A Decision-Theoretic Public Health Framework for Heated Tobacco and Nicotine Vaping Products
Abstract
1. Introduction
2. General Approach
3. Decision-Theoretic Framework
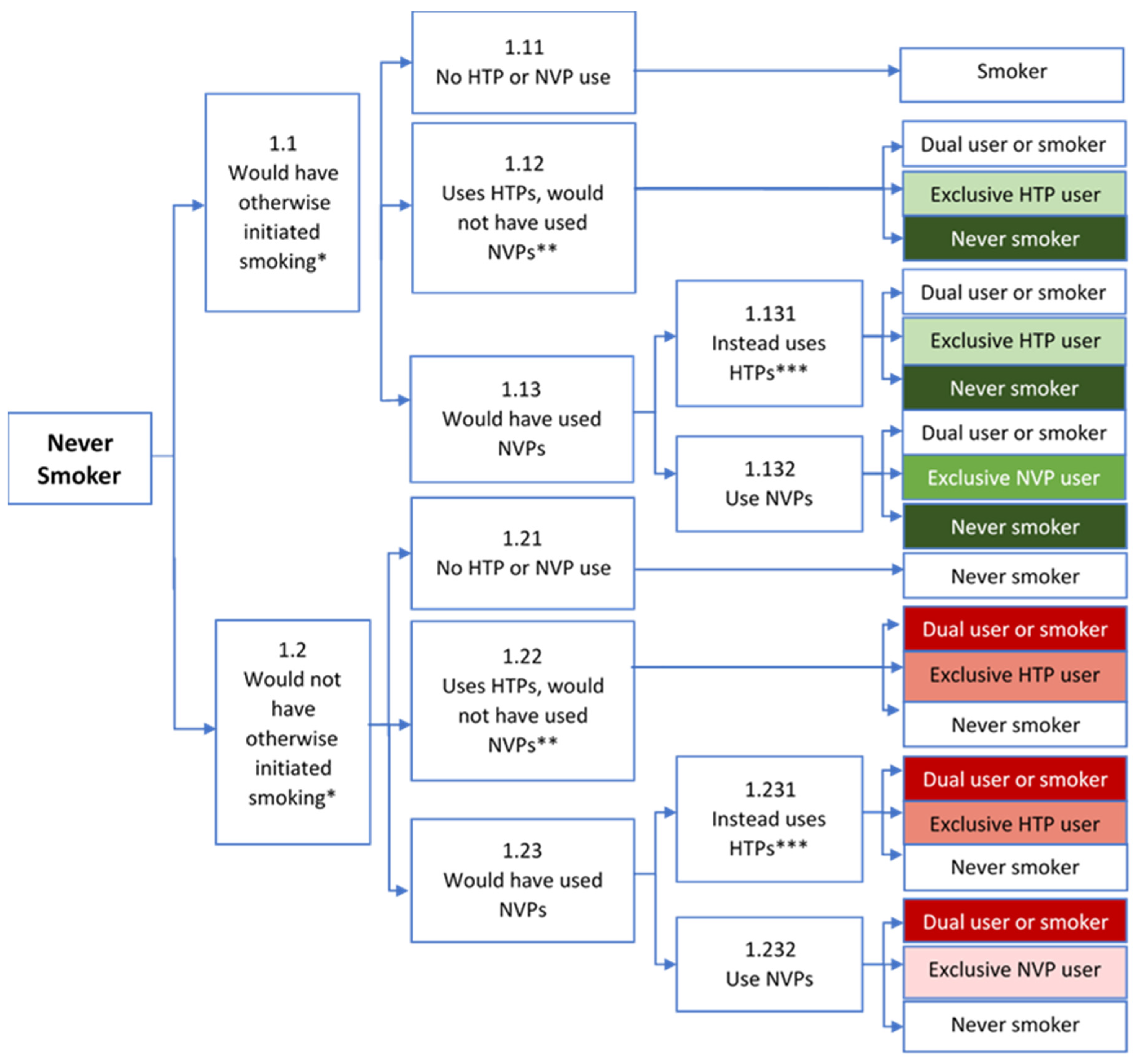
3.1. Never Users
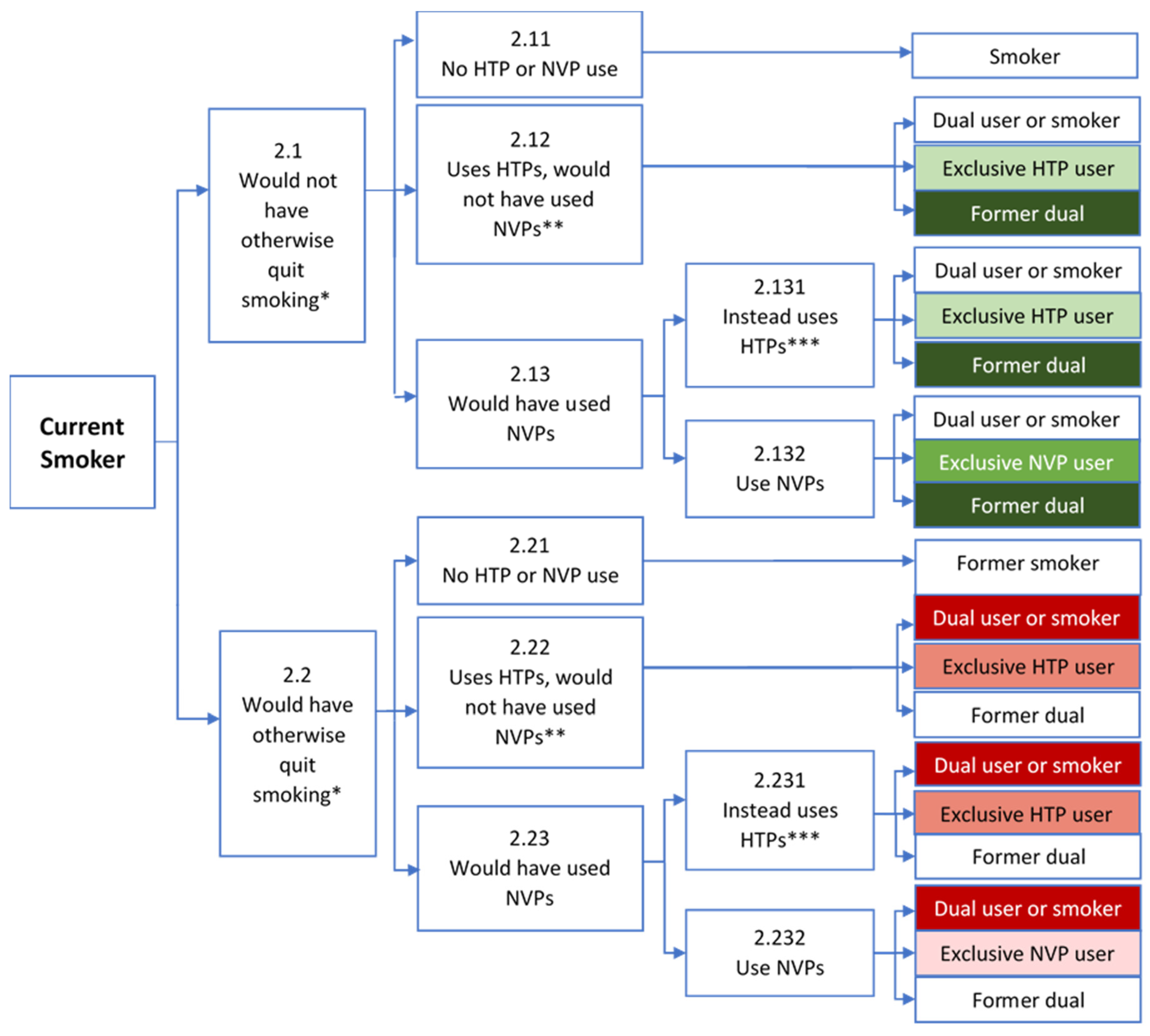
3.2. Current Smokers
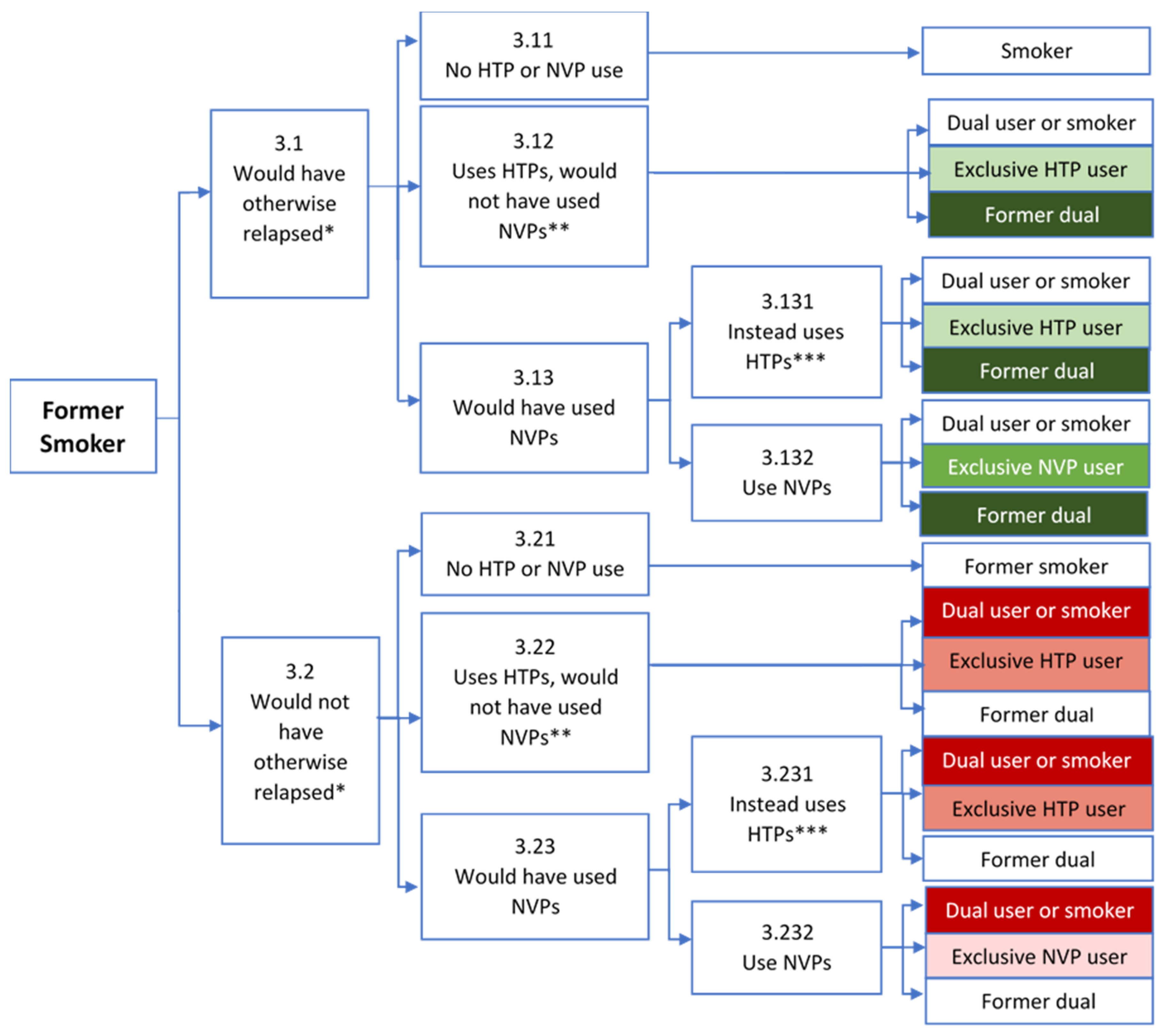
3.3. Former Smokers
3.4. Summary
4. Regulatory Framework
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Levy, D.; Sánchez-Romero, L.; Travis, N.; Yuan, Z.; Li, Y.; Skolnick, S.; Jeon, J.; Tam, J.; Meza, R. US Nicotine Vaping Product SimSmoke Simulation Model: The Effect of Vaping and Tobacco Control Policies on Smoking Prevalence and Smoking-Attributable Deaths. Int. J. Environ. Res. Public Health 2021, 18, 4876. [Google Scholar] [CrossRef] [PubMed]
- Warner, K.E. The remarkable decrease in cigarette smoking by American youth: Further evidence. Prev. Med. Rep. 2015, 2, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.T.; Warner, K.E.; Cummings, K.M.; Hammond, D.; Kuo, C.; Fong, G.T.; Thrasher, J.F.; Goniewicz, M.L.; Borland, R. Examining the relationship of vaping to smoking initiation among US youth and young adults: A reality check. Tob. Control 2019, 28, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Meza, R.; Jimenez-Mendoza, E.; Levy, D.T. Trends in Tobacco Use Among Adolescents by Grade, Sex, and Race, 1991–2019. JAMA Netw. Open 2020, 3, e2027465. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Leventhal, A.M. Prevalence of e-Cigarette Use Among Adults in the United States, 2014–2018. JAMA 2019, 322, 1824–1827. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.T.; Tam, J.; Sanchez-Romero, L.M.; Li, Y.; Yuan, Z.; Jeon, J.; Meza, R. Public health implications of vaping in the USA: The smoking and vaping simulation model. Popul. Health Metrics 2021, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.T.; Yuan, Z.; Li, Y.; Mays, D.; Sanchez-Romero, L.M. An Examination of the Variation in Estimates of E-Cigarette Prevalence among U.S. Adults. Int. J. Environ. Res. Public Health 2019, 16, 3164. [Google Scholar] [CrossRef] [PubMed]
- Gentzke, A.S.; Wang, T.W.; Jamal, A.; Park-Lee, E.; Ren, C.; Cullen, K.A.; Neff, L. Tobacco Product Use Among Middle and High School Students—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Beard, E.; West, R.; Michie, S.; Brown, J. Association of prevalence of electronic cigarette use with smoking cessation and cigarette consumption in England: A time–series analysis between 2006 and 2017. Addiction 2019, 115, 961–974. [Google Scholar] [CrossRef]
- Filippidis, F.T.; Laverty, A.A.; Gerovasili, V.; Vardavas, C.I. Two-year trends and predictors of e-cigarette use in 27 European Union member states. Tob. Control 2017, 26, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Bauld, L.; MacKintosh, A.M.; Eastwood, B.; Ford, A.; Moore, G.; Dockrell, M.; Arnott, D.; Cheeseman, H.; McNeill, A. Young People’s Use of E-Cigarettes across the United Kingdom: Findings from Five Surveys 2015–2017. Int. J. Environ. Res. Public Health 2017, 14, 973. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.; Reid, J.L.; Burkhalter, R.; O’Connor, R.J.; Goniewicz, M.L.; Wackowski, O.A.; Thrasher, J.F.; Hitchman, S.C. Trends in e-cigarette brands, devices and the nicotine profile of products used by youth in England, Canada and the USA: 2017–2019. Tob. Control 2021. online ahead of print. [Google Scholar] [CrossRef]
- Aleyan, S.; Hitchman, S.C.; Ferro, M.A.; Leatherdale, S.T. Trends and predictors of exclusive e-cigarette use, exclusive smoking and dual use among youth in Canada. Addict. Behav. 2020, 109, 106481. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.G.; Aleyan, S.; Battista, K.; Leatherdale, S.T. Trends in youth e-cigarette and cigarette use between 2013 and 2019: Insights from repeat cross-sectional data from the COMPASS study. Can J. Public Health 2021, 112, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A.; Aida, J.; Kusama, T.; Tabuchi, T.; Tsuboya, T.; Sugiyama, K.; Yamamoto, T.; Osaka, K. Heated Tobacco Products Have Reached Younger or More Affluent People in Japan. J. Epidemiol. 2020, 31, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, A.; Kuwabara, Y.; Fujii, M.; Imamoto, A.; Osaki, Y.; Minobe, R.; Maezato, H.; Nakayama, H.; Takimura, T.; Higuchi, S. Heated Tobacco Product Smokers in Japan Identified by a Population-Based Survey. J. Epidemiol. 2020, 30, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Stoklosa, M.; Cahn, Z.; Liber, A.; Nargis, N.; Drope, J. Effect of IQOS introduction on cigarette sales: Evidence of decline and replacement. Tob. Control 2019, 29, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Tabuchi, T. Use of Multiple Tobacco and Tobacco-Like Products Including Heated Tobacco and E-Cigarettes in Japan: A Cross-Sectional Assessment of the 2017 JASTIS Study. Int. J. Environ. Res. Public Health 2020, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Tabuchi, T.; Kunugita, N. Rapid increase in heated tobacco product (HTP) use from 2015 to 2019: From the Japan ‘Society and New Tobacco’ Internet Survey (JASTIS). Tob. Control 2020, 30, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Cummings, K.M.; Nahhas, G.J.; Sweanor, D.T. What Is Accounting for the Rapid Decline in Cigarette Sales in Japan? Int. J. Environ. Res. Public Health 2020, 17, 3570. [Google Scholar] [CrossRef] [PubMed]
- Odani, S.; Tabuchi, T. Prevalence of heated tobacco product use in Japan: The 2020 JASTIS study. Tob. Control 2022, 31, e64–e65. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Ryu, D.H.; Park, S.-W. Heated tobacco products: Cigarette complements, not substitutes. Drug Alcohol Depend. 2019, 204, 107576. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Lee, S.; Cho, H.-J. Prevalence and predictors of heated tobacco product use and its relationship with attempts to quit cigarette smoking among Korean adolescents. Tob. Control 2021, 30, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, H.; Lee, S.; Paek, Y.-J. Awareness, experience and prevalence of heated tobacco product, IQOS, among young Korean adults. Tob. Control 2018, 27, s74–s77. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Cho, S.-I. Heated tobacco product use among Korean adolescents. Tob. Control 2020, 29, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, M.S.; Seelam, R.; Tucker, J.S.; Rodriguez, A.; Shih, R.A.; D’Amico, E.J. Correlates of Awareness and Use of Heated Tobacco Products in a Sample of US Young Adults in 2018–2019. Nicotine Tob. Res. 2020, 22, 2178–2187. [Google Scholar] [CrossRef]
- Ratajczak, A.; Jankowski, P.; Strus, P.; Feleszko, W. Heat Not Burn Tobacco Product-A New Global Trend: Impact of Heat-Not-Burn Tobacco Products on Public Health, a Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 409. [Google Scholar] [CrossRef]
- McKelvey, K.; Popova, L.; Kim, M.; Chaffee, B.W.; Vijayaraghavan, M.; Ling, P.; Halpern-Felsher, B. Heated tobacco products likely appeal to adolescents and young adults. Tob. Control 2018, 27, s41–s47. [Google Scholar] [CrossRef]
- Laverty, A.A.; Vardavas, C.I.; Filippidis, F.T. Prevalence and reasons for use of Heated Tobacco Products (HTP) in Europe: An analysis of Eurobarometer data in 28 countries. Lancet Reg. Health-Eur. 2021, 8, 100159. [Google Scholar] [CrossRef]
- Gallus, S.; Lugo, A.; Liu, X.; Borroni, E.; Clancy, L.; Gorini, G.; Lopez, M.J.; Odone, A.; Przewozniak, K.; Tigova, O.; et al. Use and Awareness of Heated Tobacco Products in Europe. J. Epidemiol. 2021, 32, 139–144. [Google Scholar] [CrossRef]
- Tattan-Birch, H.; Brown, J.; Shahab, L.; Jackson, S.E. Trends in use of e-cigarette device types and heated tobacco products from 2016 to 2020 in England. Sci. Rep. 2021, 11, 13203. [Google Scholar] [CrossRef]
- Liber, A.C.; Cadham, C.; Cummings, M.; Levy, D.T.; Pesko, M. Poland is not replicating the HTP experience in Japan: A cautionary note. Tob. Control 2021, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Abroms, L.; Levine, H.; Romm, K.; Wysota, C.; Broniatowski, D.; Bar-Zeev, Y.; Berg, C. Anticipating IQOS market expansion in the United States. Tob. Prev. Cessat. 2022, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.J.; Bar-Zeev, Y.; Levine, H. Informing iQOS Regulations in the United States: A Synthesis of What We Know. SAGE Open 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.J.; Romm, K.F.; Patterson, B.; Wysota, C.N. Heated Tobacco Product Awareness, Use, and Perceptions in a Sample of Young Adults in the United States. Nicotine Tob. Res. 2021, 23, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Azagba, S.; Shan, L. Heated Tobacco Products: Awareness and Ever Use among, U.S. Adults. Am. J. Prev. Med. 2021, 60, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.T.; Cummings, K.M.; Villanti, A.C.; Niaura, R.; Abrams, D.B.; Fong, G.T.; Borland, R. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction 2017, 112, 8–17. [Google Scholar] [CrossRef]
- Levy, D.T.; Borland, R.; Villanti, A.C.; Niaura, R.; Yuan, Z.; Zhang, Y.; Meza, R.; Holford, T.R.; Fong, G.T.; Cummings, K.M.; et al. The Application of a Decision-Theoretic Model to Estimate the Public Health Impact of Vaporized Nicotine Product Initiation in the United States. Nicotine Tob. Res. 2017, 19, 149–159. [Google Scholar] [CrossRef]
- Levy, D.T.; Yuan, Z.; Li, Y.; Alberg, A.J.; Cummings, K.M. A modeling approach to gauging the effects of nicotine vaping product use on cessation from cigarettes: What do we know, what do we need to know? Addiction 2019, 114 (Suppl. 1), 86–96. [Google Scholar] [CrossRef]
- Fairchild, A.L.; Bayer, R.; Lee, J.S. The E-Cigarette Debate: What Counts as Evidence? Am. J. Public Health 2019, 109, 1000–1006. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Smith, D.M.; Edwards, K.C.; Blount, B.C.; Caldwell, K.L.; Feng, J.; Wang, L.; Christensen, C.; Ambrose, B.; Borek, N.; et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open 2018, 1, e185937. [Google Scholar] [CrossRef]
- Nutt, D.J.; Phillips, L.D.; Balfour, D.; Curran, H.V.; Dockrell, M.; Foulds, J.; Fagerstrom, K.; Letlape, K.; Polosa, R.; Ramsey, J.; et al. E-cigarettes are less harmful than smoking. Lancet 2016, 387, 1160–1162. [Google Scholar] [CrossRef]
- McNeill, A.; Brose, L.; Calder, R.; Bauld, L.; Robson, D. Evidence Review of E-Cigarettes and Heated Tobacco Products 2018. A Report Commissioned by Public Health England; Public Health England: London, UK, 2018.
- Eissenberg, T.; Bhatnagar, A.; Chapman, S.; Jordt, S.-E.; Shihadeh, A.; Soule, E.K. Invalidity of an Oft-Cited Estimate of the Relative Harms of Electronic Cigarettes. Am. J. Public Health 2020, 110, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Billimek, J. Estimating the Health Impacts of Tobacco Harm Reduction Policies: A Simulation Modeling Approach. Risk Anal. 2005, 25, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Feirman, S.P.; Donaldson, E.; Glasser, A.M.; Pearson, J.L.; Niaura, R.; Rose, S.W.; Abrams, D.B.; Villanti, A.C. Mathematical Modeling in Tobacco Control Research: Initial Results From a Systematic Review. Nicotine Tob. Res. 2016, 18, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.N.; Abrams, D.; Bachand, A.; Baker, G.; Black, R.; Camacho, O.; Curtin, G.; Djurdjevic, S.; Hill, A.; Mendez, D.; et al. Estimating the Population Health Impact of Recently Introduced Modified Risk Tobacco Products: A Comparison of Different Approaches. Nicotine Tob. Res. 2021, 23, 426–437. [Google Scholar] [CrossRef]
- Lee, P.N.; Djurdjevic, S.; Weitkunat, R.; Baker, G. Estimating the population health impact of introducing a reduced-risk tobacco product into Japan. The effect of differing assumptions, and some comparisons with the U.S. Regul. Toxicol. Pharmacol. 2018, 100, 92–104. [Google Scholar] [CrossRef]
- Camacho, O.M.; Hill, A.; Fiebelkorn, S.; Jones, J.D.; Prasad, K.; Proctor, C.; Murphy, J. Modeling the Population Health Impacts of Heated Tobacco Products in Japan. Tob. Regul. Sci. 2021, 7, 221–231. [Google Scholar] [CrossRef]
- Bachand, A.M.; Sulsky, S.I.; Curtin, G.M. Assessing the Likelihood and Magnitude of a Population Health Benefit Following the Market Introduction of a Modified-Risk Tobacco Product: Enhancements to the Dynamic Population Modeler, DPM(+1). Risk Anal. 2018, 38, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.; Warner, K.E. A Magic Bullet? The Potential Impact of E-Cigarettes on the Toll of Cigarette Smoking. Nicotine Tob. Res. 2021, 23, 654–661. [Google Scholar] [CrossRef]
- Cherng, S.T.; Tam, J.; Christine, P.J.; Meza, R. Modeling the Effects of E-cigarettes on Smoking Behavior: Implications for Future Adult Smoking Prevalence. Epidemiology 2016, 27, 819–826. [Google Scholar] [CrossRef][Green Version]
- Niaura, R.; Rich, I.; Johnson, A.L.; Villanti, A.; Romberg, A.R.; Hair, E.C.; Vallone, D.M.; Abrams, D.B. Young Adult Tobacco and E-cigarette Use Transitions: Examining Stability Using Multistate Modeling. Nicotine Tob. Res. 2020, 22, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Altria Launches Iqos Tobacco Device in US, and the Timing Couldn’t Be Better. CNBC News. 4 October 2019. Available online: https://www.cnbc.com/2019/10/04/altria-launches-iqos-tobacco-device-in-us-and-the-timing-couldnt-be-better.html (accessed on 5 January 2020).
- Herzog, B. MO’s Competitive Moat Widens with iQOS—We See Huge Opportunity for iQOS to Take Significant Share as FDA Cracks Down; Wells Fargo Securities Research: New York, NY, USA, 2019. [Google Scholar]
- Glantz, S.A. Heated tobacco products: The example of IQOS. Tob. Control 2018, 27, s1–s6. [Google Scholar] [CrossRef] [PubMed]
- Bosilkovska, M.; Tran, C.T.; de La Bourdonnaye, G.; Taranu, B.; Benzimra, M.; Haziza, C. Exposure to harmful and potentially harmful constituents decreased in smokers switching to Carbon-Heated Tobacco Product. Toxicol. Lett. 2020, 330, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Drovandi, A.; Salem, S.; Barker, D.; Booth, D.; Kairuz, T. Human Biomarker Exposure From Cigarettes Versus Novel Heat-Not-Burn Devices: A Systematic Review and Meta-Analysis. Nicotine Tob. Res. 2020, 22, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Yannovits, N.; Sarri, T.; Voudris, V.; Poulas, K. Nicotine Delivery to the Aerosol of a Heat-Not-Burn Tobacco Product: Comparison With a Tobacco Cigarette and E-Cigarettes. Nicotine Tob. Res. 2018, 20, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Lüdicke, F.; Ansari, S.M.; Lama, N.; Blanc, N.; Bosilkovska, M.; Donelli, A.; Picavet, P.; Baker, G.; Haziza, C.; Peitsch, M.; et al. Effects of Switching to a Heat-Not-Burn Tobacco Product on Biologically Relevant Biomarkers to Assess a Candidate Modified Risk Tobacco Product: A Randomized Trial. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Simonavicius, E.; McNeill, A.; Shahab, L.; Brose, L.S. Heat-not-burn tobacco products: A systematic literature review. Tob. Control 2019, 28, 582–594. [Google Scholar] [CrossRef]
- Mallock, N.; Pieper, E.; Hutzler, C.; Henkler-Stephani, F.; Luch, A. Heated Tobacco Products: A Review of Current Knowledge and Initial Assessments. Front. Public Health 2019, 7, 287. [Google Scholar] [CrossRef]
- St Helen, G.; Jacob Iii, P.; Nardone, N.; Benowitz, N.L. IQOS: Examination of Philip Morris International’s claim of reduced exposure. Tob. Control 2018, 27, s30–s36. [Google Scholar] [CrossRef]
- Znyk, M.; Jurewicz, J.; Kaleta, D. Exposure to Heated Tobacco Products and Adverse Health Effects, a Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6651. [Google Scholar] [CrossRef]
- Tattan-Birch, H.; Jackson, S.; Shahab, L.; Hartmann-Boyce, J.; Kock, L.; Simonavicius, E.; Brose, L.; Brown, J. Heated tobacco products for smoking cessation and reducing smoking prevalence. Cochrane Database Syst. Rev. 2022, 1, CD013790. [Google Scholar] [CrossRef] [PubMed]
- Dautzenberg, B.; Dautzenberg, M.D. Systematic analysis of the scientific literature on heated tobacco. Rev. Mal. Respir. 2019, 36, 82–103. [Google Scholar] [CrossRef] [PubMed]
- Wells Fargo Securities Equity Research. MO: Continues Its Aggressive Pivot & We’re on Board! Wells Fargo and Company: New York, NY, USA, 2019. [Google Scholar]
- Levy, D.T.; Chaloupka, F.; Lindblom, E.N.; Sweanor, D.T.; O’Connor, R.J.; Shang, C.; Borland, R. The US Cigarette Industry: An Economic and Marketing Perspective. Tob. Regul. Sci. 2019, 5, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.T.; Lindblom, E.N.; Sweanor, D.T.; Chaloupka, F.; O’Connor, R.J.; Shang, C.; Palley, T.; Fong, G.T.; Cummings, M.K.; Goniewicz, M.L.; et al. An Economic Analysis of the Pre-Deeming US Market for Nicotine Vaping Products. Tob. Regul. Sci. 2019, 5, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.T.; Sánchez-Romero, L.M.; Douglas, C.E.; Sweanor, D.T. An Analysis of the Altria-Juul Labs Deal: Antitrust and Population Health Implications. J. Compet. Law Econ. 2021, 17, 458–492. [Google Scholar] [CrossRef] [PubMed]
- Pirie, K.; Peto, R.; Reeves, G.K.; Green, J.; Beral, V. The 21st century hazards of smoking and benefits of stopping: A prospective study of one million women in the UK. Lancet 2013, 381, 133–141. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010.
- Jha, P.; Ramasundarahettige, C.; Landsman, V.; Rostron, B.; Thun, M.; Anderson, R.N.; McAfee, T.; Peto, R. 21st-Century Hazards of Smoking and Benefits of Cessation in the United States. N. Engl. J. Med. 2013, 368, 341–350. [Google Scholar] [CrossRef]
- Royal College of Physicians. Nicotine without Smoke: Tobacco Harm Reduction; RCP: London, UK, 2016. [Google Scholar]
- Glasser, A.M.; Collins, L.; Pearson, J.L.; Abudayyeh, H.; Niaura, R.S.; Abrams, D.B.; Villanti, A.C. Overview of Electronic Nicotine Delivery Systems: A Systematic Review. Am. J. Prev. Med. 2017, 52, e33–e66. [Google Scholar] [CrossRef]
- Shahab, L.; Goniewicz, M.L.; Blount, B.C.; Brown, J.; McNeill, A.; Alwis, K.U.; Feng, J.; Wang, L.; West, R. Nicotine, Carcinogen, and Toxin Exposure in Long-Term E-Cigarette and Nicotine Replacement Therapy Users: A Cross-sectional Study. Ann. Intern. Med. 2017, 166, 390–400. [Google Scholar] [CrossRef]
- Shahab, L.; Goniewicz, M.L.; Blount, B.C.; Brown, J.; West, R. E-Cigarettes and Toxin Exposure. Ann. Intern. Med. 2017, 167, 525–526. [Google Scholar] [CrossRef]
- Levy, D.T.; Meza, R.; Yuan, Z.; Li, Y.; Cadham, C.; Sanchez-Romero, L.M.; Travis, N.; Knoll, M.; Liber, A.C.; Mistry, R.; et al. Public health impact of a US ban on menthol in cigarettes and cigars: A simulation study. Tob. Control 2021, 1. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, T.; Pena, I.; Temesgen, N.; Glantz, S.A. Association between Electronic Cigarette Use and Myocardial Infarction. Am. J. Prev. Med. 2018, 55, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, D.N.; Glantz, S.A. Association of E-Cigarette Use With Respiratory Disease Among Adults: A Longitudinal Analysis. Am. J. Prev. Med. 2020, 58, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Stephens, W. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tob. Control 2017, 27, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Yannovits, N.; Sarri, T.; Voudris, V.; Poulas, K.; Leischow, S.J. Carbonyl emissions from a novel heated tobacco product (IQOS): Comparison with an e-cigarette and a tobacco cigarette. Addiction 2018, 113, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Glantz, S.A. PMI’s own in vivo clinical data on biomarkers of potential harm in Americans show that IQOS is not detectably different from conventional cigarettes. Tob. Control 2018, 27, s9–s12. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Romero, L.M.; Cadham, C.J.; Hirschtick, J.L.; Mattingly, D.J.; Cho, B.; Fleischer, N.L.; Brouwer, A.; Mistry, R.; Land, S.R.; Jeon, J.; et al. A Comparison of Tobacco Product Prevalence by Different Frequency of Use Thresholds across Three US Surveys. BMC Public Health 2021, 21, 1203. [Google Scholar] [CrossRef] [PubMed]
- Halpern-Felsher, B. Point-of-sale marketing of heated tobacco products in Israel: Cause for concern. Isr. J. Health Policy Res. 2019, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.L.; Blanchflower, T.M.; O’Brien, K.F.; Averett, P.E.; Cofie, L.E.; Gregory, K.R. Evolving IQOS packaging designs change perceptions of product appeal, uniqueness, quality and safety: A randomised experiment, 2018, USA. Tob. Control 2019, 28, e52–e55. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.; Baiocchi, M.; Halpern-Felsher, B. PMI’s heated tobacco products marketing claims of reduced risk and reduced exposure may entice youth to try and continue using these products. Tob. Control 2020, 29, e18–e24. [Google Scholar] [CrossRef] [PubMed]
- Hair, E.C.; Bennett, M.; Sheen, E.; Cantrell, J.; Briggs, J.; Fenn, Z.; Willett, J.G.; Vallone, D. Examining perceptions about IQOS heated tobacco product: Consumer studies in Japan and Switzerland. Tob. Control 2018, 27, s70–s73. [Google Scholar] [CrossRef] [PubMed]
- Kim, M. Philip Morris International introduces new heat-not-burn product, IQOS, in South Korea. Tob. Control 2018, 27, e76–e78. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.; Popova, L.; Kim, M.; Lempert, L.K.; Chaffee, B.W.; Vijayaraghavan, M.; Ling, P.; Halpern-Felsher, B. IQOS labelling will mislead consumers. Tob. Control 2018, 27, s48–s54. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.J.; Kislev, S. IQOS campaign in Israel. Tob. Control 2018, 27, s78–s81. [Google Scholar] [CrossRef] [PubMed]
- Mathers, A.; Schwartz, R.; O’Connor, S.; Fung, M.; Diemert, L. Marketing IQOS in a dark market. Tob. Control 2019, 28, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Talbot, E.M.; Giovenco, D.P.; Grana, R.; Hrywna, M.; Ganz, O. Cross-promotion of nicotine pouches by leading cigarette brands. Tob. Control 2021, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hrywna, M.; Lewis, M.J.; Ling, P.M. Nicotine Pouch Unit Sales in the US from 2016 to 2020. JAMA 2021, 326, 2330–2331. [Google Scholar] [CrossRef]
- Marynak, K.; Emery, S.; King, B.A. Nicotine Pouch Unit Sales in the US from 2016 to 2020—Reply. JAMA 2021, 326, 2331. [Google Scholar] [CrossRef]
- Marynak, K.L.; Wang, X.; Borowiecki, M.; Kim, Y.; Tynan, M.A.; Emery, S.; King, B.A. Nicotine Pouch Unit Sales in the US, 2016–2020. JAMA 2021, 326, 566–568. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Tabuchi, T. Heated tobacco product use and combustible cigarette smoking relapse/initiation among former/never smokers in Japan: The JASTIS 2019 study with 1-year follow-up. Tob. Control 2021. [Google Scholar] [CrossRef]
- Selya, A.S.; Rose, J.S.; Dierker, L.; Hedeker, D.; Mermelstein, R.J. Evaluating the mutual pathways among electronic cigarette use, conventional smoking and nicotine dependence. Addiction 2018, 113, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Shahab, L.; Beard, E.; Brown, J. Association of initial e-cigarette and other tobacco product use with subsequent cigarette smoking in adolescents: A cross-sectional, matched control study. Tob. Control 2021, 30, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.K.; Okawa, S.; Meza, R.; Katanoda, K.; Tabuchi, T. Nicotine dependence of cigarette and heated tobacco users in Japan, 2019: A cross-sectional analysis of the JASTIS Study. Tob. Control 2021, 31, e50–e56. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Li, W.H.C.; Luo, Y.H.; Na Liang, T.; Ho, L.L.K.; Cheung, A.T.; Song, P. The association between heated tobacco product use and cigarette cessation outcomes among youth smokers: A prospective cohort study. J. Subst. Abus. Treat. 2022, 132, 108599. [Google Scholar] [CrossRef] [PubMed]
- Etter, J.-F.; Eissenberg, T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015, 147, 68–75. [Google Scholar] [CrossRef]
- Liu, G.; Wasserman, E.; Kong, L.; Foulds, J. A comparison of nicotine dependence among exclusive E-cigarette and cigarette users in the PATH study. Prev. Med. 2017, 104, 86–91. [Google Scholar] [CrossRef]
- Shiffman, S.; Sembower, M.A. Dependence on e-cigarettes and cigarettes in a cross-sectional study of US adults. Addiction 2020, 115, 1924–1931. [Google Scholar] [CrossRef]
- Henriksen, L. Comprehensive tobacco marketing restrictions: Promotion, packaging, price and place. Tob. Control 2012, 21, 147–153. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.J.; Shin, S.H.; Park, E.; Oh, J.-K.; Park, E.Y.; Lim, M.K. Perceptions of Heated Tobacco Products (HTPs) and Intention to Quit among Adult Tobacco Users in Korea. J. Epidemiol. 2021, 32, 357–362. [Google Scholar] [CrossRef]
- Kanai, M.; Kanai, O.; Tabuchi, T.; Mio, T. Association of heated tobacco product use with tobacco use cessation in a Japanese workplace: A prospective study. Thorax 2021, 76, 615–617. [Google Scholar] [CrossRef]
- Luk, T.T.; Weng, X.; Wu, Y.S.; Chan, H.L.; Lau, C.Y.; Kwong, A.C.-S.; Lai, V.W.-Y.; Lam, T.H.; Wang, M.P. Association of heated tobacco product use with smoking cessation in Chinese cigarette smokers in Hong Kong: A prospective study. Tob. Control 2021, 30, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Kim, C.-Y.; Lee, K.; Kim, S. Are Heated Tobacco Product Users Less Likely to Quit than Cigarette Smokers? Findings from THINK (Tobacco and Health IN Korea) Study. Int. J. Environ. Res. Public Health 2020, 17, 8622. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Kimm, H.; Lee, J.-A.; Lee, C.-M.; Cho, H.-J. Heated tobacco product use and its relationship to quitting combustible cigarettes in Korean adults. PLoS ONE 2021, 16, e0251243. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; McRobbie, H.; Lindson, N.; Bullen, C.; Begh, R.; Theodoulou, A.; Notley, C.; Rigotti, N.A.; Turner, T.; Butler, A.R.; et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2021, 9, CD010216. [Google Scholar] [CrossRef]
- Kiyohara, K.; Tabuchi, T. Use of heated tobacco products in smoke-free locations in Japan: The JASTIS 2019 study. Tob. Control 2020, 31, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Sutanto, E.; Smith, D.M.; Hitchman, S.C.; Gravely, S.; Yong, H.-H.; Borland, R.; O’Connor, R.J.; Cummings, K.M.; Fong, G.T.; et al. Characterizing Heated Tobacco Product Use among Adult Cigarette Smokers and Nicotine Vaping Product Users in the 2018 ITC Four Country Smoking & Vaping Survey. Nicotine Tob. Res. 2021, 24, 493–502. [Google Scholar] [CrossRef]
- Lee, J.A.; Lee, C.; Cho, H.-J. Use of heated tobacco products where their use is prohibited. Tob. Control 2021. [Google Scholar] [CrossRef]
- Sutanto, E.; Smith, D.M.; Miller, C.; O’Connor, R.J.; Hyland, A.; Tabuchi, T.; Quah, A.C.K.; Cummings, K.M.; Xu, S.; Fong, G.T.; et al. Use of Heated Tobacco Products within Indoor Spaces: Findings from the 2018 ITC Japan Survey. Int. J. Environ. Res. Public Health 2019, 16, 4862. [Google Scholar] [CrossRef]
- Levy, D.T.; Douglas, C.E.; Sanchez-Romero, L.M.; Cummings, K.M.; Sweanor, D.T. An Analysis of the FTC’s Attempt to Stop the Altria-Juul Labs Deal. Tob. Regul. Sci. 2020, 6, 302–325. [Google Scholar] [CrossRef]
- Du, P.; Fan, T.; Yingst, J.; Veldheer, S.; Hrabovsky, S.; Chen, C.; Foulds, J. Changes in E-Cigarette Use Behaviors and Dependence in Long-term E-Cigarette Users. Am. J. Prev. Med. 2019, 57, 374–383. [Google Scholar] [CrossRef]
- Levy, D.T.; Tam, J.; Kuo, C.; Fong, G.T.; Chaloupka, F. The Impact of Implementing Tobacco Control Policies: The 2017 Tobacco Control Policy Scorecard. J. Public Health Manag. Pract. 2018, 24, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Bauer, J.; Lee, H. The Use of Simulation Models to Examine the Effect of Public Policies in a Dynamic Social System. Am. J. Public Health 2006, 96, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.T.; Mabry, P.L.; Graham, A.L.; Orleans, C.T.; Abrams, D.B. Reaching Healthy People 2010 by 2013: A SimSmoke Simulation. Am. J. Prev. Med. 2010, 38, S373–S381. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.T.; Mabry, P.L.; Graham, A.L.; Orleans, C.T.; Abrams, D.B. Exploring Scenarios to Dramatically Reduce Smoking Prevalence: A Simulation Model of the Three-Part Cessation Process. Am. J. Public Health 2010, 100, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.T.; Chaloupka, F.; Gitchell, J. The effects of tobacco control policies on smoking rates: A tobacco control scorecard. J. Public Health Manag. Pract. 2004, 10, 338–353. [Google Scholar] [CrossRef]
- Chaloupka, F.J.; Sweanor, D.; Warner, K.E. Differential Taxes for Differential Risks—Toward Reduced Harm from Nicotine-Yielding Products. N. Engl. J. Med. 2015, 373, 594–597. [Google Scholar] [CrossRef]
- Gaca, M.; Williamson, J.; Digard, H.; Adams, L.; Hawkridge, L.; Proctor, C. Bridging: Accelerating Regulatory Acceptance of Reduced-Risk Tobacco and Nicotine Products. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2022, 24, 1371–1378. [Google Scholar] [CrossRef]
- Premarket Tobacco Product Marketing Granted Orders. 2022. Available online: https://www.fda.gov/tobacco-products/premarket-tobacco-product-applications/premarket-tobacco-product-marketing-granted-orders (accessed on 28 May 2020).
- Czoli, C.D.; Fong, G.T.; Mays, D.; Hammond, D. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob. Control 2017, 26, e49–e58. [Google Scholar] [CrossRef]
- Huang, J.; Feng, B.; Weaver, S.R.; Pechacek, T.F.; Slovic, P.; Eriksen, M.P. Changing Perceptions of Harm of e-Cigarette vs Cigarette Use Among Adults in 2 US National Surveys From 2012 to 2017. JAMA Netw. Open 2019, 2, e191047. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, L.T.; Sweanor, D.T. ‘Not harmless’ messages without comparisons disserve consumers, potential consumers, and public health approaches to tobacco/nicotine products. Addict. Behav. 2018, 76, 390–391. [Google Scholar] [CrossRef]
- Kozlowski, L.T.; Sweanor, D.T. Young or adult users of multiple tobacco/nicotine products urgently need to be informed of meaningful differences in product risks. Addict. Behav. 2018, 76, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Liber, A.C. Using Regulatory Stances to See All the Commercial Determinants of Health. In The Milbank Quartrly; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Kim, S.; Selya, A.S. The Relationship Between Electronic Cigarette Use and Conventional Cigarette Smoking Is Largely Attributable to Shared Risk Factors. Nicotine Tob. Res. 2020, 22, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Selya, A.S.; Foxon, F. Trends in electronic cigarette use and conventional smoking: Quantifying a possible ‘diversion’ effect among US adolescents. Addiction 2021, 116, 1848–1858. [Google Scholar] [CrossRef]
- Khouja, J.N.; Suddell, S.F.; Peters, S.E.; Taylor, A.E.; Munafò, M.R. Is e-cigarette use in non-smoking young adults associated with later smoking? A systematic review and meta-analysis. Tob. Control 2020, 30, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, E.; Shang, C. The pass-through of excise taxes to market prices of heated tobacco products (HTPs) and cigarettes: A cross-country analysis. Eur. J. Health Econ. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lund, K.E.; Vedøy, T.F. A conceptual framework for assessing the public health effects from snus and novel non-combustible nicotine products. Nord. Stud. Alcohol Drugs 2021, 38, 586–604. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, D.T.; Cadham, C.J.; Li, Y.; Yuan, Z.; Liber, A.C.; Oh, H.; Travis, N.; Issabakhsh, M.; Sweanor, D.T.; Sánchez-Romero, L.M.; et al. A Decision-Theoretic Public Health Framework for Heated Tobacco and Nicotine Vaping Products. Int. J. Environ. Res. Public Health 2022, 19, 13431. https://doi.org/10.3390/ijerph192013431
Levy DT, Cadham CJ, Li Y, Yuan Z, Liber AC, Oh H, Travis N, Issabakhsh M, Sweanor DT, Sánchez-Romero LM, et al. A Decision-Theoretic Public Health Framework for Heated Tobacco and Nicotine Vaping Products. International Journal of Environmental Research and Public Health. 2022; 19(20):13431. https://doi.org/10.3390/ijerph192013431
Chicago/Turabian StyleLevy, David T., Christopher J. Cadham, Yameng Li, Zhe Yuan, Alex C. Liber, Hayoung Oh, Nargiz Travis, Mona Issabakhsh, David T. Sweanor, Luz Maria Sánchez-Romero, and et al. 2022. "A Decision-Theoretic Public Health Framework for Heated Tobacco and Nicotine Vaping Products" International Journal of Environmental Research and Public Health 19, no. 20: 13431. https://doi.org/10.3390/ijerph192013431
APA StyleLevy, D. T., Cadham, C. J., Li, Y., Yuan, Z., Liber, A. C., Oh, H., Travis, N., Issabakhsh, M., Sweanor, D. T., Sánchez-Romero, L. M., Meza, R., & Cummings, K. M. (2022). A Decision-Theoretic Public Health Framework for Heated Tobacco and Nicotine Vaping Products. International Journal of Environmental Research and Public Health, 19(20), 13431. https://doi.org/10.3390/ijerph192013431







