Fabrication of PbO2 Electrodes with Different Doses of Er Doping for Sulfonamides Degradation
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Electrode Preparation
2.3. Electrode Characterization
2.4. Electrocatalytic Degradation of SMR
2.5. Analytical and Calculation Methods
3. Results and Discussion
3.1. Characteristics of Er-PbO2 Anodes
3.1.1. Life Comparison between Titanium Nanotubes and Titanium Plates
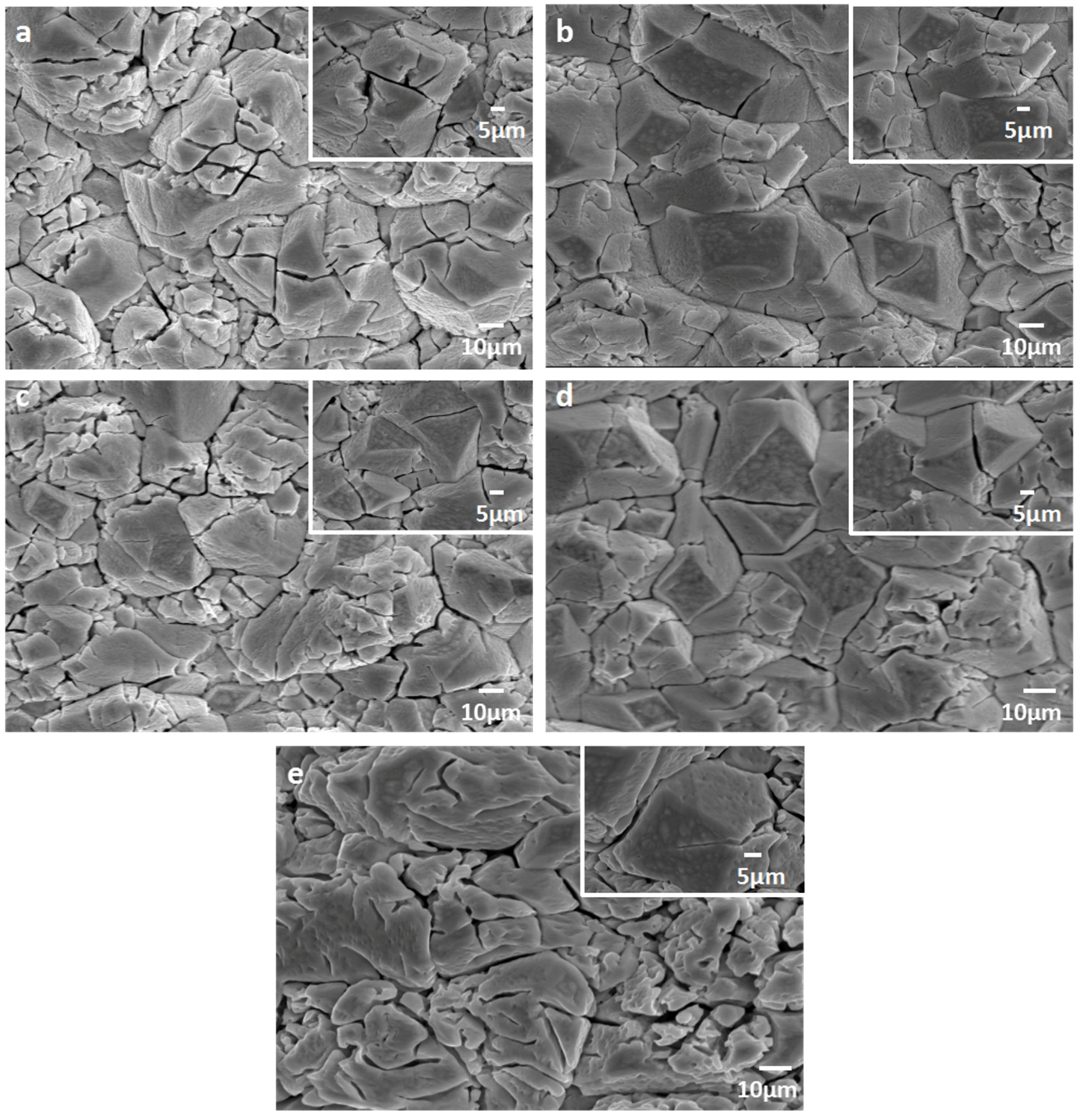
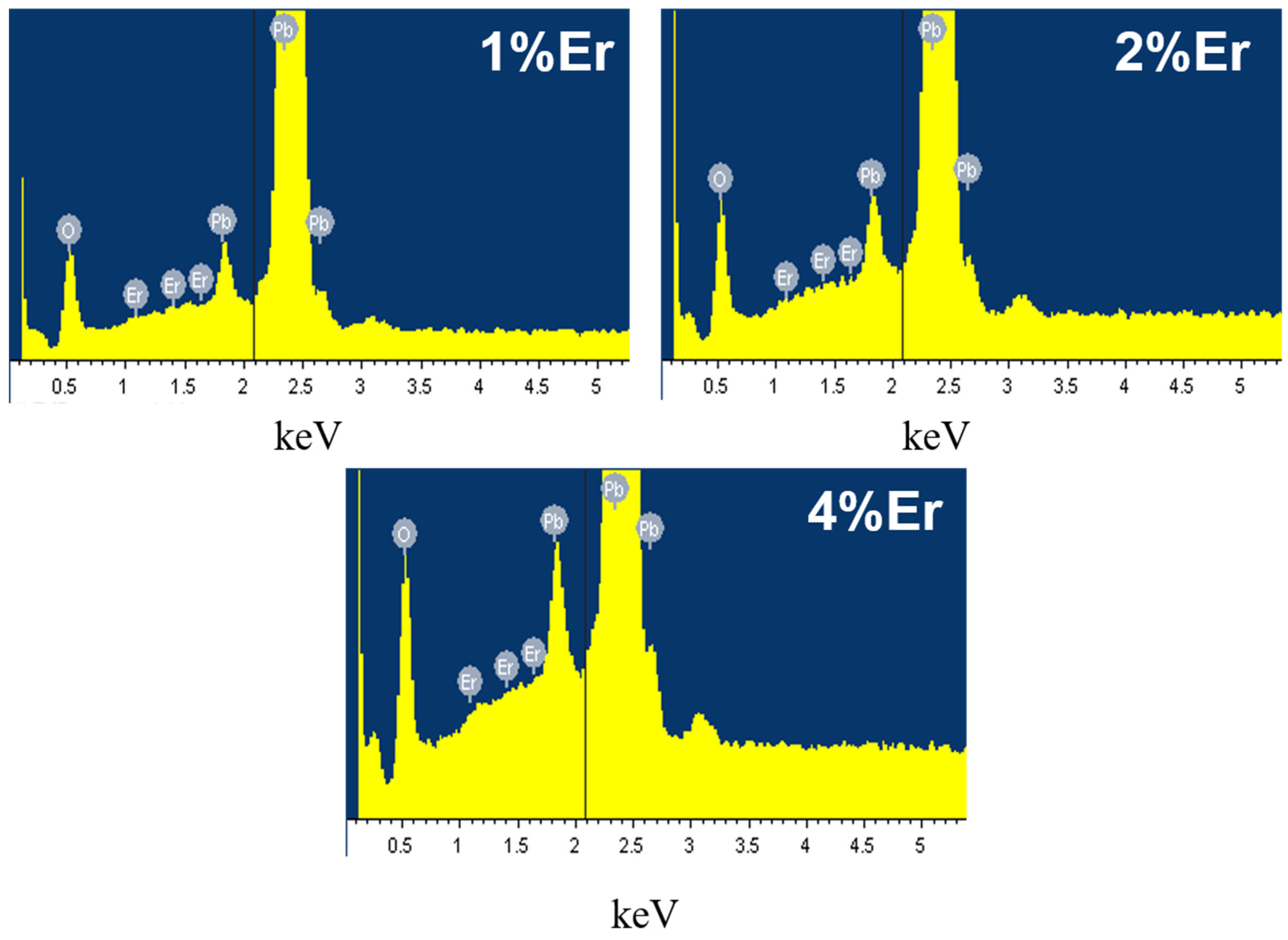
3.1.2. SEM-EDS Analysis of Er-PbO2 Anodes
3.1.3. XRD Analysis of Er-PbO2 Anodes
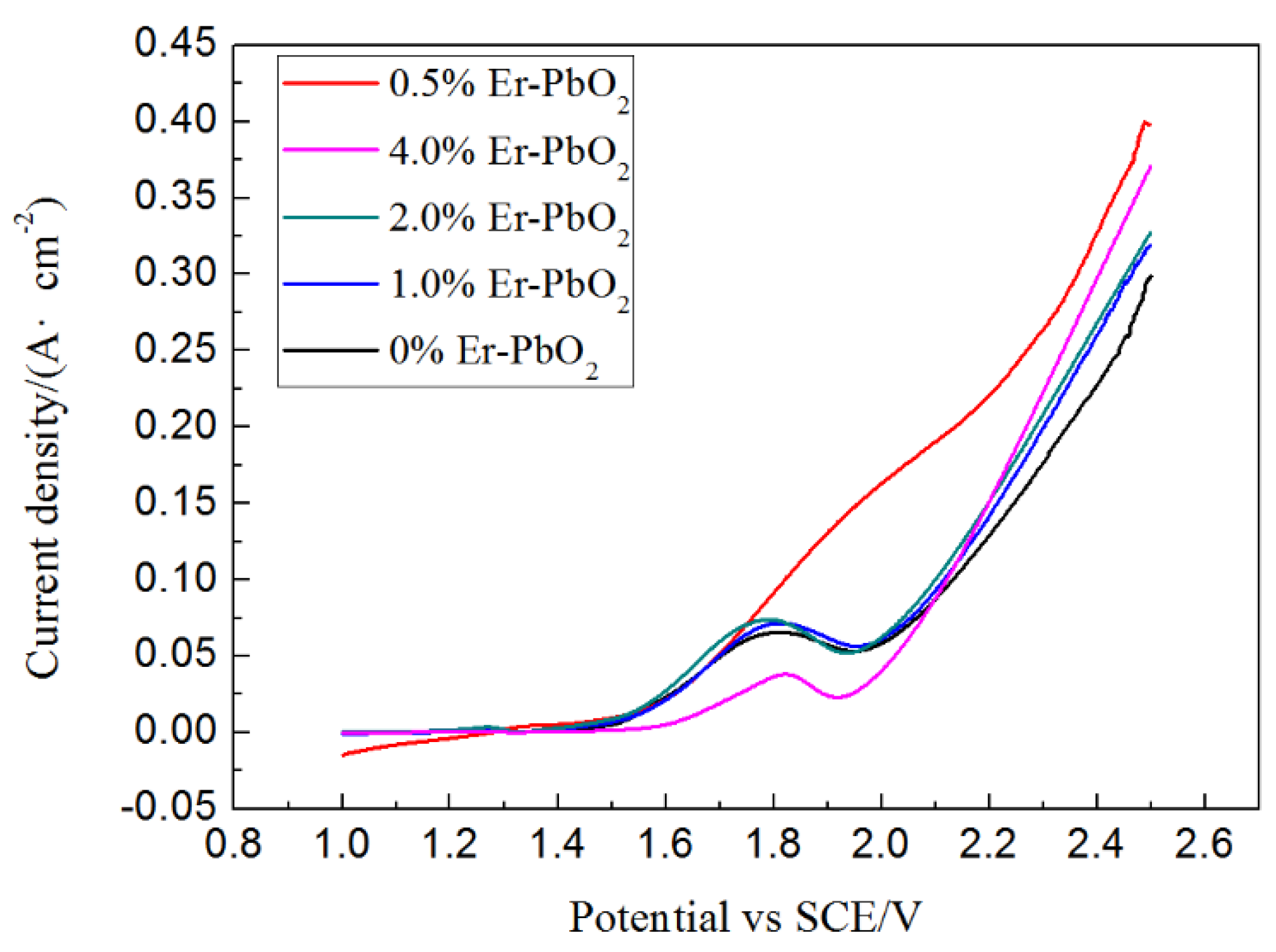
3.1.4. OEP Analysis of Er-PbO2 Anodes
3.2. Electrochemical Oxidation of SMR
3.2.1. SMR Degradation of Er-PbO2 Anodes
3.2.2. Effect of Current Density
3.2.3. Effect of pH Value
3.3. Degradation Mechanism of SMR
3.3.1. DFT Calculation
3.3.2. Intermediates Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oturan, N.; Wu, J.; Zhang, H.; Sharma, V.K.; Oturan, M.A. Electrocatalytic destruction of the antibiotic tetracycline in aqueous medium by electrochemical advanced oxidation processes: Effect of electrode materials. Appl. Catal. B Environ. 2013, 140, 92–97. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Liu, Y.W.; Wei, Q.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Sobczak, A.; Makowski, A. Photocatalytic degradation of sulfa drugs with TiO2, Fe salts and TiO2/FeCl3 in aquatic environment-Kinetics and degradation pathway. Appl. Catal. B Environ. 2009, 90, 516–525. [Google Scholar] [CrossRef]
- Wang, S.L.; Wang, H. Adsorption behavior of antibiotic in soil environment: A critical review. Front. Env. Sci. Eng. 2015, 9, 565–574. [Google Scholar] [CrossRef]
- Uslu, M.O.; Balcioglu, I.A. Comparison of the ozonation and Fenton process performances for the treatment of antibiotic containing manure. Sci. Total Environ. 2009, 407, 3450–3458. [Google Scholar] [CrossRef]
- Hermosilla, D.; Han, C.; Nadagouda, M.N.; Machala, L.; Gasco, A.; Campo, P.; Dionysiou, D.D. Environmentally friendly synthesized and magnetically recoverable designed ferrite photo-catalysts for wastewater treatment applications. J. Hazard. Mater. 2020, 381, 121200. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.Z.; Li, A.M. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Kitazono, Y.; Ihara, I.; Yoshida, G.; Toyoda, K.; Umetsu, K. Selective degradation of tetracycline antibiotics present in raw milk by electrochemical method. J. Hazard. Mater. 2012, 243, 112–116. [Google Scholar] [CrossRef]
- Sires, I.; Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E.; Martinez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166, 603–643. [Google Scholar] [CrossRef]
- Salazar, C.; Contreras, N.; Mansilla, H.D.; Yanez, J.; Salazar, R. Electrochemical degradation of the antihypertensive losartan in aqueous medium by electro-oxidation with boron-doped diamond electrode. J. Hazard. Mater. 2016, 319, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, C.Z.; Lu, J.; Hu, C.J.; Peng, S.C.; Chen, T.H. Electro-catalytic degradation of bisphenol A with modified Co3O4/beta-PbO2/Ti electrode. Electrochim. Acta 2014, 118, 169–175. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, T.; Hu, Z.X.; Zhou, L.; Zhou, M.H. TiO2-NTs/SnO2-Sb anode for efficient electrocatalytic degradation of organic pollutants: Effect of TiO2-NTs architecture. Sep. Purif. Technol. 2013, 102, 180–186. [Google Scholar] [CrossRef]
- Lin, H.; Niu, J.F.; Xu, J.L.; Li, Y.; Pan, Y.H. Electrochemical mineralization of sulfamethoxazole by Ti/SnO2-Sb/Ce-PbO2 anode: Kinetics, reaction pathways, and energy cost evolution. Electrochim. Acta 2013, 97, 167–174. [Google Scholar] [CrossRef]
- An, H.; Li, Q.; Tao, D.J.; Cui, H.; Xu, X.T.; Ding, L.; Sun, L.; Zhai, J.P. The synthesis and characterization of Ti/SnO2-Sb2O3/PbO2 electrodes: The influence of morphology caused by different electrochemical deposition time. Appl. Surf. Sci. 2011, 258, 218–224. [Google Scholar] [CrossRef]
- Dai, Q.Z.; Xia, Y.J.; Chen, J.M. Mechanism of enhanced electrochemical degradation of highly concentrated aspirin wastewater using a rare earth La-Y co-doped PbO2 electrode. Electrochim. Acta 2016, 188, 871–881. [Google Scholar] [CrossRef]
- Yao, Y.W.; Li, M.Y.; Yang, Y.; Cui, L.L.; Guo, L. Electrochemical degradation of insecticide hexazinone with Bi-doped PbO2 electrode: Influencing factors, intermediates and degradation mechanism. Chemosphere 2019, 216, 812–822. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, F.W.; Xu, M.; Zhu, C.G.; Fang, W.Y.; Wei, Y.J. Electrocatalytic degradation of methylene blue on Co doped Ti/TiO2 nanotube/PbO2 anodes prepared by pulse electrodeposition. J. Electroanal. Chem. 2015, 759, 158–166. [Google Scholar] [CrossRef]
- Xu, M.; Mao, Y.L.; Song, W.L.; OuYang, X.M.; Hu, Y.H.; Wei, Y.J.; Zhu, C.G.; Fang, W.Y.; Shao, B.C.; Lu, R.; et al. Preparation and characterization of Fe-Ce co-doped Ti/TiO2 NTs/PbO2 nanocomposite electrodes for efficient electrocatalytic degradation of organic pollutants. J. Electroanal. Chem. 2018, 823, 193–202. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.B.; Jiang, C.J.; Li, J.Y.; Yu, G.; Deng, S.B.; Lu, S.Y.; Zhang, P.X.; Zhu, C.Z.; Zhuo, Q.F. Electrochemical mineralization of perfluorooctane sulfonate by novel F and Sb co-doped Ti/SnO2 electrode containing Sn-Sb interlayer. Chem. Eng. J. 2017, 316, 296–304. [Google Scholar] [CrossRef]
- Niu, J.F.; Lin, H.; Xu, J.L.; Wu, H.; Li, Y.Y. Electrochemical Mineralization of Perfluorocarboxylic Acids (PFCAs) by Ce-Doped Modified Porous Nanocrystalline PbO2 Film Electrode. Environ. Sci. Technol. 2012, 46, 10191–10198. [Google Scholar] [CrossRef]
- Dai, Q.Z.; Shen, H.; Xia, Y.J.; Chen, F.; Wang, J.D.; Chen, J.M. The application of a novel Ti/SnO2-Sb2O3/PTFE-La-Ce-beta-PbO2 anode on the degradation of cationic gold yellow X-GL in sono-electrochemical oxidation system. Sep. Purif. Technol. 2013, 104, 9–16. [Google Scholar] [CrossRef]
- Dai, Q.Z.; Li, W.D.; Shen, H.; Chen, J.M. Enhanced Mineralization of Cationic Red X-GRL with Rare Earth doped materials: Gd for example. Int. J. Electrochem. Sci. 2013, 8, 12287–12295. [Google Scholar]
- Wang, Y.; Shen, Z.Y.; Chen, X.C. Effects of experimental parameters on 2,4-dichlorphenol degradation over Er-chitosan-PbO2 electrode. J. Hazard. Mater. 2010, 178, 867–874. [Google Scholar] [CrossRef]
- Li, S.H.; Liu, Y.K.; Wu, Y.M.; Chen, W.W.; Qin, Z.J.; Gong, N.L.; Yu, D.P. Highly sensitive formaldehyde resistive sensor based on a single Er-doped SnO2 nanobelt. Phys. B Condens. Matter 2016, 489, 33–38. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Wu, J.; Niu, J. Insights into the electrochemical degradation of sulfamethoxazole and its metabolite by Ti/SnO2-Sb/Er-PbO2 anode. Chin. Chem. Lett. 2020, 31, 2673–2677. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Li, Z.L.; Hao, C.T.; Zhang, Y.C.; Chai, S.N.; Han, G.P.; Xu, H.N.; Lu, J.S.; Dang, Y.; Sun, X.Q.; et al. Electrocatalysis enhancement of alpha, beta-PbO2 nanocrystals induced via rare earth Er(III) doping strategy: Principle, degradation application and electrocatalytic mechanism. Electrochim. Acta 2020, 333, 135535. [Google Scholar] [CrossRef]
- Wang, W.Y.; Duan, X.Y.; Sui, X.Y.; Wang, Q.; Xu, F.; Chang, L.M. Surface characterization and electrochemical properties of PbO2/SnO2 composite anodes for electrocatalytic oxidation of m-nitrophenol. Electrochim. Acta 2020, 335, 135649. [Google Scholar] [CrossRef]
- Yao, Y.; Teng, G.; Yang, Y.; Ren, B.; Cui, L. Electrochemical degradation of neutral red on PbO2/α-Al2O3 composite electrodes: Electrode characterization, byproducts and degradation mechanism. Separ. Purif. Technol. 2019, 227, 115684. [Google Scholar] [CrossRef]
- Yao, Y.; Teng, G.; Yang, Y.; Huang, C.; Liu, B.; Guo, L. Electrochemical oxidation of acetamiprid using Yb-doped PbO2 electrodes: Electrode characterization, influencing factors and degradation pathways. Separ. Purif. Technol. 2019, 211, 456–466. [Google Scholar] [CrossRef]
- Gao, B.; Sun, M.; Ding, W.; Ding, Z.; Liu, W. Decoration of γ-graphyne on TiO2 nanotube arrays: Improved photoelectrochemical and photoelectrocatalytic properties. Appl. Catal. B Environ. 2021, 281, 119492. [Google Scholar] [CrossRef]
- Ansari, A.; Nematollahi, D. A comprehensive study on the electrocatalytic degradation, electrochemical behavior and degradation mechanism of malachite green using electrodeposited nanostructured beta-PbO2 electrodes. Water Res. 2018, 144, 462–473. [Google Scholar] [CrossRef]
- Qiu, J.P.; Tang, J.T.; Shen, J.; Wu, C.W.; Qian, M.Q.; He, Z.Q.; Chen, J.M.; Song, S. Preparation of a silver electrode with a three-dimensional surface and its performance in the electrochemical reduction of carbon dioxide. Electrochim. Acta 2016, 203, 99–108. [Google Scholar]
- Xu, H.; Shao, D.; Zhang, Q.; Yang, H.H.; Wei, Y. Preparation and characterization of PbO2 electrodes from electro-deposition solutions with different copper concentration. Rsc Adv. 2014, 4, 25011–25017. [Google Scholar]
- Souri, Z.; Ansari, A.; Nematollahi, D.; Mazloum-Ardakani, M. Electrocatalytic degradation of dibenzoazepine drugs by fluorine doped beta-PbO2 electrode: New insight into the electrochemical oxidation and mineralization mechanisms. J. Electroanal. Chem. 2020, 862, 114037. [Google Scholar] [CrossRef]
- Kong, J.T.; Shi, S.Y.; Kong, L.C.; Zhu, X.P.; Ni, J.R. Preparation and characterization of PbO2 electrodes doped with different rare earth oxides. Electrochim. Acta 2007, 53, 2048–2054. [Google Scholar] [CrossRef]
- Chang, L.M.; Zhou, Y.; Duan, X.Y.; Liu, W.; Xu, D.D. Preparation and characterization of carbon nanotube and Bi co-doped PbO2 electrode. J. Taiwan Inst. Chem. Eng. 2014, 45, 1338–1346. [Google Scholar] [CrossRef]
- Xia, Y.J.; Bian, X.Z.; Xia, Y.; Zhou, W.; Wang, L.; Fan, S.Q.; Xiong, P.; Zhan, T.T.; Dai, Q.Z.; Chen, J.M. Effect of indium doping on the PbO2 electrode for the enhanced electrochemical oxidation of aspirin: An electrode comparative study. Sep. Purif. Technol. 2020, 237, 116321. [Google Scholar] [CrossRef]
- Tahar, N.B.; Savall, A. Electropolymerization of phenol on a vitreous carbon electrode in acidic aqueous solution at different temperatures. J. Appl. Electrochem. 2011, 41, 983–989. [Google Scholar] [CrossRef][Green Version]
- Xia, Y.J.; Dai, Q.Z. Electrochemical degradation of antibiotic levofloxacin by PbO2 electrode: Kinetics, energy demands and reaction pathways. Chemosphere. 2018, 205, 215–222. [Google Scholar] [CrossRef]
- Bian, X.Z.; Xia, Y.; Zhan, T.T.; Wang, L.; Zhou, W.; Dai, Q.Z.; Chen, J.M. Electrochemical removal of amoxicillin using a Cu doped PbO2 electrode: Electrode characterization, operational parameters optimization and degradation mechanism. Chemosphere 2019, 233, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, Q.N.; Yang, B.; Li, Z.J.; Lei, L.C. Electrochemical treatment of artificial humidity condensate by large-scale boron doped diamond electrode. Sep. Purif. Technol. 2014, 138, 13–20. [Google Scholar] [CrossRef]
- Fan, Y.; Ai, Z.H.; Zhang, L.Z. Design of an electro-Fenton system with a novel sandwich film cathode for wastewater treatment. J. Hazard. Mater. 2010, 176, 678–684. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.F.; Xu, Z.S.; Niu, J.F.; Hou, L.A. Electrochemical degradation of enrofloxacin by lead dioxide anode: Kinetics, mechanism and toxicity evaluation. Chem. Eng. J. 2017, 326, 911–920. [Google Scholar] [CrossRef]
- Hazhir, N.; Kiani, F.; Tahermansouri, H.; Hasan Saraei, A.G.; Koohyar, F. Prediction of Thermodynamic and Structural Properties of Sulfamerazine and Sulfamethazine in Water Using DFT and ab Initio Methods. J. Mex. Chem. Soc. 2018, 62, 1–13. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Oturan, N.; Wang, Y.; Chen, L.; Oturan, M.A. Application of response surface methodology to the removal of the antibiotic tetracycline by electrochemical process using carbon-felt cathode and DSA (Ti/RuO2-IrO2) anode. Chemosphere 2012, 87, 614–620. [Google Scholar] [CrossRef]
- Hai, H.; Xing, X.; Li, S.; Xia, S.; Xia, J. Electrochemical oxidation of sulfamethoxazole in BDD anode system: Degradation kinetics, mechanisms and toxicity evaluation. Sci. Total Environ. 2020, 738, 139909. [Google Scholar] [CrossRef]
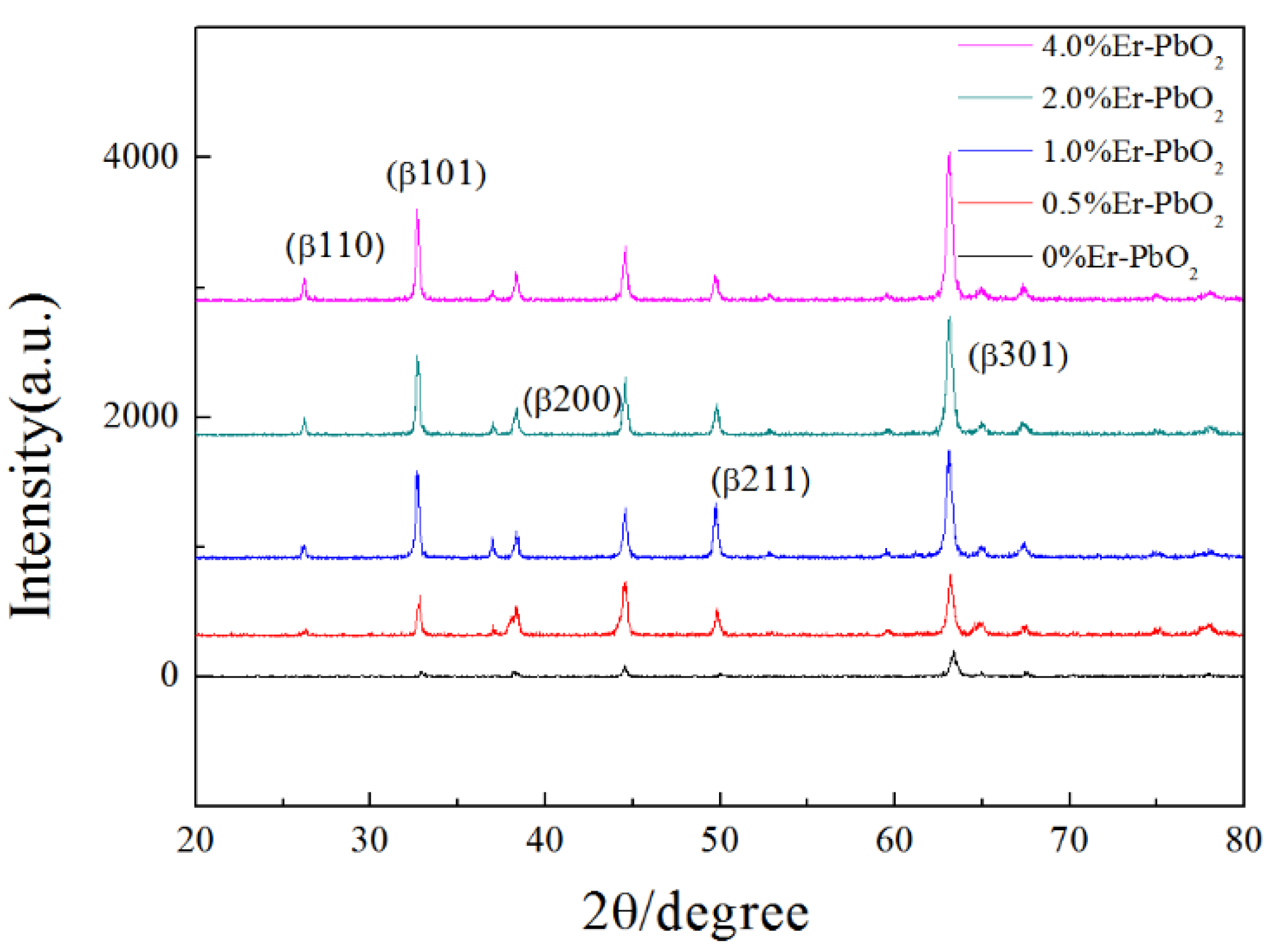
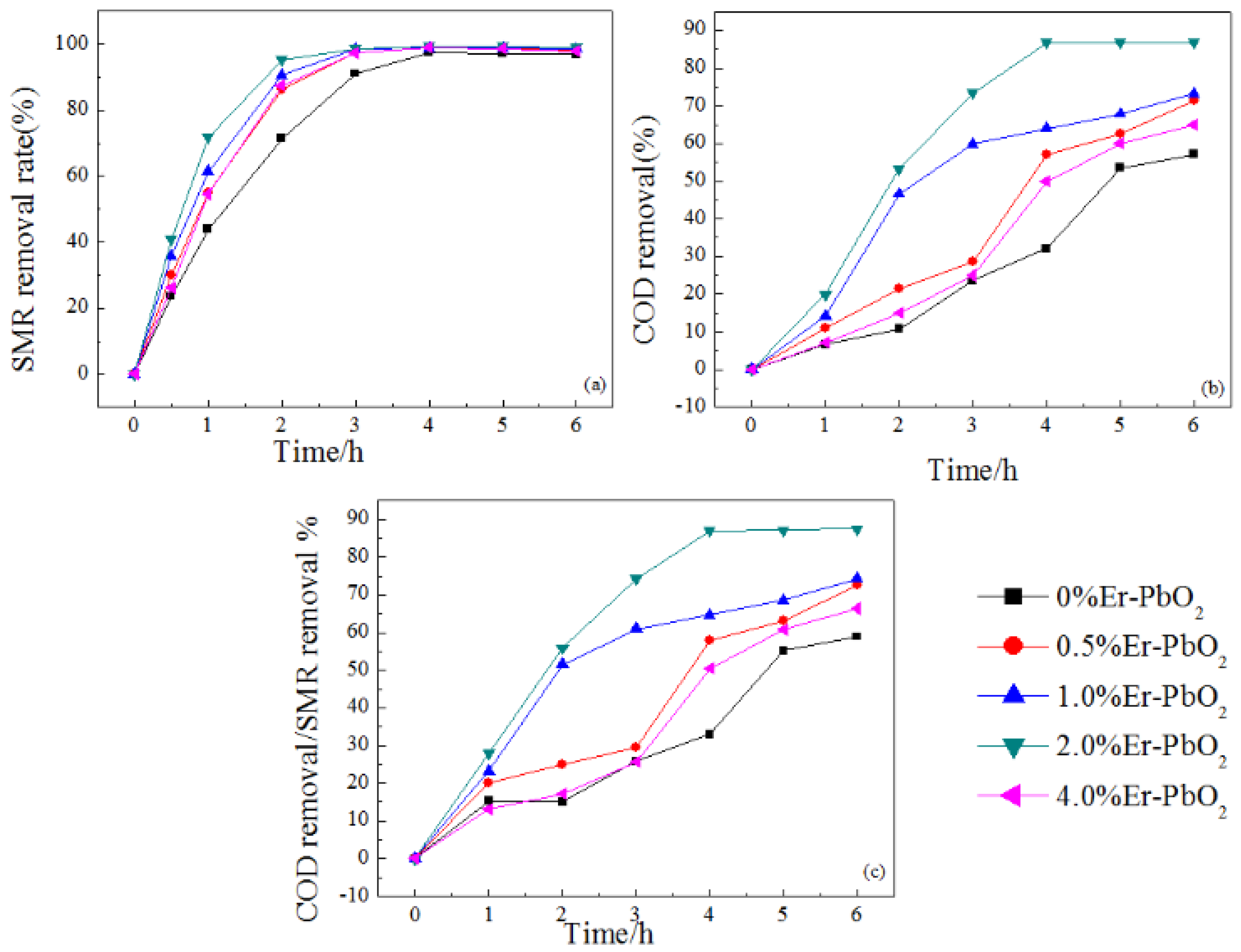
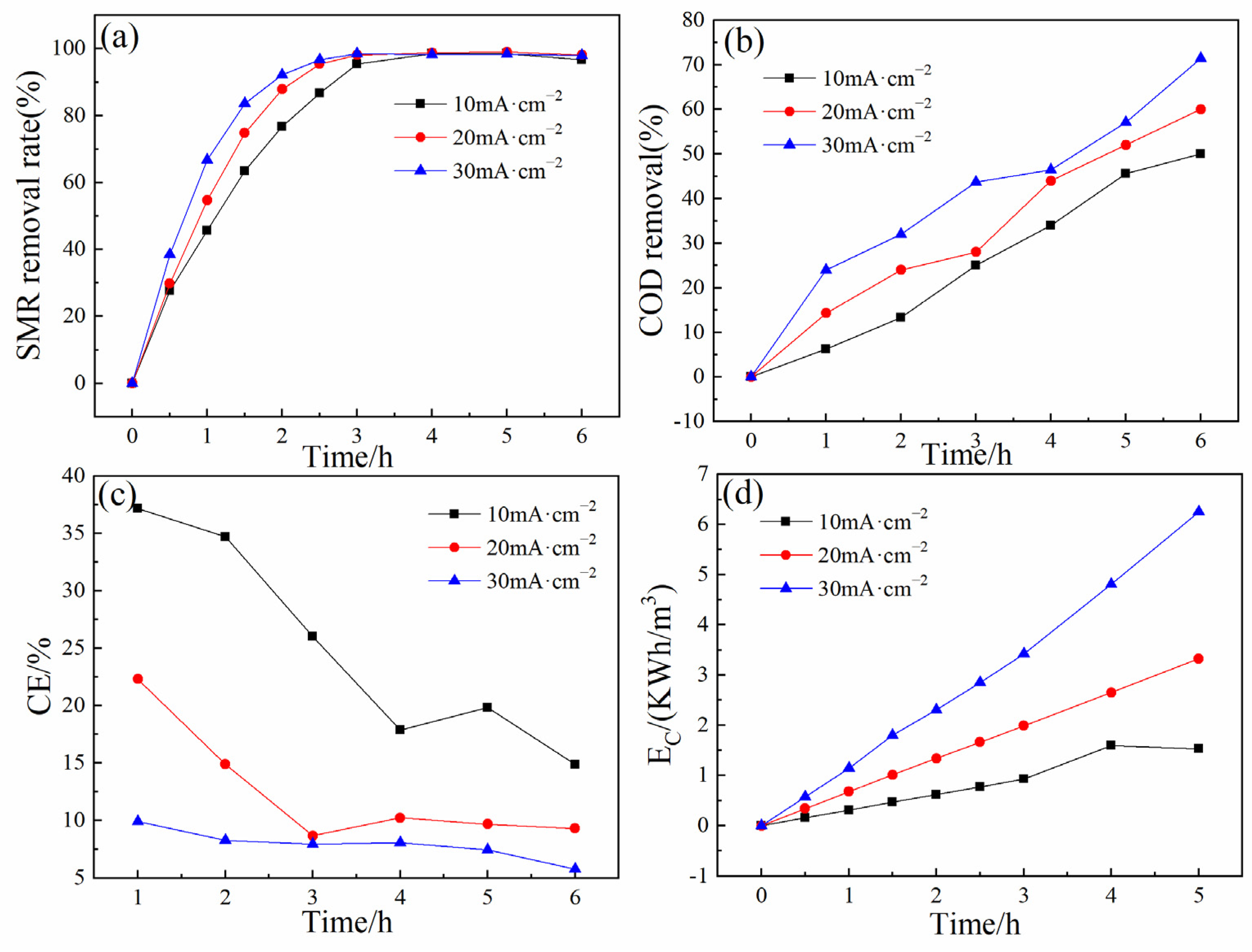
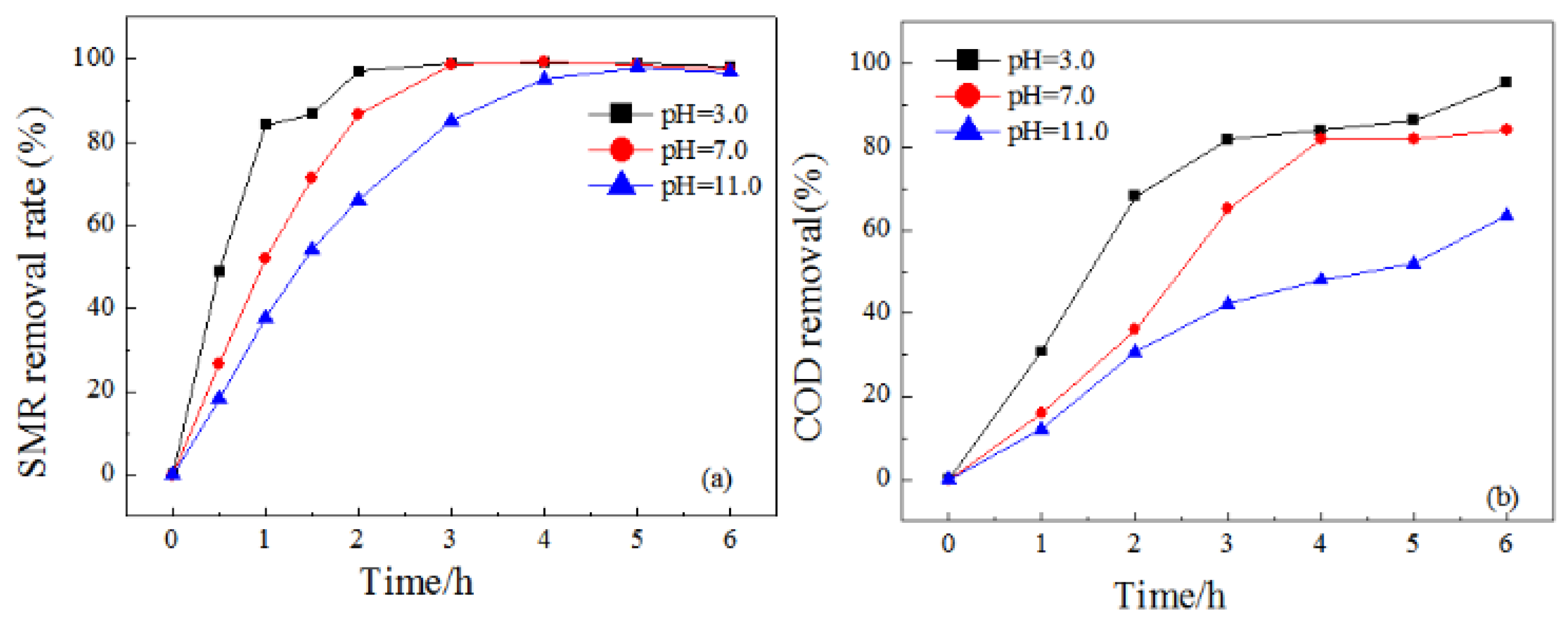
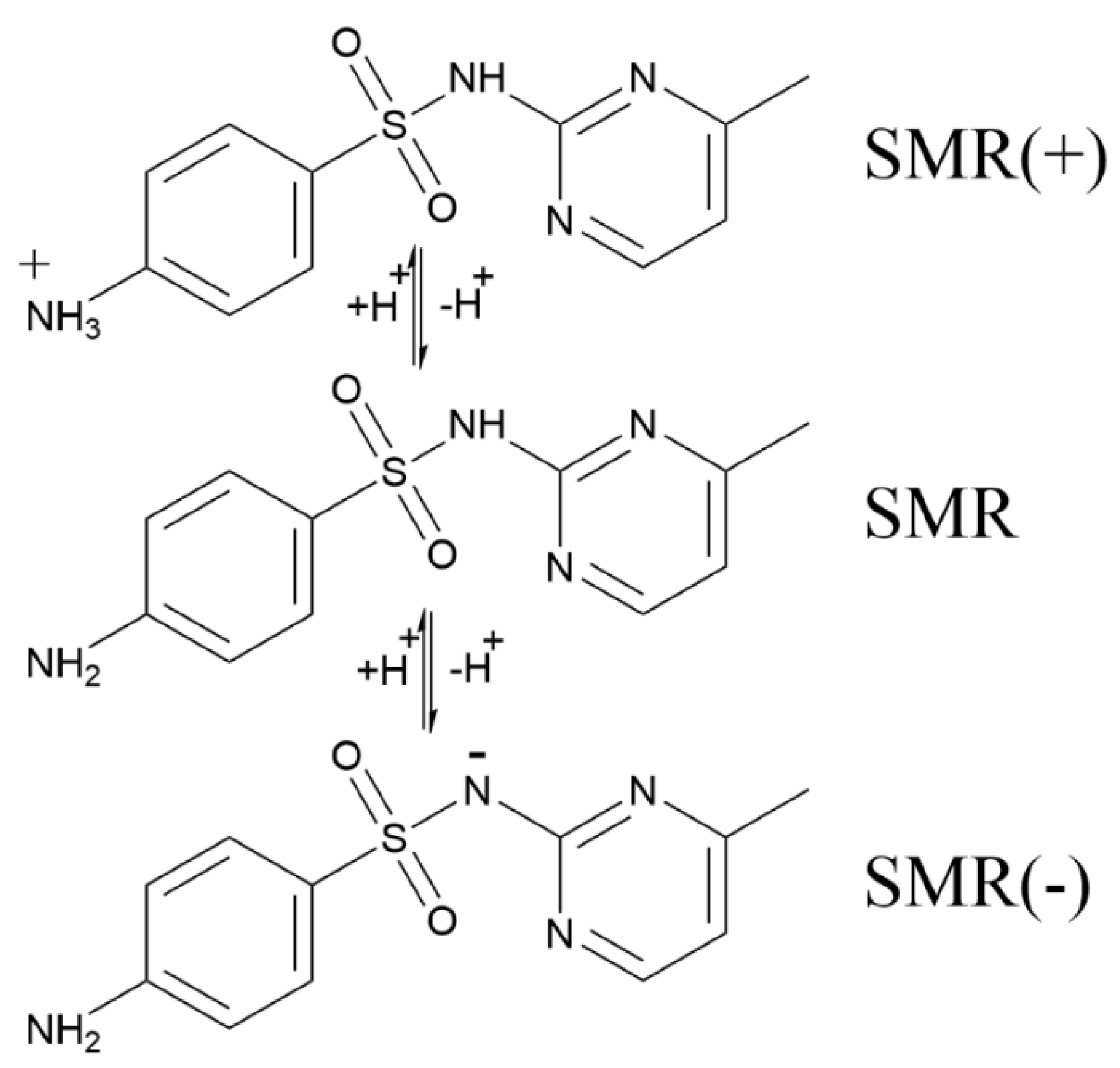
| Electrodes | WHM (301) | D (nm) |
|---|---|---|
| 0% PbO2 | 0.39072 | 43.43 |
| 0.5% Er-PbO2 | 0.40659 | 41.73 |
| 1.0% Er-PbO2 | 0.42429 | 39.99 |
| 2.0% Er-PbO2 | 0.42462 | 39.96 |
| 4.0% Er-PbO2 | 0.40481 | 41.92 |
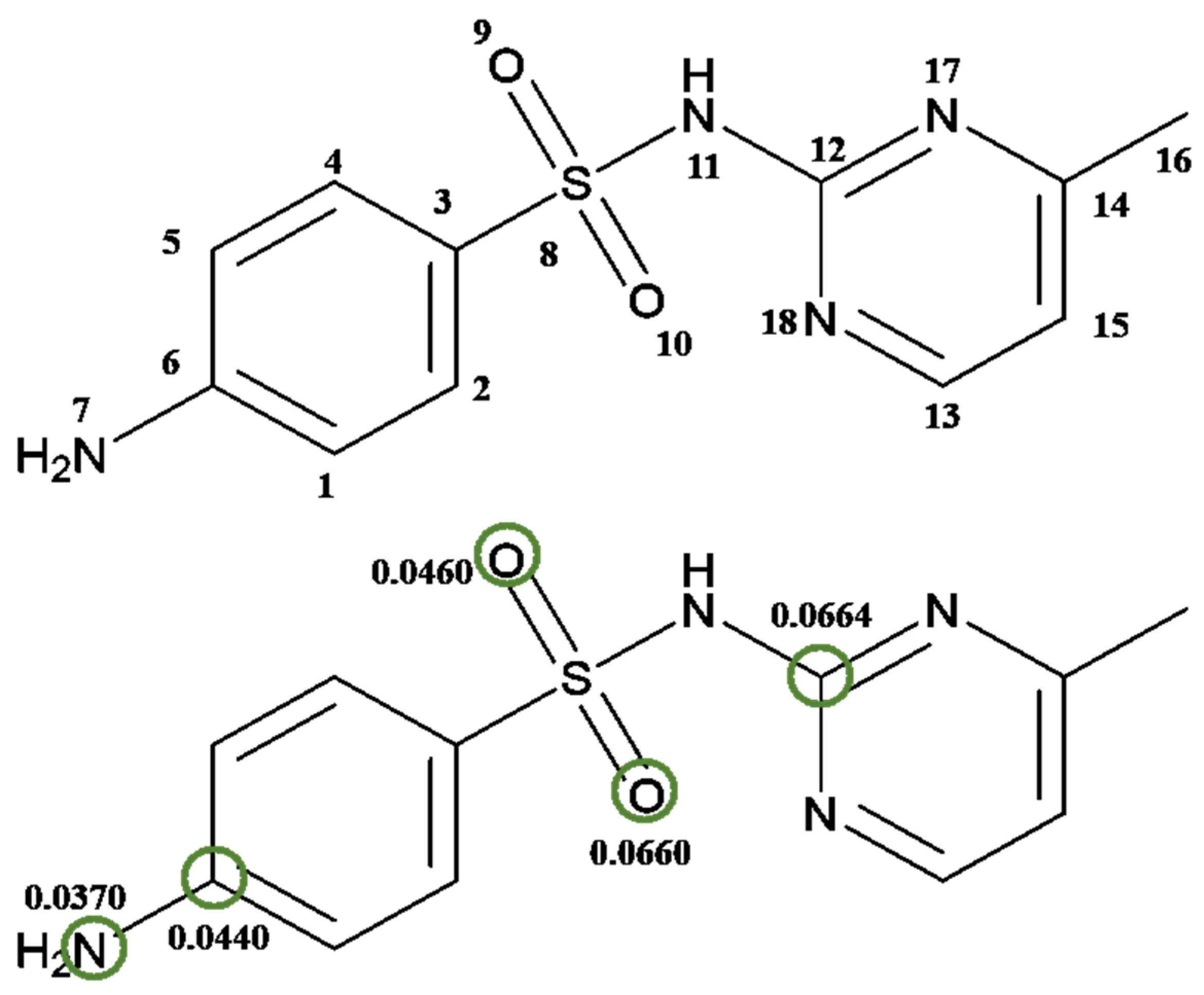
| Atom | qk (N − 1) | qk (N) | qk (N + 1) | |
|---|---|---|---|---|
| 1C | −0.084593 | −0.118527 | −0.111559 | 0.013483 |
| 2C | −0.044314 | −0.056261 | −0.095393 | 0.0255395 |
| 3C | −0.180775 | −0.199223 | −0.182452 | 0.0008385 |
| 4C | −0.069064 | −0.080553 | −0.098221 | 0.0145785 |
| 5C | −0.079208 | −0.114647 | −0.115776 | 0.018284 |
| 6C | 0.343255 | 0.298817 | 0.255246 | 0.0440045 |
| 7N | −0.582625 | −0.655931 | −0.656688 | 0.0370315 |
| 8S | 1.285632 | 1.242465 | 0.925813 | 0.1799095 |
| 9O | −0.500962 | −0.551447 | −0.592888 | 0.045963 |
| 10O | −0.467286 | −0.517021 | −0.599261 | 0.0659875 |
| 11N | −0.681021 | −0.698172 | −0.597924 | −0.0415485 |
| 12C | 0.666093 | 0.652561 | 0.533312 | 0.0663905 |
| 13C | 0.133549 | 0.124984 | 0.113774 | 0.0098875 |
| 14C | 0.300235 | 0.297406 | 0.301063 | −0.000414 |
| 15C | −0.118096 | −0.139383 | −0.157266 | 0.019585 |
| 16C | −0.370212 | −0.363920 | −0.363191 | −0.0035105 |
| 17N | −0.490604 | −0.516130 | −0.528766 | 0.019081 |
| 18N | −0.476218 | −0.468657 | −0.448271 | −0.0139735 |
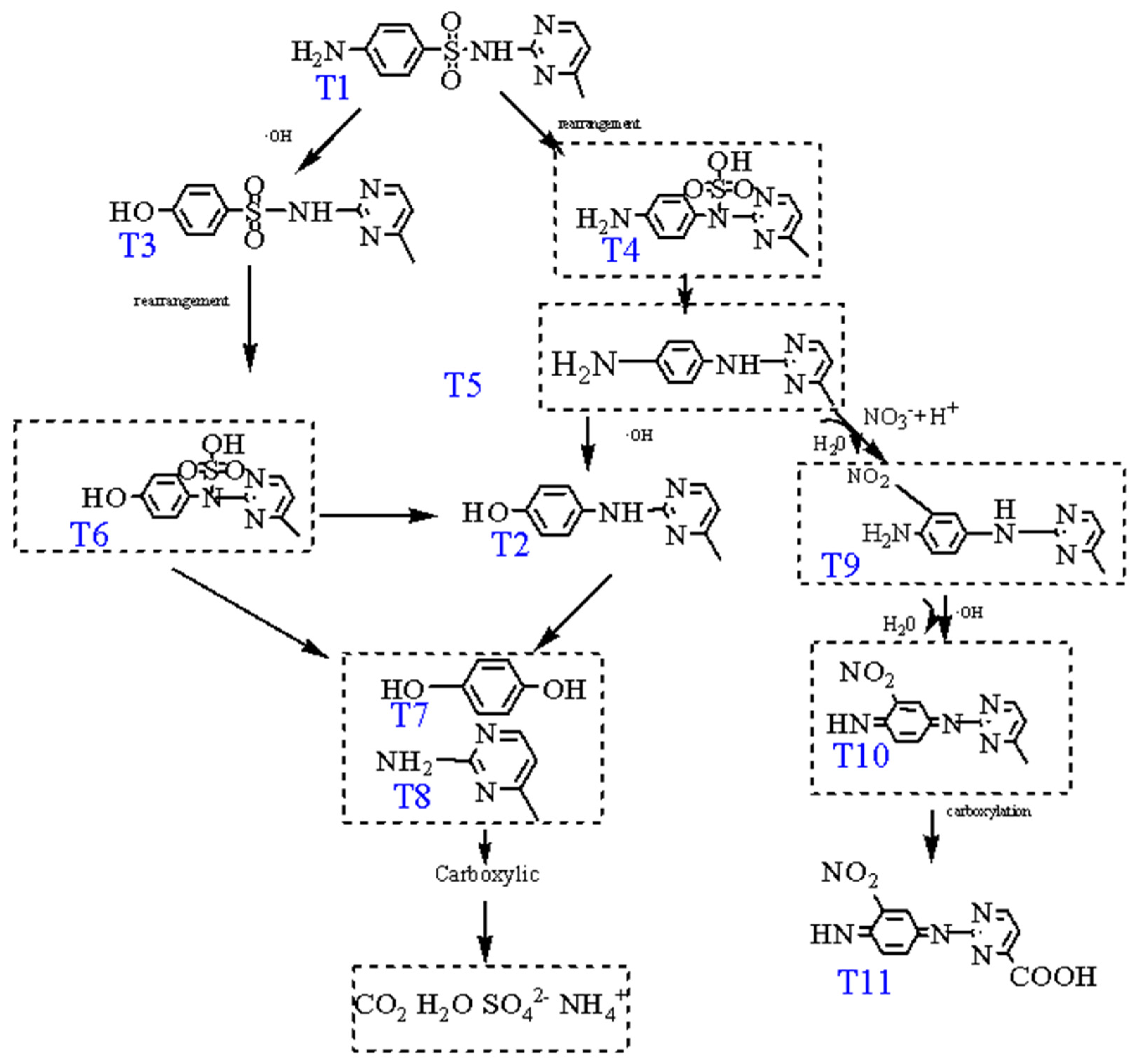
| Number | Molecular Formula | m/z | Molecular Weight | Molecular Structure |
|---|---|---|---|---|
| T1 | C11H12N4O2S | 265.0756 | 264 | 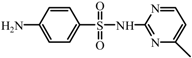 |
| T2 | C11H11N3O | 202.0982 | 201 | 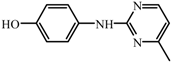 |
| T3 | C11H11N3O3S | 266.0775 | 265 | 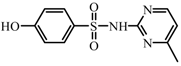 |
| T11 | C11H7N5O4 | 274.2759 | 273 | 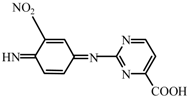 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, T.; Wei, C.; Chen, H.; Xu, J.; Wu, Y.; Xing, X. Fabrication of PbO2 Electrodes with Different Doses of Er Doping for Sulfonamides Degradation. Int. J. Environ. Res. Public Health 2022, 19, 13503. https://doi.org/10.3390/ijerph192013503
Zheng T, Wei C, Chen H, Xu J, Wu Y, Xing X. Fabrication of PbO2 Electrodes with Different Doses of Er Doping for Sulfonamides Degradation. International Journal of Environmental Research and Public Health. 2022; 19(20):13503. https://doi.org/10.3390/ijerph192013503
Chicago/Turabian StyleZheng, Tianyu, Chunli Wei, Hanzhi Chen, Jin Xu, Yanhong Wu, and Xuan Xing. 2022. "Fabrication of PbO2 Electrodes with Different Doses of Er Doping for Sulfonamides Degradation" International Journal of Environmental Research and Public Health 19, no. 20: 13503. https://doi.org/10.3390/ijerph192013503
APA StyleZheng, T., Wei, C., Chen, H., Xu, J., Wu, Y., & Xing, X. (2022). Fabrication of PbO2 Electrodes with Different Doses of Er Doping for Sulfonamides Degradation. International Journal of Environmental Research and Public Health, 19(20), 13503. https://doi.org/10.3390/ijerph192013503







