Ultrasonographic Evaluation of Skin Toxicity Following Radiotherapy of Breast Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Review Protocol and Selection Criteria
2.2. Search Strategy and Selection Process
2.3. Quality Assessment
2.4. Data Review and Extraction
3. Results
3.1. Study Selection and Quality Assessment
3.2. Study Characteristics
3.3. Ultrasound Protocol
3.4. US Skin Parameters and Locations of the Measurements
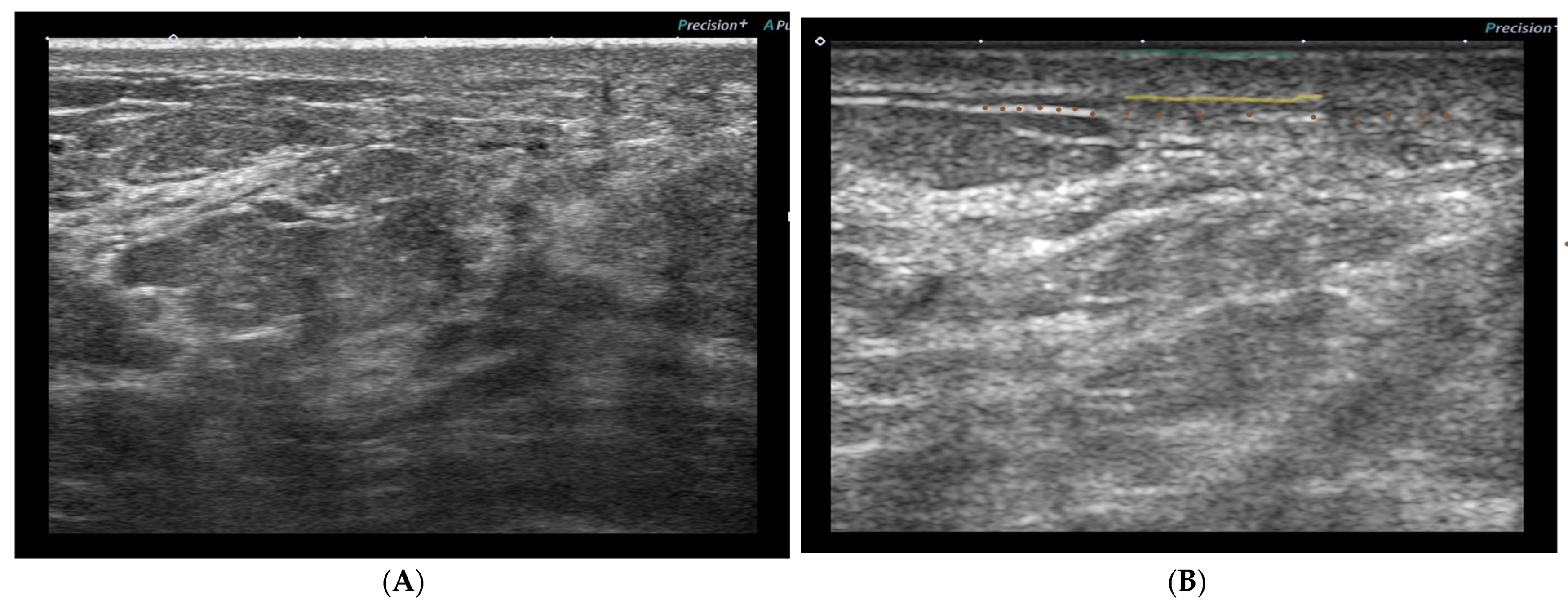
3.5. Ultrasonographic Skin Toxicity Changes
- Entry echoes—no significant differences between nonirradiated and irradiated skin for early or late reactions.
- Signal intensity—significant reduction of the signal intensity of the upper and lower corium in the early and late reactions but was more distinct in the early reactions.
- Reduction of echogenicity—no significant difference between early and late reactions for the upper corium, but, for the lower corium, differences were significant (more distinct in the early reactions).
- Border structure—no significant difference in the border structure between the dermis and subcutaneous tissue of the irradiated skin compared to the nonirradiated skin.
- The dermis–subcutaneous boundary was less well defined in the treated breast than in the untreated breast.
- Decreased dermal density or echogenicity (not quantified).
3.6. Variables Associated with Skin Toxicity
3.7. Association of US Skin Changes with Clinical Assessments
3.8. US Reliability/Reproducibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, L.; Bonaccio, M.; de Gaetano, G.; Donati, M.B. Epidemiology of Breast Cancer, a Paradigm of the “Common Soil” Hypothesis. Semin. Cancer Biol. 2021, 72, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Barba, D.; León-Sosa, A.; Lugo, P.; Suquillo, D.; Torres, F.; Surre, F.; Trojman, L.; Caicedo, A. Breast Cancer, Screening and Diagnostic Tools: All You Need to Know. Crit. Rev. Oncol. 2020, 157, 103174. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, S.A.; Strasser, J. Updates in the Treatment of Breast Cancer with Radiotherapy. Surg. Oncol. Clin. N. Am. 2017, 26, 371–382. [Google Scholar] [CrossRef]
- Wright, J.L.; Parekh, A. Updates in Postmastectomy Radiation. Surg. Oncol. Clin. N. Am. 2017, 26, 383–392. [Google Scholar] [CrossRef]
- Speers, C.; Pierce, L.J. Postoperative Radiotherapy after Breast-Conserving Surgery for Early-Stage Breast Cancer a Review. JAMA Oncol. 2016, 2, 1075–1082. [Google Scholar] [CrossRef]
- Kole, A.J.; Kole, L.; Moran, M.S. Acute Radiation Dermatitis in Breast Cancer Patients: Challenges and Solutions. Breast Cancer Targets Ther. 2017, 9, 313–323. [Google Scholar] [CrossRef]
- Harper, J.L.; Franklin, L.E.; Jenrette, J.M.; Aguero, E.G. Skin Toxicity during Breast Irradiation: Pathophysiology and Management. South. Med. J. 2004, 97, 989–993. [Google Scholar] [CrossRef]
- Lin, J.Y.; Yang, X.; Serra, M.; Miller, A.H.; Godette, K.D.; Kahn, S.T.; Henry, S.; Brown, G.; Liu, T.; Torres, M.A. Full Axillary Lymph Node Dissection and Increased Breast Epidermal Thickness 1 Year after Radiation Therapy for Breast Cancer. J. Surg. Oncol. 2019, 120, 1397–1403. [Google Scholar] [CrossRef]
- Yang, X.; Ren, H.; Guo, X.; Hu, C.; Fu, J. Radiation-Induced Skin Injury: Pathogenesis, Treatment, and Management. Aging 2020, 12, 23379–23393. [Google Scholar] [CrossRef]
- Archambeau, J.O.; Pezner, R.; Wasserman, T. Pathophysiology of Irradiated Skin and Breast. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1171–1185. [Google Scholar] [CrossRef]
- Tortorelli, G.; di Murro, L.; Barbarino, R.; Cicchetti, S.; di Cristino, D.; Falco, M.D.; Fedele, D.; Ingrosso, G.; Janniello, D.; Morelli, P.; et al. Standard or Hypofractionated Radiotherapy in the Postoperative Treatment of Breast Cancer: A Retrospective Analysis of Acute Skin Toxicity and Dose Inhomogeneities. BMC Cancer 2013, 13, 230. [Google Scholar] [CrossRef]
- Atiq, A.; Atiq, M.; Naeem, H.; Saeed, N.; Abbas, M. Modern Radiation Therapy Techniques and Their Toxicities for Breast Cancer. In Breast Cancer: From Bench to Personalized Medicine; Shakil Malik, S., Masood, N., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 429–451. ISBN 978-981-19-0197-3. [Google Scholar]
- Ozyigit, G.; Gultekin, M. Current Role of Modern Radiotherapy Techniques in the Management of Breast Cancer. World J. Clin. Oncol. 2014, 5, 425–439. [Google Scholar] [CrossRef][Green Version]
- Lilla, C.; Ambrosone, C.B.; Kropp, S.; Helmbold, I.; Schmezer, P.; von Fournier, D.; Haase, W.; Sautter-Bihl, M.L.; Wenz, F.; Chang-Claude, J. Predictive Factors for Late Normal Tissue Complications Following Radiotherapy for Breast Cancer. Breast Cancer Res. Treat. 2007, 106, 143–150. [Google Scholar] [CrossRef]
- de Langhe, S.; Mulliez, T.; Veldeman, L.; Remouchamps, V.; van Greveling, A.; Gilsoul, M.; de Schepper, E.; de Ruyck, K.; de Neve, W.; Thierens, H. Factors Modifying the Risk for Developing Acute Skin Toxicity after Whole-Breast Intensity Modulated Radiotherapy. BMC Cancer 2014, 14, 711. [Google Scholar] [CrossRef]
- Méry, B.; Vallard, A.; Trone, J.C.; Pacaut, C.; Guy, J.B.; Espenel, S.; Langrand-Escure, J.; Ollier, E.; Wang, G.; Diao, P.; et al. Correlation between Anthropometric Parameters and Acute Skin Toxicity in Breast Cancer Radiotherapy Patients: A Pilot Assessment Study. Br. J. Radiol. 2015, 88, 1–6. [Google Scholar] [CrossRef]
- Vrieling, C.; Collette, L.; Fourquet, A.; Hoogenraad, W.J.; Horiot, J.C.; Jager, J.J.; Pierart, M.; Poortmans, P.M.; Struikmans, H.; van der Hulst, M.; et al. The Influence of the Boost in Breast-Conserving Therapy on Cosmetic Outcome in the EORTC “Boost versus No Boost” Trial. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 677–685. [Google Scholar] [CrossRef]
- Mukesh, M.B.; Harris, E.; Collette, S.; Coles, C.E.; Bartelink, H.; Wilkinson, J.; Evans, P.M.; Graham, P.; Haviland, J.; Poortmans, P.; et al. Normal Tissue Complication Probability (NTCP) Parameters for Breast Fibrosis: Pooled Results from Two Randomised Trials. Radiother. Oncol. 2013, 108, 293–298. [Google Scholar] [CrossRef]
- Wright, J.L.; Takita, C.; Reis, I.M.; Zhao, W.; Lee, E.; Hu, J.J. Racial Variations in Radiation-Induced Skin Toxicity Severity: Data from a Prospective Cohort Receiving Postmastectomy Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 335–343. [Google Scholar] [CrossRef]
- Fodor, A.; Brombin, C.; Mangili, P.; Tummineri, R.; Pasetti, M.; Zerbetto, F.; Longobardi, B.; Galvan, A.S.; Deantoni, C.L.; Dell’Oca, I.; et al. Toxicity of Hypofractionated Whole Breast Radiotherapy Without Boost and Timescale of Late Skin Responses in a Large Cohort of Early-Stage Breast Cancer Patients. Clin. Breast Cancer 2022, 22, e480–e487. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Q.; Hu, T.; Chen, R.; Wang, J.; Chang, H.; Cheng, J. Risk Factors Related to Acute Radiation Dermatitis in Breast Cancer Patients After Radiotherapy: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 738851. [Google Scholar] [CrossRef]
- Yee, C.; Wang, K.; Asthana, R.; Drost, L.; Lam, H.; Lee, J.; Vesprini, D.; Leung, E.; DeAngelis, C.; Chow, E. Radiation-Induced Skin Toxicity in Breast Cancer Patients: A Systematic Review of Randomised Trials. Clin. Breast Cancer 2018, 18, e825–e840. [Google Scholar] [CrossRef]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORT#C). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE).v.5.0. In Cancer Therapy Evaluation Program (CTEP); National Institutes of Health (NIH): Bethesda, MD, USA, 2017; p. 155. [Google Scholar]
- Hoeller, U.; Tribius, S.; Kuhlmey, A.; Grader, K.; Fehlauer, F.; Alberti, W. Increasing the Rate of Late Toxicity by Changing the Score? A Comparison of RTOG/EORTC and LENT/SOMA Scores. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1013–1018. [Google Scholar] [CrossRef]
- Ch’Ng, S.S.; Roddy, J.; Keen, H.I. A Systematic Review of Ultrasonography as an Outcome Measure of Skin Involvement in Systemic Sclerosis. Int. J. Rheum. Dis. 2013, 16, 264–272. [Google Scholar] [CrossRef]
- Cucoş, M.; Crişan, M.; Lenghel, M.; Dudea, M.; Croitoru, R.; Dudea, S.M. Conventional Ultrasonography and Sonoelastography in the Assessment of Plaque Psoriasis under Topical Corticosteroid Treatment–Work in Progress. Med. Ultrason. 2014, 16, 107–113. [Google Scholar] [CrossRef]
- Huang, Y.P.; Zheng, Y.P.; Leung, S.F.; Choi, A.P.C. High Frequency Ultrasound Assessment of Skin Fibrosis: Clinical Results. Ultrasound Med. Biol. 2007, 33, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Pazdrowski, J.; Dańczak-Pazdrowska, A.; Polańska, A.; Kaźmierska, J.; Barczak, W.; Szewczyk, M.; Golusiński, P.; Adamski, Z.; Żaba, R.; Golusiński, W. An Ultrasonographic Monitoring of Skin Condition in Patients Receiving Radiotherapy for Head and Neck Cancers. Ski. Res. Technol. 2019, 25, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, J.; Yoshida, E.J.; Woodhouse, S.A.; Schiff, P.B.; Wang, T.J.C.; Lu, Z.F.; Pile-Spellman, E.; Zhang, P.; Kutcher, G.J. Quantitative Ultrasonic Evaluation of Radiation-Induced Late Tissue Toxicity: Pilot Study of Breast Cancer Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Yang, X.; Noreen, S.; Chen, H.; Han, T.; Henry, S.; Mister, D.; Andic, F.; Long, Q.; Liu, T. The Impact of Axillary Lymph Node Surgery on Breast Skin Thickening During and After Radiation Therapy for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 590–596. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Yang, X.; He, J.; Lin, J.; Henry, S.; Brown, G.; Chu, L.; Godette, K.D.; Kahn, S.T.; Liu, T.; et al. Impact of Regional Nodal Irradiation and Hypofractionated Whole-Breast Radiation on Long-Term Breast Retraction and Poor Cosmetic Outcome in Breast Cancer Survivors. Clin. Breast Cancer 2020, 20, e75–e81. [Google Scholar] [CrossRef]
- Yoshida, E.J.; Chen, H.; Torres, M.; Andic, F.; Liu, H.Y.; Chen, Z.; Sun, X.; Curran, W.J.; Liu, T. Reliability of Quantitative Ultrasonic Assessment of Normal-Tissue Toxicity in Breast Cancer Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 724–731. [Google Scholar] [CrossRef]
- Yoshida, E.J.; Chen, H.; Torres, M.A.; Curran, W.J.; Liu, T. Spectrophotometer and Ultrasound Evaluation of Late Toxicity Following Breast-Cancer Radiotherapy. Med. Phys. 2011, 38, 5747–5755. [Google Scholar] [CrossRef]
- Wratten, C.R.; O’Brien, P.C.; Hamilton, C.S.; Bill, D.; Kilmurray, J.; Denham, J.W. Breast Edema in Patients Undergoing Breast-Conserving Treatment for Breast Cancer: Assessment via High Frequency Ultrasound. Breast J. 2007, 13, 266–273. [Google Scholar] [CrossRef]
- Wratten, C.; Kilmurray, J.; Wright, S.; O’Brien, P.; Back, M.; Hamilton, C.; Denham, J. A Study of High Frequency Ultrasound to Assess Cutaneous Oedema in Conservatively Managed Breast. Front. Radiat. Ther. Oncol. 2002, 37, 121–127. [Google Scholar] [CrossRef]
- Wratten, C.; Kilmurray, J.; Wright, S.; O’Brien, P.C.; Back, M.; Hamilton, C.S.; Denham, J.W. Pilot Study of High-Frequency Ultrasound to Assess Cutaneous Oedema in the Conservatively Managed Breast. Int. J. Cancer 2000, 90, 295–301. [Google Scholar] [CrossRef]
- Borm, K.J.; Kleine Vennekate, J.; Vagedes, J.; Islam, M.O.A.; Duma, M.N.; Loos, M.; Combs, S.E.; Schiller, K.; Klusen, S.; Paepke, S.; et al. A Comprehensive Prospective Comparison of Acute Skin Toxicity after Hypofractionated and Normofractionated Radiation Therapy in Breast Cancer. Cancers 2021, 13, 5826. [Google Scholar] [CrossRef]
- Landoni, V.; Giordano, C.; Marsella, A.; Saracino, B.; Petrongari, M.G.; Ferraro, A.M.; Strigari, L.; Pinnarò, P. Evidence from a Breast Cancer Hypofractionated Schedule: Late Skin Toxicity Assessed by Ultrasound. J. Exp. Clin. Cancer Res. 2013, 32, 80. [Google Scholar] [CrossRef]
- Garnier, M.; Champeaux, E.; Laurent, E.; Boehm, A.; Briard, O.; Wachter, T.; Vaillant, L.; Patat, F.; Bens, G.; Machet, L. High-Frequency Ultrasound Quantification of Acute Radiation Dermatitis: Pilot Study of Patients Undergoing Radiotherapy for Breast Cancer. Ski. Res. Technol. 2017, 23, 602–606. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, J.; Osterman, K.S.; Zhang, P.; Woodhouse, S.A.; Schiff, P.B.; Kutcher, G.J. Measurements of Radiation-Induced Skin Changes in Breast-Cancer Radiation Therapy Using Ultrasonic Imaging. In Proceedings of the 2008 International Conference on BioMedical Engineering and Informatics, Sanya, China, 27–30 May 2008; Volume 2, pp. 718–722. [Google Scholar] [CrossRef]
- Keskikuru, R.; Jukkola, A.; Nuutinen, J.; Kataja, V.; Risteli, J.; Autio, P.; Lahtinen, T. Radiation-Induced Changes in Skin Type I and III Collagen Synthesis during and after Conventionally Fractionated Radiotherapy. Radiother. Oncol. 2004, 70, 243–248. [Google Scholar] [CrossRef]
- Warszawski, A.; Röttinger, E.M.; Vogel, R.; Warszawski, N. 20 MHz Ultrasonic Imaging for Quantitative Assessment and Documentation of Early and Late Postradiation Skin Reactions in Breast Cancer Patients. Radiother. Oncol. 1998, 47, 241–247. [Google Scholar] [CrossRef]
- Schack, L.H.; Alsner, J.; Overgaard, J.; Andreassen, C.N.; Offersen, B.V. Radiation-Induced Morbidity Evaluated by High-Frequency Ultrasound. Acta Oncol. 2016, 55, 1498–1500. [Google Scholar] [CrossRef]
- Wong, S.; Kaur, A.; Back, M.; Lee, K.M.; Baggarley, S.; Lu, J.J. An Ultrasonographic Evaluation of Skin Thickness in Breast Cancer Patients after Postmastectomy Radiation Therapy. Radiat. Oncol. 2011, 6, 9. [Google Scholar] [CrossRef]
- Botar Jid, C.; Bolboacă, S.D.; Cosgarea, R.; Şenilă, S.; Rogojan, L.; Lenghel, M.; Vasilescu, D.; Dudea, S.M. Doppler Ultrasound and Strain Elastography in the Assessment of Cutaneous Melanoma: Preliminary Results. Med. Ultrason. 2015, 17, 509–514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iagnocco, A.; Kaloudi, O.; Perella, C.; Bandinelli, F.; Riccieri, V.; Vasile, M.; Porta, F.; Valesini, G.; Matucci-Cerinic, M. Ultrasound Elastography Assessment of Skin Involvement in Systemic Sclerosis: Lights and Shadows. J. Rheumatol. 2010, 37, 1688–1691. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Criteria | |
|---|---|
| P—patient | Women with breast cancer ≥18 years old |
| I—intervention | Radiotherapy |
| C—comparison | Ultrasound assessments of the irradiated skin compared with the contralateral nonirradiated or pre-RT skin measurements |
| O—outcome | Skin toxicity |
| S—the type of study | Exclusion of studies with no statistical comparisons (case study or case series) or consisting of fewer than 10 patients, as well as reviews, editorials, and non-English or nonhuman studies |
| Author(s), Year, and Country | Study Design | Demographic Information | US Machine | RT Delivery Protocol | Type of Breast Surgery | Clinical Assessment | Time of Clinical and US Assessments |
|---|---|---|---|---|---|---|---|
| (Borm et al. 2021) [39] Germany | Prospective | Total patients: 166, after hierarchal clustering: 80 Mean age: 58.6 Mean BMI: 24.9 | Philips EPIQ 7 w Probe: 18–4b MHz linear array | CF: 50.4 Gy, (n = 105) HF: 40.05 Gy, (n = 61) Photon boost except 1 patient electron | BCS (n = 71) Mastectomy (n = 8) Others (n = 1) | CTCAE (v5.0) | Before, at the end of RT, and 6 weeks post RT |
| (Landoni et al. 2013) [40] Italy | Prospective | Total patients: 89 Median age: 62, range (31–79 years) | Sequoia 512 scanner (Siemens Medical Systems, USA) Probe: 8.0–15.0 MHz linear array (15L8 W) | HF: 34 Gy in 2 weeks with an electron boost of 8 Gy | BCS | CTCAEv3 | 11.4–85.7 months post RT (median 20.5 months) |
| (Garnier et al. 2017) [41] France | Prospective | Total patients: 34 Median age: 61.5, range (53–68 years) Median BMI: 23.5 Smoking (n = 2) Median BV: 425 mL (307–577) | 1-Dermcup 2020, Atys Médical, Soucieu-en-Jarrest, France Probe: 20 MHz 2-LOGIQ7S Probe: 18 MHz | CF: 50 Gy Photon boost of 16 Gy | BCS | CTCAE v4.0 | At the end of RT |
| (Wang et al. 2020) [33] USA | Prospective | Total patients: 109 CF = 63, median age 58, range (26–75 y), mean BMI 29.8, mean BV: 1904.5 cm3 (223.3–6988.29), smoking history (n = 14) HF = 46, median age 54.5, range (38–78 years), mean BMI 31.9, mean BV: 1961.2 cm3 (472.7–4113.62), smoking history (n = 12) | NR | CF: 50 Gy (n = 63) HF: 39.9 Gy (n = 46) Combined electron and photon boost in both groups | BCS | Breast retraction assessment | Before RT, the last day of RT, 12 weeks, and 1-year post RT |
| (Torres et al. 2016) [32] USA | Prospective | Total patients: 70 Mean age 57, range (26–75 years) Mean BMI 29.3 Mean BV: 1819.9 cm3 (223.3–6988.3) Smoking history (n = 14) | NR | CF: 50 Gy 10–16 Gy electron or photon boost | BCS | RTOG | Before RT, at week 6, and 6 weeks post RT |
| (Lin et al. 2019) [9] USA | Prospective | Total patients: 66 Mean age 56.3, range (26–75 years) Mean BMI: 29.9 Mean BV: 1919.9 cm3 (223.3–6988.3) Smoking history (n = 12) | NR | CF: 50 Gy Majority received an electron boost of 10–16 Gy | BCS | NR | Before RT, week 6 of RT, and 6 weeks, 6 months, and 1 year post RT |
| (Yoshida et al. 2012) [34] USA | Prospective | Total patients: 26 Mean age 55, range (42 to 74 years) | Sonix RP (Ultrasonix Medical Corporation, BC, Canada) Probe: 12-MHz linear | CF: 50.0–50.4 Gy Electron boost of 10.0–16.0 Gy | BCS | RTOG | Acute toxicity group (n = 8): before, during, and up to 6 months post RT Late toxicity group (n = 18): received one US study ≥6 months post RT |
| (Yoshida et al. 2011) [35] USA | Prospective | Total patients: 18 Mean age 56, range (44–74 years) | Sonix RP (Ultrasonix Medical Corporation, BC, Canada) Probe and US setting: 10 MHz linear array, 1.25 cm focal length, 4 cm depth, 72% gain, and 80 dB dynamic range | CF: 50.0 to 50.4 Gy Electron boost of 10.0 to 16.0 Gy | BCS | RTOG | 6 to 92 months post RT (median, 22 months) |
| (Liu et al. 2010) [31] USA | Prospective | Total patients: 18 Mean age 56, range (44–74 years) | Sonix RP (Ultrasonix Medical Corporation, Richmond, BC, Canada) Probe: 12-MHz linear array | CF: 50.0 to 50.4 Gy Electron boost of 10.0 to 16.0 Gy | BCS | RTOG | 6 to 94 months post RT (median, 22 months) |
| (Liu et al. 2008) [42] USA | Prospective | Total patients: 12 | Sonix RP system Probe: 6 MHz linear array (L14–5/38) The RF data were acquired with a 20 MHz sampling frequency | CF: 50–50.4 Gy Electron boost of 10–16 Gy | BCS | NR | 6 to 94 months post RT (Median, 22 months) |
| (Keskikuru et al. 2004) [43] Finland | Prospective | Total patients: 21 | Dermascan, Denmark Probe: 20 MHz | CF: 50 Gy Boost not mentioned | BCS | Modified Dische classification for erythema at the end of RT; subcutaneous induration scoring at 1 and 2 years | Before RT, at 2.5 weeks, at the end of RT, and 1, 4, 7, 12, and 24 months post RT |
| (Wratten et al. 2002) [37] Australia | Prospective | Total patients: 13 age range (43–77 years) | Dermascan C, Cortex Technology, Denmark Probe: 20 MHz, an axial resolution of 60µm, a lateral resolution of 200µm, and a depth of up to 15 mm | CF: 50–64 Gy ± electron boost | BCS | Visual for the presence of oedema | Before RT and before fractions 4, 6, 9, 11, 16, 21, and 26 |
| (Wratten et al. 2000) [38] Australia | Prospective | Total patients: 11 age range (35–72 years) | Dermascan C, Cortex Technology, Denmark Probe: 20 MHz, an axial resolution of 60µm, a lateral resolution of 200µm, and a depth of up to 15 mm | CF: 44–50 Gy Electron boost of 10–20 Gy | BCS | Visual for the presence of oedema | Different time assessments for different patients: baseline, during, and post RT, including one patient up to 22 years post RT |
| (Warszawski et al. 1998) [44] Germany | Prospective | Total patients: 29 Median age: 58, range (39–72 years) | Taberna pro Medicum (DUB 20) (Luneburg, Germany) Probe: Digital linear 20 MHz, axial, and lateral resolutions of 70 and 150 mm, respectively | CF: 46–50 Gy 10 Gy electron boost for patients with BCS | BCS (n = 23) + Mastectomy (n = 6) | RTOG | ≤3 months in 18 patients and 6–135 months post RT in 11 patients |
| (Schack et al. 2016) [45] Denmark | Prospective | Total patients: 15 Median age 66, range (44–75 years) Median BV: 715 mL (177–1627) | DermaScan C from Cortex Technology ApS, Denmark Probe: 20 MHz with a maximum depth of 6 mm | 40 or 50 Gy (no details) | BCS | LENT-SOMA Scale | Baseline, post RT (median 3.0 years (1.0–4.6 years)) |
| (Wong et al. 2011) [46] Singapore | Prospective | Total patients: 32 Median age: 52.5, range (37–68 years) | Sequoia® 512 scanner (Siemens Medical Systems, USA) Probe: linear array (15L8 W), 14 MHz centre frequency, and a maximum depth of 80 mm | 46–50 Gy No boost | Mastectomy | RTOG | 16–39 months post RT (median, 27.5 months) |
| (Wratten et al. 2007) [36] Australia | Prospective | Total patients: 54 Median age: 55, range (31–74 years) | Dermascan C, Cortex Technology, Smedevaenget, Denmark Probe: 20 MHz | CF: 50–66 Gy Electron boost 10–20 Gy (n = 21) | BCS | NR | Baseline, weekly during RT, at 2 weeks, 6 weeks, 4 months, 6 months, 12 months, and 24 months post RT |
| Author(s), Year | Measured Parameters and Locations | Ultrasound Skin Findings | Main Findings |
|---|---|---|---|
| (Borm et al. 2021) [39] | Skin thickness Locations: at 12:00, 3:00, 6:00, and 9:00 around the mamilla | Healthy breast: 1.7–1.8 mm at all timepoints for both groups Treated breast: HF: 2.3 mm before RT, 2.4 mm at the end of RT, and 2.5 mm post RT CF: 2.3 mm before RT, 2.3 mm at the end of RT, and 2.5 mm post RT |
|
| (Landoni et al. 2013) [40] | Skin thickness Locations: the irradiated breast, boost region, and corresponding positions on the untreated breast | Mean skin thickness: Irradiated breast: 2.13 ± 0.72 mm Contralateral site: 1.61 ± 0.29 mm Boost region: 2.25 ± 0.79 mm. Contralateral site: 1.63 ± 0.33 mm |
|
| (Garnier et al. 2017) [41] | Dermal thickness Locations: the irradiated breast, boost region, and corresponding positions on the untreated breast | Median dermal thickness (mm): Irradiated skin: 1.7 [1.4–2.1] Contralateral site: 1.3 [1.0–1.5] Boost region: 1.7 [1.4–2.1] Contralateral site: 1.2 [1.0–1.4] |
|
| (Wang et al. 2020) [33] | Mean epidermal thickness STRA Locations: the four quadrants of both breasts | Mean STRA: Baseline CF: 1.3, HF: 1.3 During RT CF: 1.5, HF: 1.4 12 weeks post RT CF: 1.6, HF: 1.5 1 year post RT CF: 1.5, HF: 1.5 |
|
| (Torres et al. 2016) [32] | Mean epidermal thickness and STRA Locations: the four quadrants and boost region in both breasts | STRA: Baseline: 1.27 (SD 0.29). During RT: 1.52 (SD 0.46) 6 weeks post RT: 1.6 (SD 0.46). |
|
| (Lin et al. 2019) [9] | Epidermal thickness STRA Locations: the four quadrants of both breasts | Mean STRA: Baseline: 1.28 ± 0.31. At week 6: 1.55 6 weeks post RT: 1.62 6 months post RT: 1.65 ± 0.41 1 year post RT: 1.44 ± 0.38 |
|
| (Yoshida et al. 2012) [34] | Ultrasound RF data: skin thickness PCC of the hypodermis Locations: 12:00, 3:00, 6:00, 9:00, and tumour bed of both breasts | Dermal toxicity: 28.5% ± 26.6% for RTOG = 0 and 69.7% ± 39.7% for RTOG = 1 or 2 Hypodermal toxicity: 5.4% ± 35.8% for RTOG = 0 and 19.2% ± 26.2% for RTOG = 1 or 2 |
|
| (Yoshida et al. 2011) [35] | Ultrasound RF data: skin thickness and PCC Locations: 12:00, 3:00, 6:00, and 9:00 positions of both breasts | The average skin thickness: Treated breast: 2.61 mm (1.53–3.65 mm) Untreated breast: 2.05 mm (1.66–2.41 mm) Average PCC: Treated breast: 0.28 (range: 0.21–0.41) Untreated breast: 0.41 (range: 0.03–0.52) |
|
| (Liu et al. 2010) [31] | Ultrasound RF signals: skin thickness and PCC Locations: 12:00, 3:00, 6:00, and 9:00 of both breasts | Skin thickness range: Untreated breasts: 1.66–2.41 mm Treated breasts: 1.53–3.65 mm PCC range: Untreated hypodermis: 0.03 to 0.52 Treated hypodermis: 0.21 to 0.41 |
|
| (Liu et al. 2008) [42] | Ultrasound RF signals: skin thickness and PCC Locations: irradiated breast and nonirradiated breast | Average skin thickness: Irradiated skin: 3.3 ± 1.4 mm (2.01 to 5.82 mm) Nonirradiated skin: 2.2 ± 0.4 mm (1.93 to 2.75 mm) Average PCC: Irradiated hypodermis: 0.18 ± 0.08 (0.01 to 0.36) Nonirradiated hypodermis: 0.27 ± 0.10 (0.10 to 0.42) |
|
| (Keskikuru et al. 2004) [43] | Skin thickness of induced suction blisters Location: in the upper medial quadrant of both breasts | The mean skin thickness of the Irradiated breast: Before RT: 1.9 mm, 4 months: 2.1 mm 7 months: 2.00 mm 1 year: 1.9 mm 2 years: 1.7 mm Nonirradiated breast: <1.8 mm at all timepoints |
|
| (Wratten et al. 2002) [37] | Skin thickness Locations: 4 cm medial and lateral to the nipple in both breasts | Mean skin thickness of: Medial treated breast 2.23 mm Lateral treated breast 1.91 Medial untreated breast 1.38 mm Lateral untreated breast 1.16 mm |
|
| (Wratten et al. 2000) [38] | Total cutaneous thickness Locations: 4 cm medial and lateral to the nipple in both breasts | The mean total cutaneous thickness: Treated breast: 2.71 mm (range 1.42–4.66 mm, SD 0.83 mm) Untreated breast: 1.35 mm (range 0.84–1.82 mm, SD 0.21 mm) |
|
| (Warszawski et al. 1998) [44] | Entry echo of the skin Corium (dermal) thickness The echogenicity of the upper and lower corium Structure of the border between the corium and subcutis Locations: at the edge between the upper quadrants 2–3 cm above the mammilla in both breasts | Mean corium thickness (µm): Nonirradiated skin: 1683 ± 308 Early reactions: 2683 ± 721 Late reactions: 2307 ± 934 The mean echogenicity of the upper and lower corium: Nonirradiated skin: 3.63 ± 1.58 to 5.04 ± 1.56 Early reactions: 1.90 ± 1.37 to 1.93 ± 0.76 Late reactions: 2.32 ± 0.88 to 3.33 ± 1.41 Unsharp dermis-subcutaneous border: Nonirradiated skin: 5/31, 16.1% Early reactions: 15/44, 34.1% Late reactions: 8/21, 38.1% |
|
| (Schack et al. 2016) [45] | Dermis thickness Locations: 3 cm from the areola in all four quadrants of both breasts | The mean dermis thickness: Untreated breast: 1.26 mm (95% CI 1.08–1.44) Irradiated breast: 2.22 mm (95% CI 1.78–2.66) The mean difference: 0.96 mm (95% CI 0.50–1.42) |
|
| (Wong et al. 2011) [46] | Skin thickness Locations: 9 points within the medial, central, and lateral areas of both breasts | Mean skin thickness (mm): Irradiated Rt chest wall: 0.1712 Nonirradiated Rt side: 0.1845 Irradiated Lt chest wall: 0.1764 Nonirradiated Lt side: 0.1835 |
|
| (Wratten et al. 2007) [36] | Mean epidermal thickness Locations: 4 cm medial and lateral to the nipple in both breasts | Treated breast: Baseline: 1.9–2.3 mm During RT: 1.9–2.5 mm 4–6 months: 2.3–3 mm 1–2 years: 1.5–2.5 mm Untreated breast: 1.3–1.5 mm at all timepoints |
|
| Author(s), Year | US Changes in Parameter | Association with Clinical Assessment | Notes | |
|---|---|---|---|---|
| Early (≤3 Months) | Late (˃3 Months) | |||
| (Borm et al. 2021) [39] | ↑ skin thickness compared to the HB | At the end of RT: no significant difference in skin thickness but significant difference (p = 0.03) in the CTCAE score 6 weeks post RT: no significant difference in skin thickness or CTCAE score (p = 0.39) | HF is associated with a lower degree of acute RD compared to CF at the end of treatment CTCAE scores and US measurements do not reliably reflect the patient’s perception | |
| (Garnier et al. 2017) [41] | ↑ dermal thickness compared to the HB | The mean relative ↑ in dermal thickness in irradiated skin (RIDTIS) was greater for grades 2 and 3 than 1: 0.53 vs. 0.29 mm (p = 0.023) | US of dermal thickness may be a reliable tool to quantify acute RD | |
| (Wang et al. 2020) [33] | Increased STRA compared to baseline | Increased STRA compared to baseline | A significant association between STRA and breast asymmetry (p = 0.02, 0.04, <0.01 at baseline, 12 weeks, and 1 year post RT, respectively) | HF is associated with better long-term cosmetic outcomes Supraclavicular nodal irradiation and CF are associated with worse cosmetic outcomes 1 year post RT |
| (Torres et al. 2016) [32] | Significant difference compared to baseline (p < 0.001) and the end of RT (p = 0.03) | Correlated with RTOG Mean STRA is ↑ in patients with grade 2 than grade 0 at the end of RT (p = 0.001) and 6 weeks post RT (p < 0.03) | RT had a synergistic effect with lymph node surgery on breast skin thickening | |
| (Lin et al. 2019) [9] | Significant changes compared to baseline (p < 0.001) | Significant changes compared to baseline (p < 0.001) | NR | ALND has a long-term impact on breast skin thickening |
| (Yoshida et al. 2011) [35] | Significant difference of skin thickness (p < 0.001) and PCC (p < 0.001). | PCC correlated with RTOG late toxicity, but skin thickness did not (↑38.4% for RTOG grade 0, 23.8% for grade 1, and 31.1% for grade 2 toxicity); p-value NR | Quantitative US is an objective tool that assesses RT-induced tissue injury, which may improve patients’ quality of life | |
| (Yoshida et al. 2012) [34] | Significant dermal (p < 0.0001) and hypodermal toxicity (p = 0.0027) | -Significant dermal toxicity (p < 0.05) -Not significant hypodermal toxicity (p = 0.22) | Late toxicity assessments correlated with RTOG (Patients with RTOG grade 1 or 2 have greater US toxicity changes than patients with RTOG grade 0, p = 0.04 for dermal toxicity and p = 0.22 for hypodermis toxicity) | Early and late radiation-induced effects on normal tissue can be reliably assessed using the quantitative US |
| (Keskikuru et al. 2004) [43] | Significant changes in skin thickness (p < 0.05, p < 0.01 at different timepoints) | Significant changes (p < 0.05, p < 0.01 at different timepoints) until one year then declined | No significant correlations between the skin thickness and the score of erythema or subcutaneous induration | Increased collagen synthesis is associated with oedema resulting from radiation-induced damage to skin microvasculature |
| (Wratten et al. 2000) [38] | Significant changes (p < 0.001) | There was a persistent ↑ in cutaneous thickness in the treated breast | The most prominent visual breast oedema exhibited the greatest total cutaneous thickness (p-value NR) | HFUS can quantify cutaneous breast oedema accurately |
| (Wratten et al. 2002) [37] | No obvious skin thickness changes during RT (p-value NR). | The most marked cutaneous thickness was in patients with obvious visible breast oedema before RT (p-value NR) | HFUS is not an ideal, sensitive, and quantitative measure of acute RD in this group of patients | |
| (Wratten et al. 2007) [36] | A minor ↑ in epidermal thickness (p-value NR) | Significant changes (p = 0.000 with or without level 2 nodal dissection) | NR | The utility of HFUS is in a research setting when assessing interventions that aim to reduce breast oedema |
| (Warszawski et al. 1998) [44] | Significant changes in the dermis thickness and echogenicity (p < 0.001) Nonsignificant changes in the structure of the dermis-subcutis border (p = 0.07) | Significant changes in the dermis thickness (p = 0.0018) and echogenicity (p < 0.001 for lower dermis, p = 0.0027 for upper dermis) Nonsignificant changes in the structure of the dermis-subcutis border (p = 0.08) | There were discrepancies between the clinical and US assessments, mainly in the late reactions (K = −0.13, Pearson’s correlation) Early skin reactions: structural changes could be recorded by US evaluation much earlier than visible reactions by the naked eye | High resolution 20 MHz US is noninvasive, quantitative, and reproducible for assessing early and late skin reactions US skin changes depend on the time interval between completion of RT and US evaluations |
| (Landoni et al. 2013) [40] | Statistically significant difference (p < 0.001) | US assessments were in agreement with clinical assessments A significant direct correlation was found between the increment in skin thickness with fibrosis (grade ≥ 1) in the irradiated breast (p-value = 0.0236) and the boost region (p-value = 0.0164) | Late cutaneous reactions can be reliably assessed by US | |
| (Liu et al. 2008) [42] | Significant skin thickness (p = 0.005) and Pearson coefficient (p = 0.02) changes | NR | US technique is noninvasive and feasible to detect and quantify radiation-induced skin changes | |
| (Liu et al. 2010) [31] | Significant skin thickness and Pearson coefficient changes (p < 0.001) | US evaluations were consistent with RTOG scores Skin thickness correlated with RTOG late subcutaneous toxicity, and PCC correlated with late skin toxicity (p-value NR) | The quantitative US is noninvasive and objective for assessing radiation-induced changes to the skin | |
| (Schack et al. 2016) [45] | Significant differences (p = 0.0003) | The highest mean difference in dermis thickness (1.61 mm (95% CI 0.41–2.82) was in patients with clinical oedema and grade 2 induration (p = 0.02) | HFUS evaluation of the skin is not part of large-scale follow-up routines in assessing radiation-induced morbidity | |
| (Wong et al. 2011) [46] | Significant skin thickness changes of the Rt chest (p = 0.007) and Lt chest (p = 0.025) | US measurements correlated with RTOG Patients with grade 2 acute skin toxicity presented with thinner skin (mean skin thickness 0.1720 mm) compared to patients with grade 1 (0.1879 mm) (p = 0.006) | HFUS can be utilised to document quantitative skin changes following postmastectomy RT | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, F.A.; Manan, H.A.; Mustapha, A.W.M.M.; Sidek, K.; Yahya, N. Ultrasonographic Evaluation of Skin Toxicity Following Radiotherapy of Breast Cancer: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 13439. https://doi.org/10.3390/ijerph192013439
Hussein FA, Manan HA, Mustapha AWMM, Sidek K, Yahya N. Ultrasonographic Evaluation of Skin Toxicity Following Radiotherapy of Breast Cancer: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(20):13439. https://doi.org/10.3390/ijerph192013439
Chicago/Turabian StyleHussein, Fatimah Alaa, Hanani Abdul Manan, Aida W. M. Mohd Mustapha, Khairiyah Sidek, and Noorazrul Yahya. 2022. "Ultrasonographic Evaluation of Skin Toxicity Following Radiotherapy of Breast Cancer: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 20: 13439. https://doi.org/10.3390/ijerph192013439
APA StyleHussein, F. A., Manan, H. A., Mustapha, A. W. M. M., Sidek, K., & Yahya, N. (2022). Ultrasonographic Evaluation of Skin Toxicity Following Radiotherapy of Breast Cancer: A Systematic Review. International Journal of Environmental Research and Public Health, 19(20), 13439. https://doi.org/10.3390/ijerph192013439






