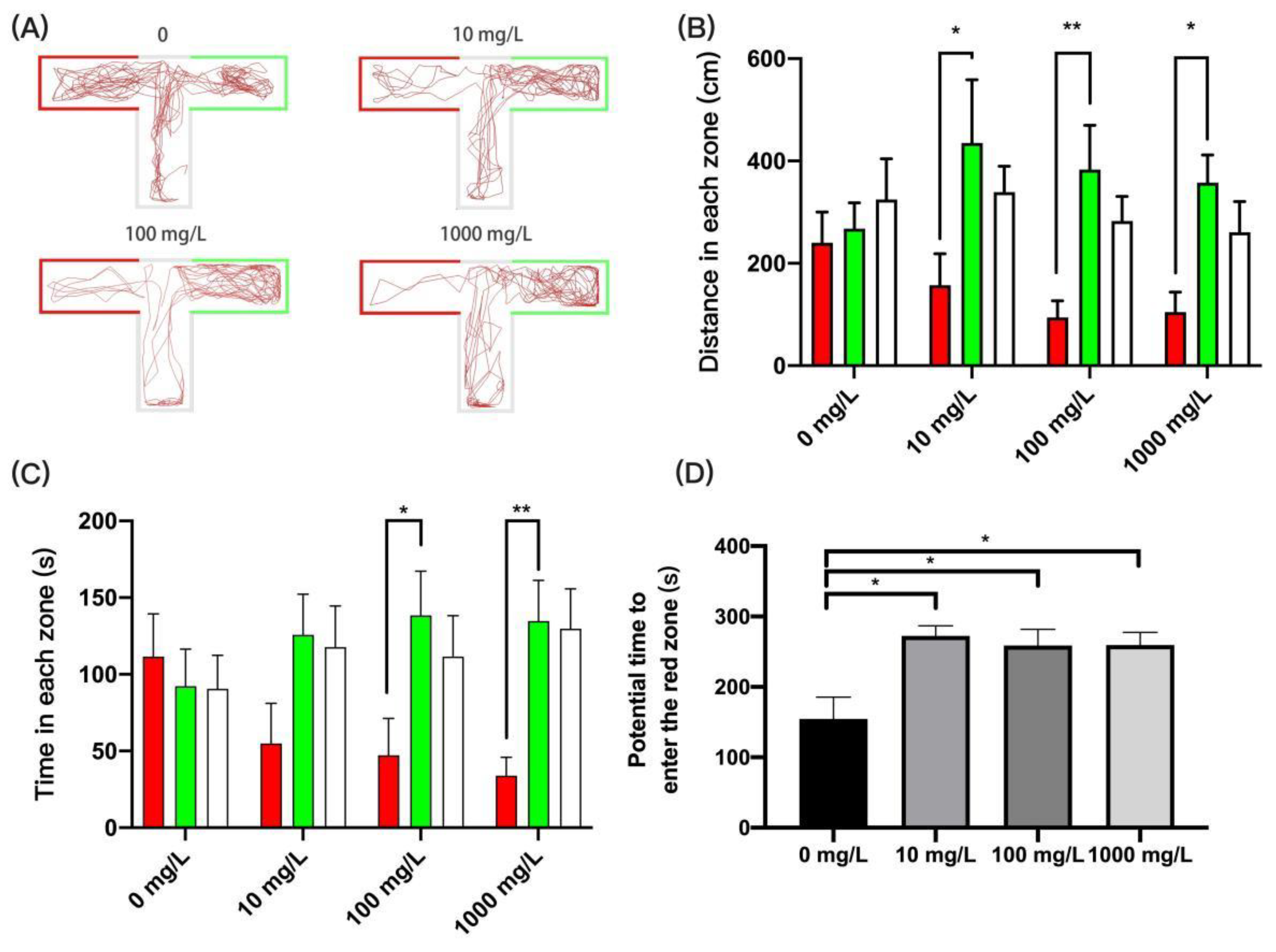
2.4.7. T-Maze Memory Test
2.5. Determining the Neurotransmitters in the Brain
2.6. Statistical Analysis
3. Results
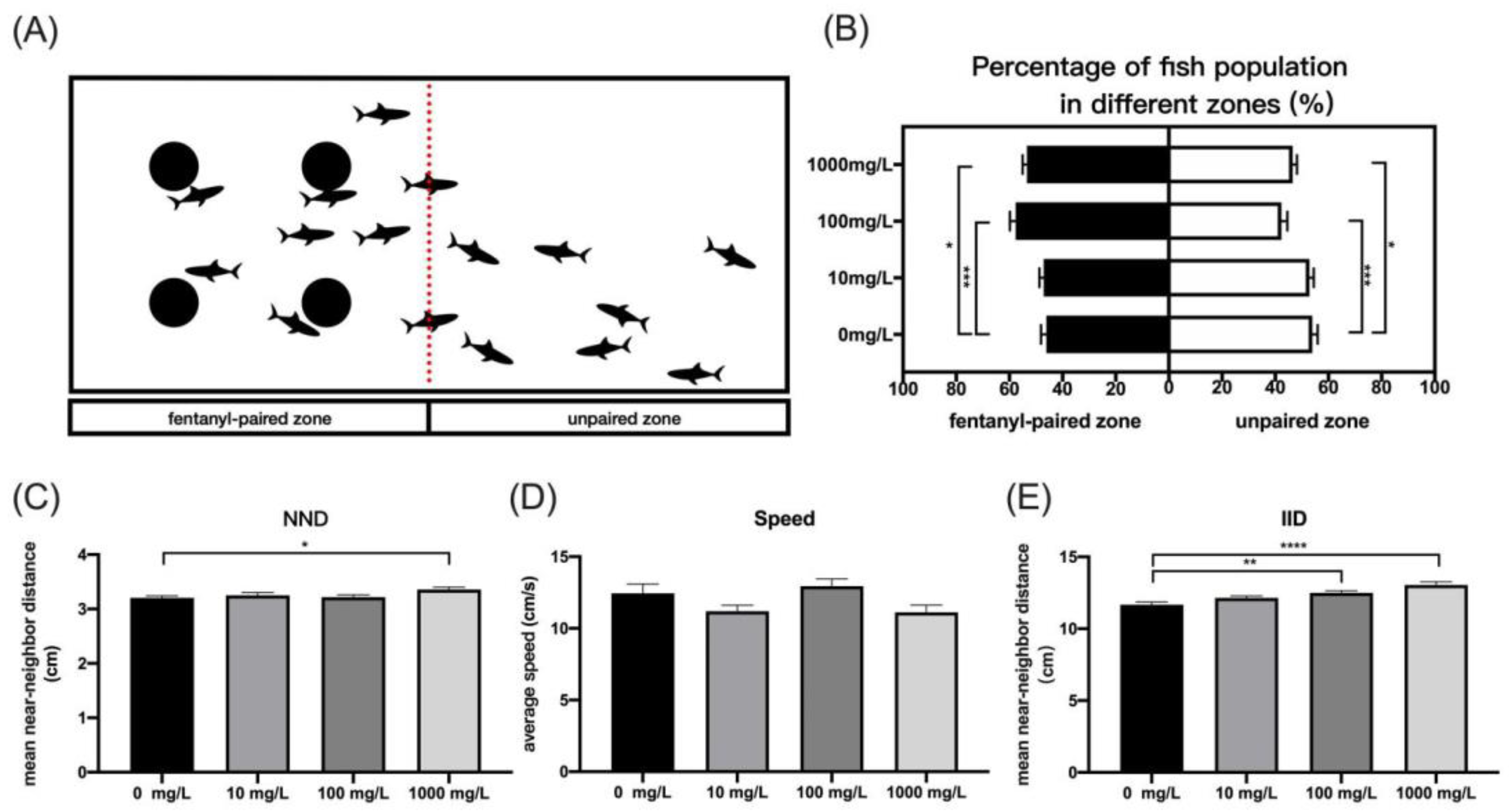
3.1. Fentanyl Induced Conditional Place Preference and Reduced Cohesion in the Shoaling Behavior of Zebrafish
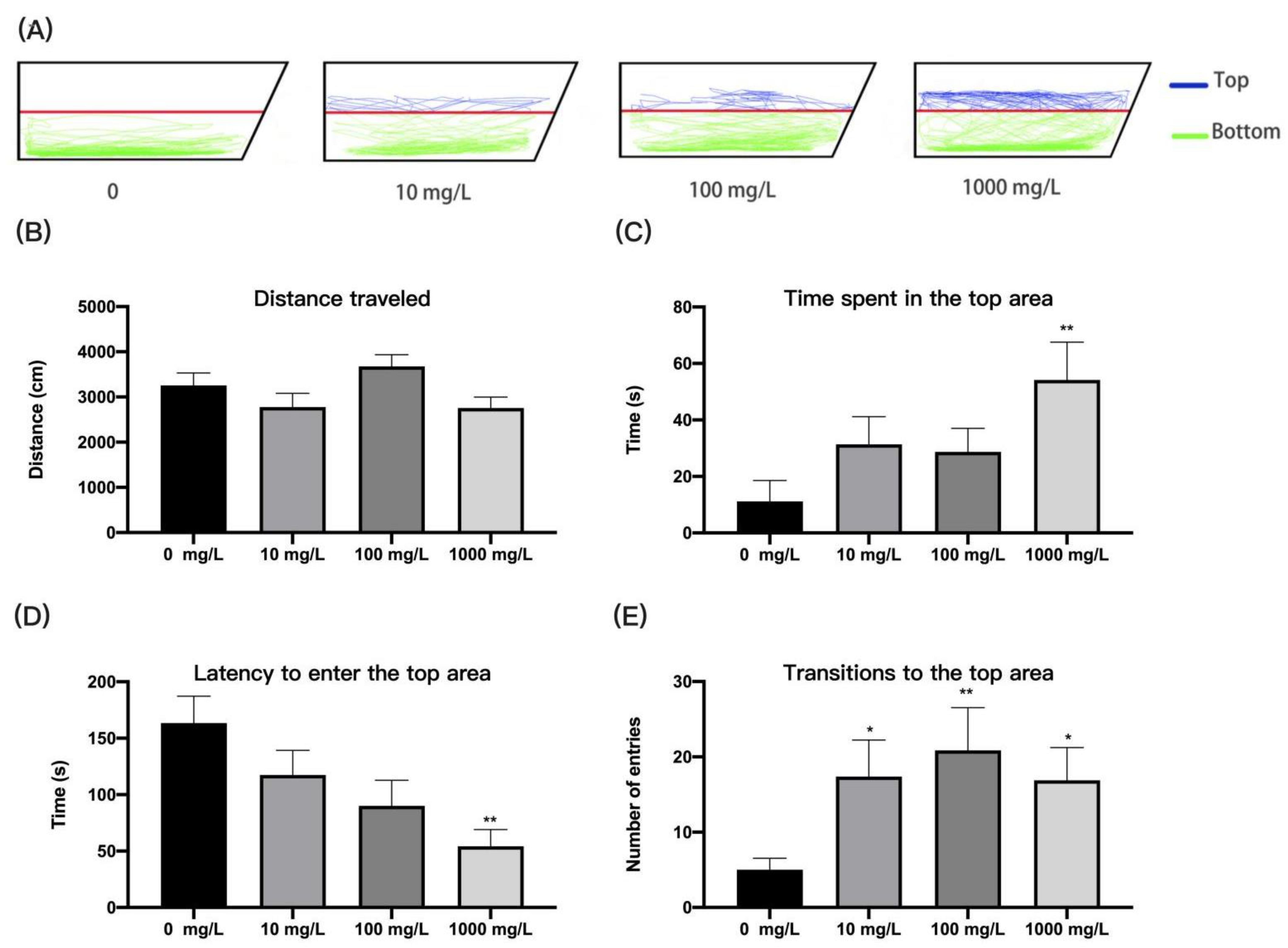
3.2. Fentanyl Reduced Individual Zebrafish Anxiety in a Novel Tank Test
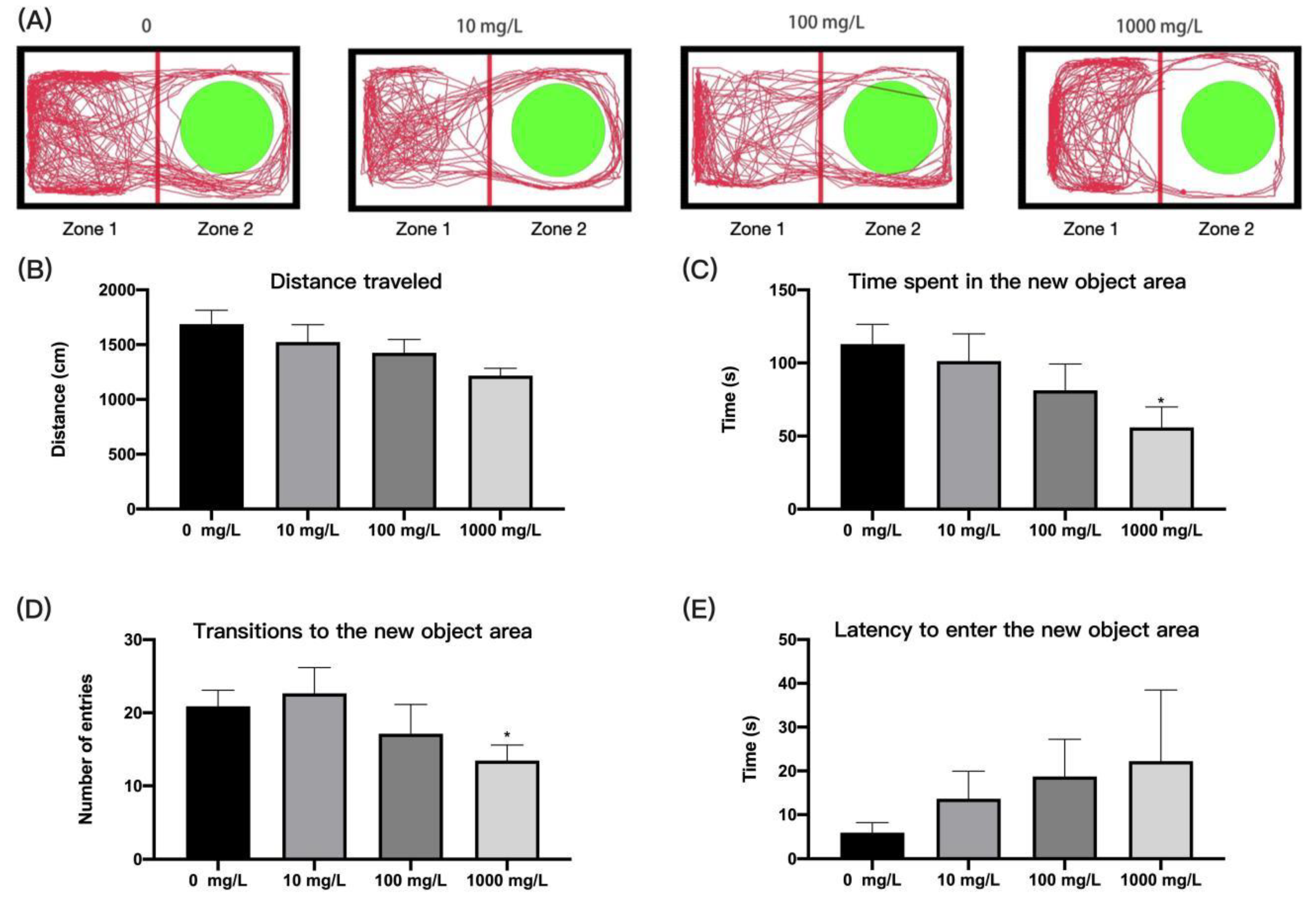
3.3. Fentanyl Reduced Individual Zebrafish Anxiety in Novel Object Exploration Test
3.4. Fentanyl Increased Aggressive Behavior of Individual Zebrafish in Mirror Attack Test
3.5. Fentanyl Reduced the Social Preferences of Individual Zebrafish in Social Preference Test
3.6. Fentanyl Impaired Learning Memory Capacity in the T-Maze Memory Test
3.7. Fentanyl Disturbs Neurotransmitter Stability in Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeo, Y.; Johnson, R.; Heng, C. The Public Health Approach to the Worsening Opioid Crisis in the United States Calls for Harm Reduction Strategies to Mitigate the Harm From Opioid Addiction and Overdose Deaths. Mil. Med. 2021, 187, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Diestelmann, M.; Zangl, A.; Herrle, I.; Koch, E.; Graw, M.; Paul, L.D. MDPV in forensic routine cases: Psychotic and aggressive behavior in relation to plasma concentrations. Forensic Sci. Int. 2018, 283, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Abate, M.A.; Smith, G.S.; Kraner, J.C.; Mock, A.R. Fentanyl and fentanyl-analog involvement in drug-related deaths. Drug Alcohol. Depend. 2019, 196, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rossen, L.M.S.P. Provisional Drug Overdose Death Counts. Available online: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm (accessed on 1 June 2022).
- Cheema, E.; McGuinness, K.; Hadi, M.A.; Paudyal, V.; Elnaem, M.H.; Alhifany, A.A.; Elrggal, M.E.; Al Hamid, A. Causes, Nature and Toxicology of Fentanyl-Associated Deaths: A Systematic Review of Deaths Reported in Peer-Reviewed Literature. J. Pain Res. 2020, 13, 3281–3294. [Google Scholar] [CrossRef]
- Peeters, L.E.J.; Vleut, I.T.; Tan, G.E.; Croes, E.A.; Bethlehem, C. Case report on postmortem fentanyl measurement after overdose with more than 67 fentanyl patches. Forensic Toxicol. 2021, 40, 199–203. [Google Scholar] [CrossRef]
- Vearrier, D.; Grundmann, O. Clinical Pharmacology, Toxicity, and Abuse Potential of Opioids. J. Clin. Pharmacol. 2021, 61 (Suppl. S2), S70–S88. [Google Scholar] [CrossRef]
- Van den Brink, W.; Pierce, M.; van Amsterdam, J. What lessons from Europe’s experience could be applied in the United States in response to the opioid addiction and overdose crisis? Addiction 2022, 117, 1197–1198. [Google Scholar] [CrossRef]
- Barash, J.A.; Ganetsky, M.; Boyle, K.L.; Raman, V.; Toce, M.S.; Kaplan, S.; Lev, M.H.; Worth, J.L.; DeMaria, A., Jr. Acute Amnestic Syndrome Associated with Fentanyl Overdose. N. Engl. J. Med. 2018, 378, 1157–1158. [Google Scholar] [CrossRef]
- Yang, W.; Chini, M.; Popplau, J.A.; Formozov, A.; Dieter, A.; Piechocinski, P.; Rais, C.; Morellini, F.; Sporns, O.; Hanganu-Opatz, I.L.; et al. Anesthetics fragment hippocampal network activity, alter spine dynamics, and affect memory consolidation. PLoS Biol. 2021, 19, e3001146. [Google Scholar] [CrossRef]
- Solis, E., Jr.; Cameron-Burr, K.T.; Shaham, Y.; Kiyatkin, E.A. Fentanyl-Induced Brain Hypoxia Triggers Brain Hyperglycemia and Biphasic Changes in Brain Temperature. Neuropsychopharmacology 2018, 43, 810–819. [Google Scholar] [CrossRef]
- Baehr, C.; Kelcher, A.H.; Khaimraj, A.; Reed, D.E.; Pandit, S.G.; AuCoin, D.; Averick, S.; Pravetoni, M. Monoclonal Antibodies Counteract Opioid-Induced Behavioral and Toxic Effects in Mice and Rats. J. Pharmacol. Exp. Ther. 2020, 375, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, I.E.; Cunningham, K.A. Self-administered fentanyl profoundly impacts rat brain innate immune targets. Neuropsychopharmacology 2021, 46, 247. [Google Scholar] [CrossRef] [PubMed]
- Haouzi, P.; Mellen, N.; McCann, M.; Sternick, M.; Guck, D.; Tubbs, N. Evidence for the emergence of an opioid-resistant respiratory rhythm following fentanyl overdose. Respir. Physiol. Neurobiol. 2020, 277, 103428. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Kumar, D.; Kumar, P.; Gupta, P.K.; Bhattacharya, R. Biochemical, Oxidative, and Physiological Changes Caused by Acute Exposure of Fentanyl and Its 3 Analogs in Rodents. Int. J. Toxicol. 2018, 37, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Ye, F.; Luo, Q.; Tao, Y.; Shu, H. Increased Hyperalgesia and Proinflammatory Cytokines in the Spinal Cord and Dorsal Root Ganglion After Surgery and/or Fentanyl Administration in Rats. Anesth. Analg. 2018, 126, 289–297. [Google Scholar] [CrossRef]
- Muller, T.E.; Fontana, B.D.; Bertoncello, K.T.; Franscescon, F.; Mezzomo, N.J.; Canzian, J.; Stefanello, F.V.; Parker, M.O.; Gerlai, R.; Rosemberg, D.B. Understanding the neurobiological effects of drug abuse: Lessons from zebrafish models. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109873. [Google Scholar] [CrossRef]
- Cooman, T.; Bergeron, S.A.; Coltogirone, R.; Horstick, E.; Arroyo, L. Evaluation of fentanyl toxicity and metabolism using a zebrafish model. J. Appl. Toxicol. 2022, 42, 706–714. [Google Scholar] [CrossRef]
- Kirla, K.T.; Erhart, C.; Groh, K.J.; Stadnicka-Michalak, J.; Eggen, R.I.L.; Schirmer, K.; Kraemer, T. Zebrafish early life stages as alternative model to study ‘designer drugs’: Concordance with mammals in response to opioids. Toxicol. Appl. Pharmacol. 2021, 419, 115483. [Google Scholar] [CrossRef]
- Zaig, S.; da Silveira Scarpellini, C.; Montandon, G. Respiratory depression and analgesia by opioid drugs in freely behaving larval zebrafish. eLife 2021, 10, e63407. [Google Scholar] [CrossRef]
- Pesavento, S.; Bilel, S.; Murari, M.; Gottardo, R.; Arfe, R.; Tirri, M.; Panato, A.; Tagliaro, F.; Marti, M. Zebrafish larvae: A new model to study behavioural effects and metabolism of fentanyl, in comparison to a traditional mice model. Med. Sci. Law 2022, 62, 188–198. [Google Scholar] [CrossRef]
- Miller, N.; Gerlai, R. Quantification of shoaling behaviour in zebrafish (Danio rerio). Behav. Brain Res. 2007, 184, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, T.M. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict. Biol. 2007, 12, 227–462. [Google Scholar] [CrossRef] [PubMed]
- Brock, A.J.; Goody, S.M.G.; Mead, A.N.; Sudwarts, A.; Parker, M.O.; Brennan, C.H. Assessing the Value of the Zebrafish Conditioned Place Preference Model for Predicting Human Abuse Potential. J. Pharmacol. Exp. Ther. 2017, 363, 66–79. [Google Scholar] [CrossRef]
- Maaswinkel, H.; Zhu, L.; Weng, W. Assessing social engagement in heterogeneous groups of zebrafish: A new paradigm for autism-like behavioral responses. PLoS ONE 2013, 8, e75955. [Google Scholar]
- Seguret, A.; Collignon, B.; Cazenille, L.; Chemtob, Y.; Halloy, J. Loose social organisation of AB strain zebrafish groups in a two-patch environment. PLoS ONE 2019, 14, e0206193. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Croft, D.P.; James, R. Social network theory in the behavioural sciences: Potential applications. Behav. Ecol. Sociobiol. 2007, 62, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.Y.; Gerlai, R. Shoaling in zebrafish: What we don’t know. Rev. Neurosci. 2011, 22, 17–25. [Google Scholar] [CrossRef]
- Ponzoni, L.; Braida, D.; Bondiolotti, G.; Sala, M. The Non-Peptide Arginine-Vasopressin v1a Selective Receptor Antagonist, SR49059, Blocks the Rewarding, Prosocial, and Anxiolytic Effects of 3,4-Methylenedioxymethamphetamine and Its Derivatives in Zebra Fish. Front. Psychiatry 2017, 8, 146. [Google Scholar] [CrossRef]
- Braida, D.; Limonta, V.; Pegorini, S.; Zani, A.; Guerini-Rocco, C.; Gori, E.; Sala, M. Hallucinatory and rewarding effect of salvinorin A in zebrafish: Kappa-opioid and CB1-cannabinoid receptor involvement. Psychopharmacology 2007, 190, 441–448. [Google Scholar] [CrossRef]
- Stewart, A.; Riehl, R.; Wong, K.; Green, J.; Cosgrove, J.; Vollmer, K.; Kyzar, E.; Hart, P.; Allain, A.; Cachat, J.; et al. Behavioral effects of MDMA (‘ecstasy’) on adult zebrafish. Behav. Pharmacol. 2011, 22, 275–280. [Google Scholar] [CrossRef]
- Wilson, J.M.; Bunte, R.M.; Carty, A.J. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio). J. Am. Assoc. Lab Anim. Sci. 2009, 48, 785–789. [Google Scholar] [PubMed]
- Bui Thi, N.H.; Nguyen Thi, N.A.; Audira, G.; Siregar, P.; Liang, S.T.; Huang, J.C.; Hsiao, C.D. Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish. Int. J. Mol. Sci. 2020, 21, 1844. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Li, X.; Han, G.; Du, L.; Li, M. Zebrafish neuro-behavioral profiles altered by acesulfame (ACE) within the range of “no observed effect concentrations (NOECs)”. Chemosphere 2020, 243, 125431. [Google Scholar] [CrossRef] [PubMed]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef]
- Zabegalov, K.N.; Kolesnikova, T.O.; Khatsko, S.L.; Volgin, A.D.; Yakovlev, O.A.; Amstislavskaya, T.G.; Friend, A.J.; Bao, W.; Alekseeva, P.A.; Lakstygal, A.M.; et al. Understanding zebrafish aggressive behavior. Behav. Process. 2019, 158, 200–210. [Google Scholar] [CrossRef]
- Chen, J.; Tanguay, R.L.; Simonich, M.; Nie, S.; Zhao, Y.; Li, L.; Bai, C.; Dong, Q.; Huang, C.; Lin, K. TBBPA chronic exposure produces sex-specific neurobehavioral and social interaction changes in adult zebrafish. Neurotoxicol. Teratol. 2016, 56, 9–15. [Google Scholar] [CrossRef]
- Wojnicz, A.; Avendano Ortiz, J.; Casas, A.I.; Freitas, A.E.; López, M.G.; Ruiz-Nuno, A. Simultaneous determination of 8 neurotransmitters and their metabolite levels in rat brain using liquid chromatography in tandem with mass spectrometry: Application to the murine Nrf2 model of depression. Clin. Chim. Acta 2016, 453, 174–181. [Google Scholar] [CrossRef]
- Bosse, G.D.; Peterson, R.T. Development of an opioid self-administration assay to study drug seeking in zebrafish. Behav. Brain Res. 2017, 335, 158–166. [Google Scholar] [CrossRef]
- Lin, Y.; Li, H.; Peng, J.; Li, C.; Zhu, C.; Zhou, Y.; Chen, Z.; Li, J.; Luo, C.; Mo, Z. Decrease of morphine-CPP by sinomenine via mediation of tyrosine hydroxylase, NMDA receptor subunit 2B and opioid receptor in the zebrafish brain. Pak. J. Pharm. Sci. 2021, 34, 1659–1665. [Google Scholar]
- Jiang, M.; Chen, Y.; Li, C.; Peng, Q.; Fang, M.; Liu, W.; Kang, Q.; Lin, Y.; Yung, K.K.; Mo, Z. Inhibiting effects of rhynchophylline on zebrafish methamphetamine dependence are associated with amelioration of neurotransmitters content and down-regulation of TH and NR2B expression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 68, 31–43. [Google Scholar] [CrossRef]
- Clayman, C.L.; Malloy, E.J.; Kearns, D.N.; Connaughton, V.P. Differential behavioral effects of ethanol pre-exposure in male and female zebrafish (Danio rerio). Behav. Brain Res. 2017, 335, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Pisera-Fuster, A.; Rocco, L.; Faillace, M.P.; Bernabeu, R. Sensitization-dependent nicotine place preference in the adult zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, F.V.; Muller, T.E.; Franscescon, F.; Quadros, V.A.; Souza, T.P.; Canzian, J.; Leitemperger, J.; Loro, V.L.; Rosemberg, D.B. Taurine modulates behavioral effects of intermittent ethanol exposure without changing brain monoamine oxidase activity in zebrafish: Attenuation of shoal- and anxiety-like responses, and abolishment of memory acquisition deficit. Pharmacol. Biochem. Behav. 2021, 209, 173256. [Google Scholar] [CrossRef] [PubMed]
- Gemmer, A.; Mirkes, K.; Anneser, L.; Eilers, T.; Kibat, C.; Mathuru, A.; Ryu, S.; Schuman, E. Oxytocin receptors influence the development and maintenance of social behavior in zebrafish (Danio rerio). Sci. Rep. 2022, 12, 4322. [Google Scholar] [CrossRef]
- McAroe, C.L.; Craig, C.M.; Holland, R.A. Shoaling promotes place over response learning but does not facilitate individual learning of that strategy in zebrafish (Danio rerio). BMC Zool. 2017, 2, 2. [Google Scholar] [CrossRef]
- Bretaud, S.; Li, Q.; Lockwood, B.L.; Kobayashi, K.; Lin, E.; Guo, S. A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience 2007, 146, 1109–1116. [Google Scholar] [CrossRef]
- Sbragaglia, V.; Klamser, P.P.; Romanczuk, P.; Arlinghaus, R. Evolutionary Impact of Size-Selective Harvesting on Shoaling Behavior: Individual-Level Mechanisms and Possible Consequences for Natural and Fishing Mortality. Am. Nat. 2022, 199, 480–495. [Google Scholar] [CrossRef]
- Franscescon, F.; Souza, T.P.; Muller, T.E.; Michelotti, P.; Canzian, J.; Stefanello, F.V.; Rosemberg, D.B. Taurine prevents MK-801-induced shoal dispersion and altered cortisol responses in zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110399. [Google Scholar] [CrossRef]
- Saszik, S.M.; Smith, C.M. The impact of stress on social behavior in adult zebrafish (Danio rerio). Behav. Pharmacol. 2018, 29, 53–59. [Google Scholar] [CrossRef]
- Stork, O.; Ji, F.Y.; Kaneko, K.; Stork, S.; Yoshinobu, Y.; Moriya, T.; Shibata, S.; Obata, K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000, 865, 45–58. [Google Scholar] [CrossRef]
- Ricci, L.A.; Grimes, J.M.; Knyshevski, I.; Melloni, R.H. Repeated cocaine exposure during adolescence alters glutamic acid decarboxylase-65 (GAD65) immunoreactivity in hamster brain: Correlation with offensive aggression. Brain Res. 2005, 1035, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Onarheim, T.; Janczak, A.M.; Nordgreen, J. The Effects of Social vs. Individual Housing of Zebrafish on Whole-Body Cortisol and Behavior in Two Tests of Anxiety. Front. Vet. Sci. 2022, 9, 859848. [Google Scholar] [CrossRef] [PubMed]
- Neiffer, D.L.; Stamper, M.A. Fish sedation, analgesia, anesthesia, and euthanasia: Considerations, methods, and types of drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Bessiere, B.; Laboureyras, E.; Ben Boujema, M.; Laulin, J.P.; Simonnet, G. A high-dose of fentanyl induced delayed anxiety-like behavior in rats. Prevention by a NMDA receptor antagonist and nitrous oxide (N(2)O). Pharmacol. Biochem. Behav. 2012, 102, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Alipio, J.B.; Brockett, A.T.; Fox, M.E.; Tennyson, S.S.; deBettencourt, C.A.; El-Metwally, D.; Francis, N.A.; Kanold, P.O.; Lobo, M.K.; Roesch, M.R.; et al. Enduring consequences of perinatal fentanyl exposure in mice. Addict. Biol. 2021, 26, e12895. [Google Scholar] [CrossRef]
- Bruzzone, M.; Gatto, E.; Lucon Xiccato, T.; Dalla Valle, L.; Fontana, C.M.; Meneghetti, G.; Bisazza, A. Measuring recognition memory in zebrafish larvae: Issues and limitations. PeerJ 2020, 8, e8890. [Google Scholar] [CrossRef]
- Sim, H.I.; Kim, D.H.; Kim, M. Cellular messenger molecules mediating addictive drug-induced cognitive impairment: Cannabinoids, ketamine, methamphetamine, and cocaine. Future J. Pharm. Sci. 2022, 8, 19. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Ogg, T.W. Alfentanil and memory function. A comparison with fentanyl for day case termination of pregnancy. Anaesthesia 1985, 40, 537–540. [Google Scholar] [CrossRef]
- Kolodny, A.; Courtwright, D.T.; Hwang, C.S.; Kreiner, P.; Eadie, J.L.; Clark, T.W.; Alexander, G.C. The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Annu. Rev. Public Health 2015, 36, 559–574. [Google Scholar]
- Da, Y.; Luo, S.; Tian, Y. Real-Time Monitoring of Neurotransmitters in the Brain of Living Animals. ACS Appl. Mater. Interfaces, 2022; Epub ahead of print. [Google Scholar] [CrossRef]
- Rogers, R.D. The roles of dopamine and serotonin in decision making: Evidence from pharmacological experiments in humans. Neuropsychopharmacology 2011, 36, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Huang, L.C.; Lin, S.H.; Yang, Y.K. Dopaminergic and glutamatergic biomarkers disruption in addiction and regulation by exercise: A mini review. Biomarkers 2022, 27, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Salahinejad, A.; Jamwal, A.; Chivers, D.P.; Niyogi, S. Chronic Dietary Selenomethionine Exposure Induces Oxidative Stress, Dopaminergic Dysfunction, and Cognitive Impairment in Adult Zebrafish (Danio rerio). Environ. Sci. Technol. 2017, 51, 12879–12888. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Salahinejad, A.; Ferrari, M.C.O.; Niyogi, S.; Chivers, D.P. Dopaminergic dysregulation and impaired associative learning behavior in zebrafish during chronic dietary exposure to selenium. Environ. Pollut. 2018, 237, 174–185. [Google Scholar] [CrossRef]
- Attaran, A.; Salahinejad, A.; Crane, A.L.; Niyogi, S.; Chivers, D.P. Chronic exposure to dietary selenomethionine dysregulates the genes involved in serotonergic neurotransmission and alters social and antipredator behaviours in zebrafish (Danio rerio). Environ. Pollut. 2019, 246, 837–844. [Google Scholar] [CrossRef]
- Faria, M.; Prats, E.; Bellot, M.; Gomez-Canela, C.; Raldua, D. Pharmacological Modulation of Serotonin Levels in Zebrafish Larvae: Lessons for Identifying Environmental Neurotoxicants Targeting the Serotonergic System. Toxics 2021, 9, 118. [Google Scholar] [CrossRef]
- Serra, P.A.; Susini, G.; Rocchitta, G.; Migheli, R.; Dessanti, G.; Miele, E.; Desole, M.S.; Miele, M. Effects of sufentanil on the release and metabolism of dopamine and ascorbic acid and glutamate release in the striatum of freely moving rats. Neurosci. Lett. 2003, 344, 9–12. [Google Scholar] [CrossRef]
- Sustkova-Fiserova, M.; Charalambous, C.; Havlickova, T.; Lapka, M.; Jerabek, P.; Puskina, N.; Syslova, K. Alterations in Rat Accumbens Endocannabinoid and GABA Content during Fentanyl Treatment: The Role of Ghrelin. Int. J. Mol. Sci. 2017, 18, 2486. [Google Scholar] [CrossRef]
- Qureshi, T.; Bjorkmo, M.; Nordengen, K.; Gundersen, V.; Utheim, T.P.; Watne, L.O.; Storm-Mathisen, J.; Hassel, B.; Chaudhry, F.A. Slc38a1 Conveys Astroglia-Derived Glutamine into GABAergic Interneurons for Neurotransmitter GABA Synthesis. Cells 2020, 9, 1686. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, A.; Fu, L.; Liu, M.; Li, K.; Chian, S.; Yao, W.; Wang, B.; Wang, J. Fentanyl Induces Novel Conditioned Place Preference in Adult Zebrafish, Disrupts Neurotransmitter Homeostasis, and Triggers Behavioral Changes. Int. J. Environ. Res. Public Health 2022, 19, 13533. https://doi.org/10.3390/ijerph192013533
Wu Y, Wang A, Fu L, Liu M, Li K, Chian S, Yao W, Wang B, Wang J. Fentanyl Induces Novel Conditioned Place Preference in Adult Zebrafish, Disrupts Neurotransmitter Homeostasis, and Triggers Behavioral Changes. International Journal of Environmental Research and Public Health. 2022; 19(20):13533. https://doi.org/10.3390/ijerph192013533
Chicago/Turabian StyleWu, Yuanzhao, Anli Wang, Lixiang Fu, Meng Liu, Kang Li, Song Chian, Weixuan Yao, Binjie Wang, and Jiye Wang. 2022. "Fentanyl Induces Novel Conditioned Place Preference in Adult Zebrafish, Disrupts Neurotransmitter Homeostasis, and Triggers Behavioral Changes" International Journal of Environmental Research and Public Health 19, no. 20: 13533. https://doi.org/10.3390/ijerph192013533
APA StyleWu, Y., Wang, A., Fu, L., Liu, M., Li, K., Chian, S., Yao, W., Wang, B., & Wang, J. (2022). Fentanyl Induces Novel Conditioned Place Preference in Adult Zebrafish, Disrupts Neurotransmitter Homeostasis, and Triggers Behavioral Changes. International Journal of Environmental Research and Public Health, 19(20), 13533. https://doi.org/10.3390/ijerph192013533




