Preliminary Assessment of a Flexible Multi-Sensor Wearable System Based on Fiber Bragg Gratings for Respiratory Monitoring of Hemiplegic Patients
Abstract
1. Introduction
2. Materials and Methods
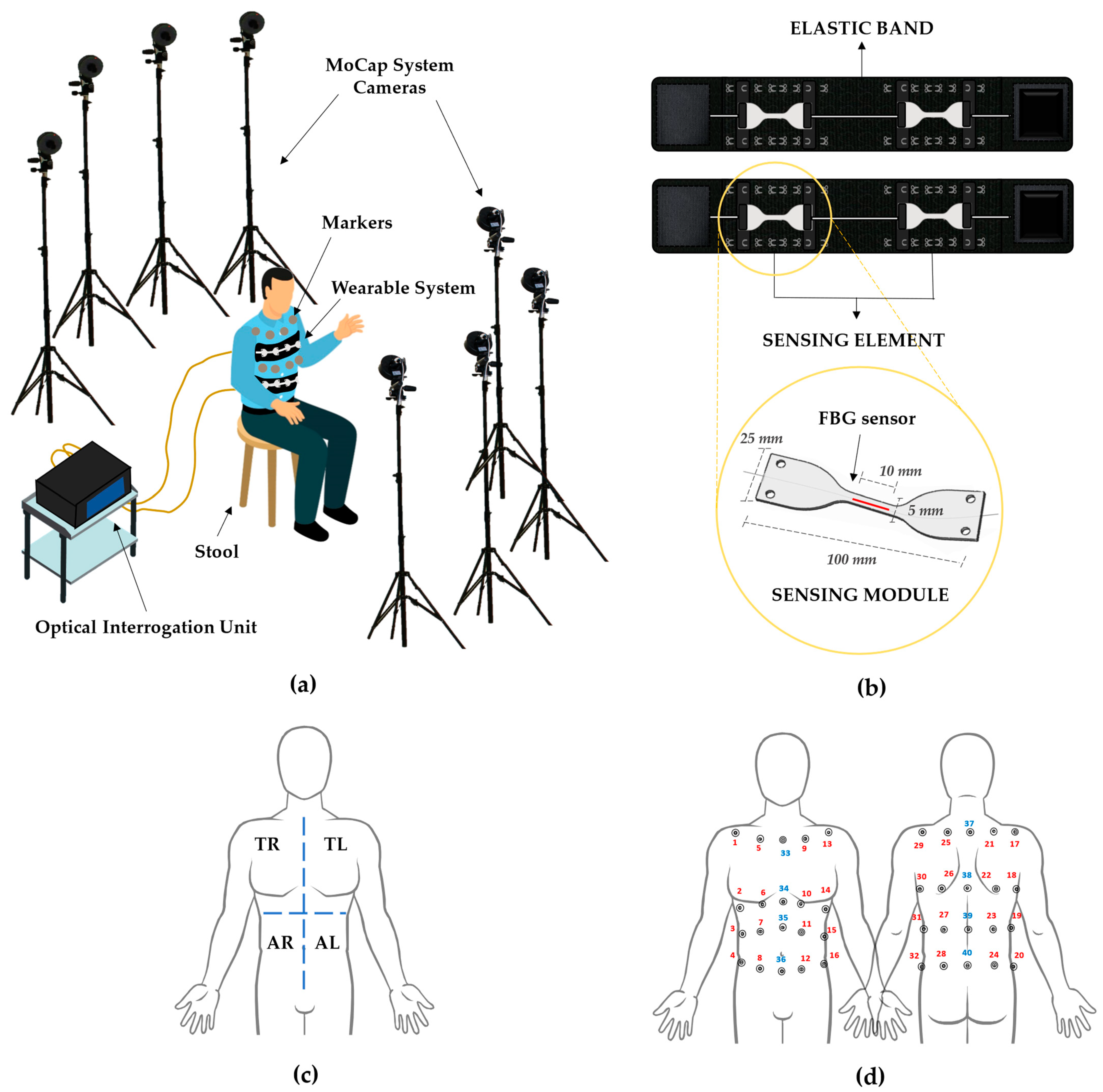
2.1. Experimental Set-Up
- Stool: a stool placed in the center of the dedicated area allows the volunteers to sit.
- Motion Capture System: a stereophotogrammetric MoCap system was exploited as a reference device for chest wall kinematics and respiratory activity. The system (BTS D-Smart, produced by BTS Bio-Engineering S.r.l., Milan, Italy) consists of eight cameras, installed at approximately 2 m from the stool, four forward and four rearward, as shown in Figure 1a. An amount of 1 m3 of calibrated volume was obtained. The trajectories of 40 photo-reflective hemispherical markers with a diameter of 12 mm (applied to the volunteers’ torsos, as detailed in Section 2.2) were collected with a sampling rate of 60 Hz by means of the tracker software provided by BTS (BTS, Bioengineering S.r.l., Milan, Italy);
- Wearable System: the wearable system (represented in Figure 1b, upper image) comes as two stretchable elastic bands to be worn around the chest and abdomen, to each of which a sensing element is attached by means of an anchoring system consisting of hooks to be secured to metallic loops sewed on the elastic bands. Every sensing element is composed of two multiplexed sensing modules (SMs), which come as dumbbell-shaped flexible matrices (see Figure 1b, bottom image) made of a commercial bi-phasic polymer (i.e., DragonSkinTM 20, Smooth-on-Inc.) with an overall length of 100 mm, and a width of 25 mm at the edges and 5 mm in the narrow portion. Each SM encloses in its center a 10-mm-length fiber Bragg grating (FBG) sensor (Broptics Technology Inc., reflectivity >99.5%, polarization-dependent loss <0.15 dB, insertion loss <0.2 dB, and cladding mode loss <0.2 dB). FBGs are resonant structures whose length is typically in the range of millimeters, and they are located in specific sections of the fiber optic via laser beam inscription. These structures reflect to the source a narrow portion of light that is centered around a particular wavelength (i.e., Bragg wavelength—λB), different for each FBG. λB meets the following condition of dependence on the effective refractive index (ηeff) and the grating period (Λ) [38]:λB (ε, ΔT) = 2·ηeff (ε, ΔT) · Λ (ε, ΔT)
- 4.
- Optical Interrogation Unit: an optical interrogation unit (si255, developed by Micron Optics Inc., Atlanta, GA, USA, wavelength range of 1460–1620 nm) was employed to interrogate the FBGs contained in the sensing modules. The interrogation unit supplies the sensors with broadband polarized light and collects the ΔλB values at a 1 kHz sampling rate.
2.2. Population and Experimental Protocol
- 5.
- Trial 1: 5 s of apnea followed by 40 s of eupnea, maintaining the upright sitting position with the hands resting on thighs;
- 6.
- Trial 2: 5 s of apnea followed by 30 s of tachypnea (to the best of each subject’s ability), maintaining the upright sitting position, hands resting on thighs.
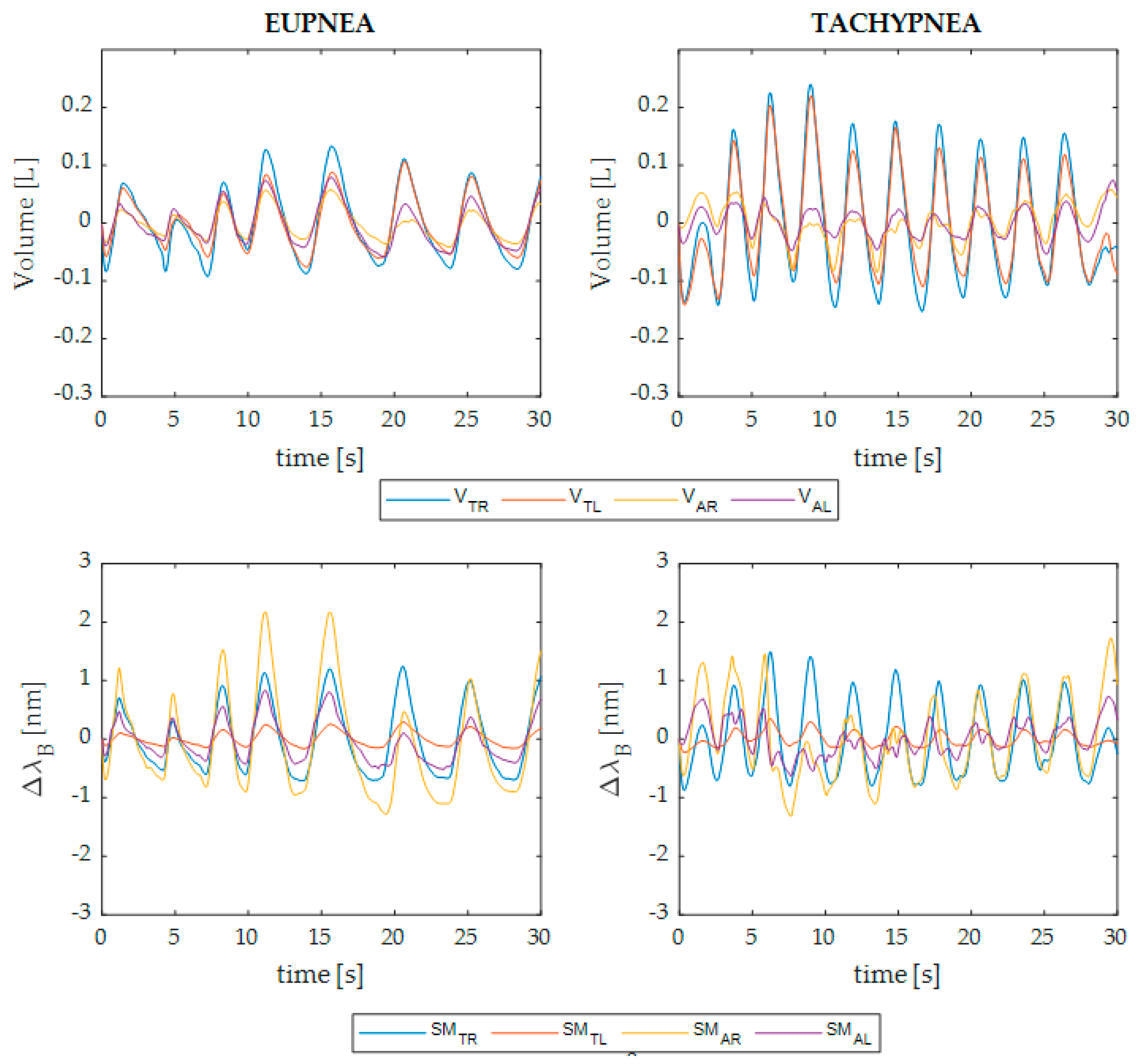
3. Data Analysis and Results
3.1. Preliminary Data Processing
- Thoracic compartment signal: sum of the signals related to the TR and TL (i.e., SMTR + SMTL for the wearable system and VTR + VTL for the MoCap);
- Abdominal compartment signal: sum of the signals related to the AR and AL (i.e., SMAB + SMAB for the wearable system and VAB + VAB for the MoCap);
- Plegic compartment signal: sum of the thoracic and abdominal signals related to the affected side of each patient;
- Non-plegic compartment signal: sum of the thoracic and abdominal signals related to the non-affected side of each patient;
- Summed signal: sum of all four torso areas’ signals (i.e., SMTR + SMTL + SMAB + SMAB for the wearable system and VTOT for the MoCap).
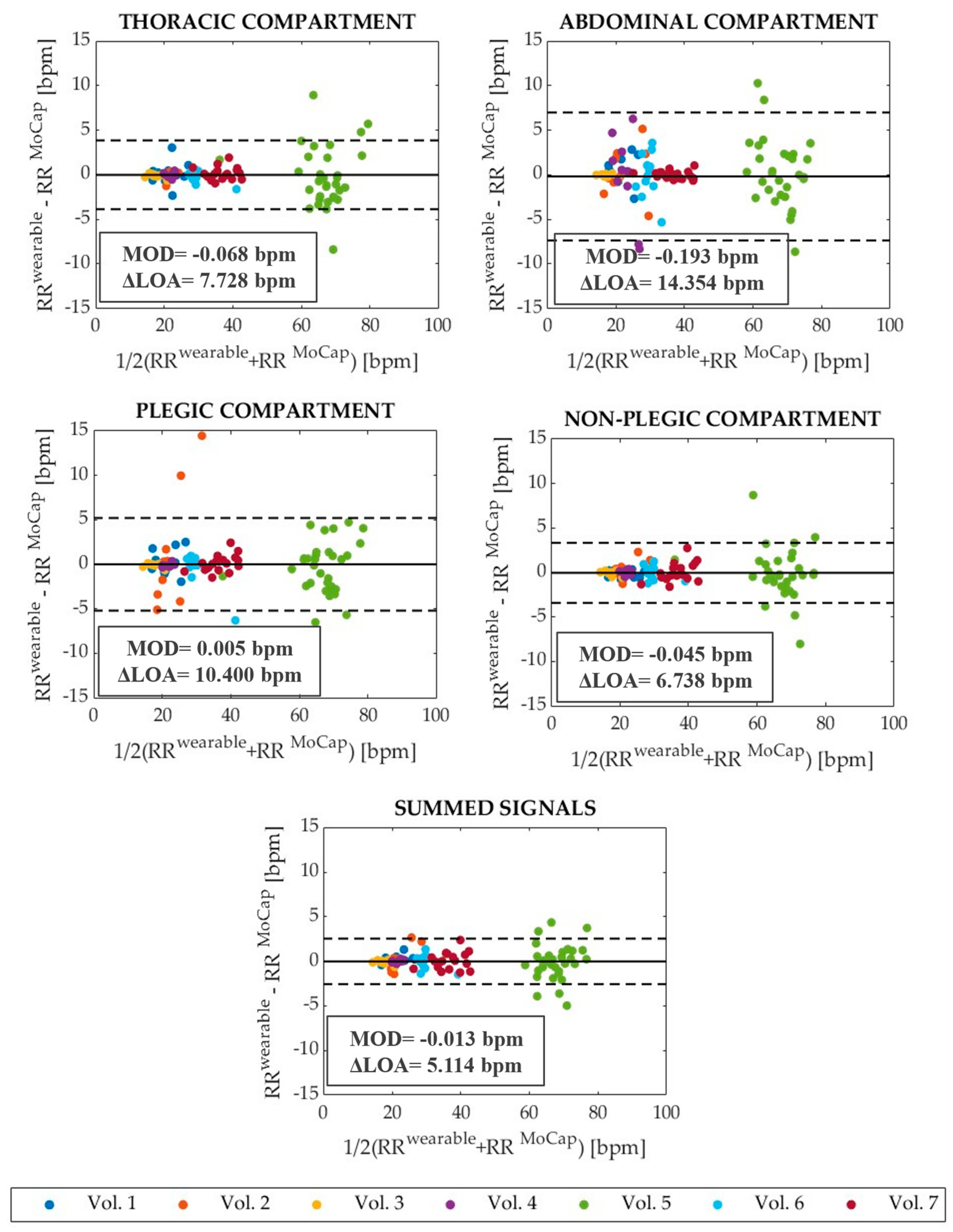
3.2. Assessment of the Wearable System in Respiratory Rate Estimation
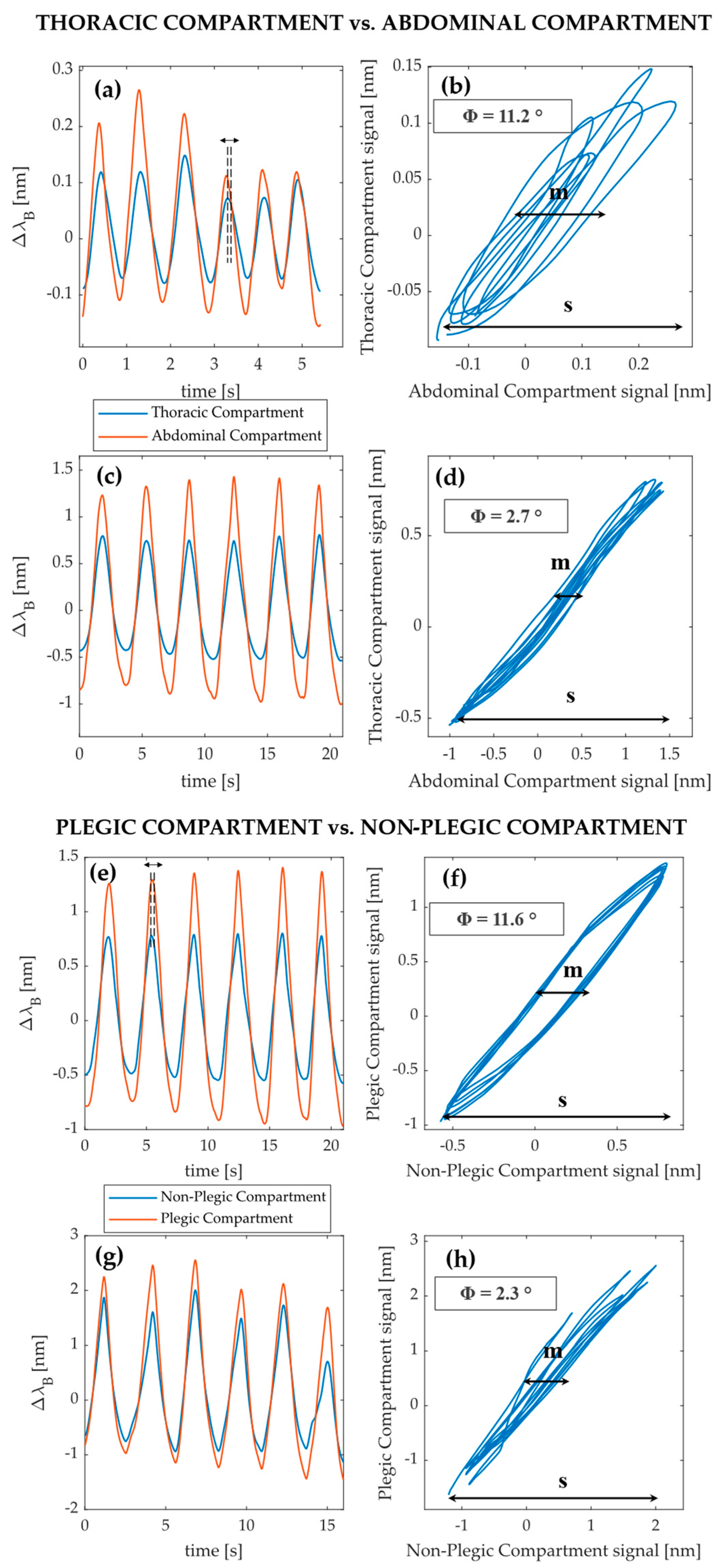
3.3. Explorative Investigation on Respiratory Asynchronies between Compartments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Kim, J.; Thayabaranathan, T.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; Owolabi, M.; Pandian, J. Global stroke statistics 2019. Int. J. Stroke 2020, 15, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Annoni, J.-M.; Ackermann, D.; Kesselring, J. Respiratory function in chronic hemiplegia. Int. Disabil. Stud. 1990, 12, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Lanini, B.; Bianchi, R.; Romagnoli, I.; Coli, C.; Binazzi, B.; Gigliotti, F.; Pizzi, A.; Grippo, A.; Scano, G. Chest wall kinematics in patients with hemiplegia. Am. J. Respir. Crit. Care Med. 2003, 168, 109–113. [Google Scholar] [CrossRef]
- Fluck, D.C. Chest movements in hemiplegia. Clin. Sci. 1966, 31, 383–388. [Google Scholar] [PubMed]
- Cohen, E.; Mier, A.; Heywood, P.; Murphy, K.; Boultbee, J.; Guz, A. Diaphragmatic movement in hemiplegic patients measured by ultrasonography. Thorax 1994, 49, 890–895. [Google Scholar] [CrossRef]
- Teixeira-Salmela, L.F.; Parreira, V.F.; Britto, R.R.; Brant, T.C.; Inácio, É.P.; Alcântara, T.O.; Carvalho, I.F. Respiratory pressures and thoracoabdominal motion in community-dwelling chronic stroke survivors. Arch. Phys. Med. Rehabil. 2005, 86, 1974–1978. [Google Scholar] [CrossRef]
- Prisk, G.K.; Hammer, J.; Newth, C.J.L. Techniques for measurement of thoracoabdominal asynchrony. Pediatr. Pulmonol. 2002, 34, 462–472. [Google Scholar] [CrossRef]
- Hammer, J.; Newth, C.J.L. Assessment of thoraco-abdominal asynchrony. Paediatr. Respir. Rev. 2009, 10, 75–80. [Google Scholar] [CrossRef]
- Aliverti, A.; Quaranta, M.; Chakrabarti, B.; Albuquerque, A.L.P.; Calverley, P.M. Paradoxical movement of the lower ribcage at rest and during exercise in COPD patients. Eur. Respir. J. 2009, 33, 49–60. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Grimby, G. Respiration in tetraplegia and in hemiplegia: A review. Int. Rehabil. Med. 1984, 6, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Florêncio, R.B.; da Nobrega, A.J.S.; Lima, Í.N.D.F.; Gualdi, L.P.; Cabral, E.E.; Fagundes, M.L.L.C.; Aliverti, A.; Resqueti, V.R.; de Fregonezi, G.A.F. Chest wall volume and asynchrony in stroke and Parkinson’s disease subjects: A case-control study. PLoS ONE 2019, 14, e0216641. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Linderholm, H.; Wilson, A.F. Restrictive ventilatory dysfunction in stroke: Its relation to locomotor function. Scand. J. Rehabil. Med. Suppl. 1983, 9, 118–124. [Google Scholar] [PubMed]
- Kim, B.R.; Chun, M.H.; Kang, S.H. Change of Respiratory Function following Rehabilitation in Acute Hemiplegic Stroke Patients. J. Korean Acad. Rehabil. Med. 2009, 33, 21–28. [Google Scholar]
- Tipton, M.J.; Harper, A.; Paton, J.F.R.; Costello, J.T. The human ventilatory response to stress: Rate or depth? J. Physiol. 2017, 595, 5729–5752. [Google Scholar] [CrossRef]
- Massaroni, C.; Nicolò, A.; Sacchetti, M.; Schena, E. Contactless Methods For Measuring Respiratory Rate: A Review. IEEE Sens. J. 2020, 21, 12821–12839. [Google Scholar] [CrossRef]
- Henderson, A.M.; Mosse, C.A.; Armstrong, R.F. Long-term flow measurement: An assessment of the Siemens Servo 900B expiratory flowmeter. J. Med. Eng. Technol. 1983, 7, 144–146. [Google Scholar] [CrossRef]
- Johnstone, J.A.; Ford, P.A.; Hughes, G.; Watson, T.; Garrett, A.T. Bioharness multivariable monitoring device: Part. I: Validity. J. Sport. Sci. Med. 2012, 11, 400–408. [Google Scholar]
- Massaroni, C.; Di Tocco, J.; Bravi, M.; Carnevale, A.; Presti, D.L.; Sabbadini, R.; Miccinilli, S.; Sterzi, S.; Formica, D.; Schena, E. Respiratory Monitoring During Physical Activities With a Multi-Sensor Smart Garment and Related Algorithms. IEEE Sens. J. 2019, 20, 2173–2180. [Google Scholar] [CrossRef]
- Molinaro, N.; Massaroni, C.; Presti, D.L.; Saccomandi, P.; Di Tomaso, G.; Zollo, L.; Perego, P.; Andreoni, G.; Schena, E. Wearable textile based on silver plated knitted sensor for respiratory rate monitoring. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2865–2868. [Google Scholar]
- Jang, S.; Choi, J.Y.; Yoo, E.S.; Lim, D.Y.; Lee, J.Y.; Kim, J.K.; Pang, C. Printable wet-resistive textile strain sensors using bead-blended composite ink for robustly integrative wearable electronics. Compos. Part B Eng. 2021, 210, 108674. [Google Scholar] [CrossRef]
- Zhong, J.; Li, C.; Zhu, W.; Zhou, H.; Liu, Y.; Han, X. Wearable respiratory strain monitoring system based on textile-based capacitive strain sensor. J. Phys. Conf. Ser. 2020, 1570, 12033. [Google Scholar]
- Naranjo-Hernández, D.; Talaminos-Barroso, A.; Reina-Tosina, J.; Roa, L.M.; Barbarov-Rostan, G.; Cejudo-Ramos, P.; Márquez-Martín, E.; Ortega-Ruiz, F. Smart vest for respiratory rate monitoring of COPD patients based on non-contact capacitive sensing. Sensors 2018, 18, 2144. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, A.; Marioli, D.; Sardini, E.; Serpelloni, M. Autonomous wearable system for vital signs measurement with energy-harvesting module. IEEE Trans. Instrum. Meas. 2016, 65, 1423–1434. [Google Scholar] [CrossRef]
- Teichmann, D.; Kuhn, A.; Leonhardt, S.; Walter, M. The MAIN shirt: A textile-integrated magnetic induction sensor array. Sensors 2014, 14, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Presti, D.L.; Massaroni, C.; D’Abbraccio, J.; Massari, L.; Caponero, M.; Longo, U.G.; Formica, D.; Oddo, C.; Schena, E. Wearable system based on flexible FBG for respiratory and cardiac monitoring. IEEE Sens. J. 2019, 19, 7391–7398. [Google Scholar] [CrossRef]
- Lo Presti, D.; Romano, C.; Massaroni, C.; D’Abbraccio, J.; Massari, L.; Caponero, M.A.; Oddo, C.M.; Formica, D.; Schena, E. Cardio-Respiratory Monitoring in Archery Using a Smart Textile Based on Flexible Fiber Bragg Grating Sensors. Sensors 2019, 19, 3581. [Google Scholar] [CrossRef] [PubMed]
- Presti, D.L.; Massaroni, C.; Formica, D.; Saccomandi, P.; Giurazza, F.; Caponero, M.A.; Schena, E. Smart textile based on 12 fiber Bragg gratings array for vital signs monitoring. IEEE Sens. J. 2017, 17, 6037–6043. [Google Scholar] [CrossRef]
- Zhang, C.; Miao, C.; Li, H.; Song, H.; Xu, F. Smart textile sensing system for human respiration monitoring based on fiber Bragg grating. In Proceedings of the International Symposium on Photoelectronic Detection and Imaging 2009: Material and Device Technology for Sensors; SPIE: Bellingham, WA, USA, 2009; Volume 7381, pp. 35–41. [Google Scholar]
- Silva, A.F.; Carmo, J.P.; Mendes, P.M.; Correia, J.H. Simultaneous cardiac and respiratory frequency measurement based on a single fiber Bragg grating sensor. Meas. Sci. Technol. 2011, 22, 75801. [Google Scholar] [CrossRef]
- Dziuda, Ł.; Krej, M.; Skibniewski, F.W. Fiber Bragg grating strain sensor incorporated to monitor patient vital signs during MRI. IEEE Sens. J. 2013, 13, 4986–4991. [Google Scholar] [CrossRef]
- Ciocchetti, M.; Massaroni, C.; Saccomandi, P.; Caponero, M.A.; Polimadei, A.; Formica, D.; Schena, E. Smart textile based on fiber bragg grating sensors for respiratory monitoring: Design and preliminary trials. Biosensors 2015, 5, 602–615. [Google Scholar] [CrossRef]
- Tavares, C.; Leitão, C.; Presti, D.L.; Domingues, M.F.; Alberto, N.; Silva, H.; Antunes, P. Respiratory and heart rate monitoring using an FBG 3D-printed wearable system. Biomed. Opt. Express 2022, 13, 2299–2311. [Google Scholar] [CrossRef]
- Massaroni, C.; Nicolò, A.; Lo Presti, D.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-based methods for measuring respiratory rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef] [PubMed]
- Massaroni, C.; Zaltieri, M.; Presti, L.; Nicolò, A.; Tosi, D.; Schena, E. Fiber Bragg grating sensors for cardiorespiratory monitoring: A review. IEEE Sens. J. 2020, 21, 14069–14080. [Google Scholar] [CrossRef]
- Presti, D.L.; Massaroni, C.; Leitão, C.S.J.; Domingues, M.D.F.; Sypabekova, M.; Barrera, D.; Floris, I.; Massari, L.; Oddo, C.M.; Sales, S. Fiber Bragg Gratings for medical applications and future challenges: A review. IEEE Access 2020, 8, 156863–156888. [Google Scholar] [CrossRef]
- Di Tocco, J.; Presti, D.L.; Zaltieri, M.; D’Alesio, G.; Filosa, M.; Massari, L.; Aliperta, A.; Di Rienzo, M.; Carrozza, M.C.; Ferrarin, M. A wearable system based on flexible sensors for unobtrusive respiratory monitoring in occupational settings. IEEE Sens. J. 2020, 21, 14369–14378. [Google Scholar] [CrossRef]
- Erdogan, T. Fiber grating spectra. J. Light. Technol. 1997, 15, 1277–1294. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Ferrigno, G.; Carnevali, P.; Aliverti, A.; Molteni, F.; Beulcke, G.; Pedotti, A. Three-dimensional optical analysis of chest wall motion. J. Appl. Physiol. 1994, 77, 1224–1231. [Google Scholar] [CrossRef]
- Massaroni, C.; Silvatti, A.P.; Levai, I.K.; Dickinson, J.; Winter, S.; Schena, E.; Silvestri, S. Comparison of marker models for the analysis of the volume variation and thoracoabdominal motion pattern in untrained and trained participants. J. Biomech. 2018, 76, 247–252. [Google Scholar] [CrossRef]
- Massaroni, C.; Cassetta, E.; Silvestri, S. A novel method to compute breathing volumes via motion capture systems: Design and experimental trials. J. Appl. Biomech. 2017, 33, 361–365. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]

| # Volunteer | Age [y.o.] | Sex | Affected Side | BMI [kg·m−2] | UE-FMA 1 |
|---|---|---|---|---|---|
| 1 | 73 | Male | Left | 34.6 | 37 |
| 2 | 62 | Male | Left | 26.7 | 32 |
| 3 | 46 | Female | Right | 19.3 | 33 |
| 4 | 64 | Male | Right | 32.7 | 43 |
| 5 | 33 | Female | Left | 19.1 | 55 |
| 6 | 55 | Male | Right | 17.4 | 50 |
| 7 | 43 | Male | Left | 27.6 | 34 |
| Trial 1—EUPNEA | |||||
| # Volunteer | MAPERR [%] Thoracic | MAPERR [%] Abdominal | MAPERR [%] Plegic | MAPERR [%] Non-Plegic | MAPERR [%] Summed |
| 1 | 1.51 | 1.93 | 0.86 | 2.57 | 1.60 |
| 2 | 1.78 | 0.72 | 1.91 | 1.28 | 1.15 |
| 3 | 1.47 | 0.78 | 0.62 | 2.79 | 1.08 |
| 4 | 0.69 | 0.69 | 0.79 | 0.37 | 0.52 |
| 5 | 2.03 | 1.58 | 1.87 | 1.11 | 1.08 |
| 6 | 0.73 | 3.45 | 1.46 | 1.35 | 1.34 |
| 7 | 1.07 | 2.66 | 6.09 | 5.23 | 1.52 |
| All | 1.32 | 1.77 | 1.81 | 2.08 | 1.22 |
| Trial 2—TACHYPNEA | |||||
| # Volunteer | MAPERR [%] Thoracic | MAPERR [%] Abdominal | MAPERR [%] Plegic | MAPERR [%] Non-Plegic | MAPERR [%] Summed |
| 1 | 4.25 | 5.76 | 5.68 | 1.91 | 2.06 |
| 2 | 1.31 | 8.63 | 18.55 | 3.33 | 4.41 |
| 3 | 1.08 | 0.89 | 0.87 | 1.13 | 1.00 |
| 4 | 0.97 | 14.36 | 1.05 | 1.04 | 0.52 |
| 5 | 3.93 | 5.60 | 3.38 | 2.97 | 2.18 |
| 6 | 1.70 | 5.46 | 2.44 | 2.17 | 1.94 |
| 7 | 1.39 | 0.97 | 1.87 | 2.22 | 1.94 |
| All | 2.45 | 5.61 | 4.30 | 2.33 | 2.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaltieri, M.; Massaroni, C.; Di Tocco, J.; Bravi, M.; Morrone, M.; Sterzi, S.; Caponero, M.A.; Schena, E.; Lo Presti, D. Preliminary Assessment of a Flexible Multi-Sensor Wearable System Based on Fiber Bragg Gratings for Respiratory Monitoring of Hemiplegic Patients. Int. J. Environ. Res. Public Health 2022, 19, 13525. https://doi.org/10.3390/ijerph192013525
Zaltieri M, Massaroni C, Di Tocco J, Bravi M, Morrone M, Sterzi S, Caponero MA, Schena E, Lo Presti D. Preliminary Assessment of a Flexible Multi-Sensor Wearable System Based on Fiber Bragg Gratings for Respiratory Monitoring of Hemiplegic Patients. International Journal of Environmental Research and Public Health. 2022; 19(20):13525. https://doi.org/10.3390/ijerph192013525
Chicago/Turabian StyleZaltieri, Martina, Carlo Massaroni, Joshua Di Tocco, Marco Bravi, Michelangelo Morrone, Silvia Sterzi, Michele Arturo Caponero, Emiliano Schena, and Daniela Lo Presti. 2022. "Preliminary Assessment of a Flexible Multi-Sensor Wearable System Based on Fiber Bragg Gratings for Respiratory Monitoring of Hemiplegic Patients" International Journal of Environmental Research and Public Health 19, no. 20: 13525. https://doi.org/10.3390/ijerph192013525
APA StyleZaltieri, M., Massaroni, C., Di Tocco, J., Bravi, M., Morrone, M., Sterzi, S., Caponero, M. A., Schena, E., & Lo Presti, D. (2022). Preliminary Assessment of a Flexible Multi-Sensor Wearable System Based on Fiber Bragg Gratings for Respiratory Monitoring of Hemiplegic Patients. International Journal of Environmental Research and Public Health, 19(20), 13525. https://doi.org/10.3390/ijerph192013525












