Effects of 8-Week Exhausting Deep Knee Flexion Flywheel Training on Persistent Quadriceps Weakness in Well-Trained Athletes Following Anterior Cruciate Ligament Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Subjects
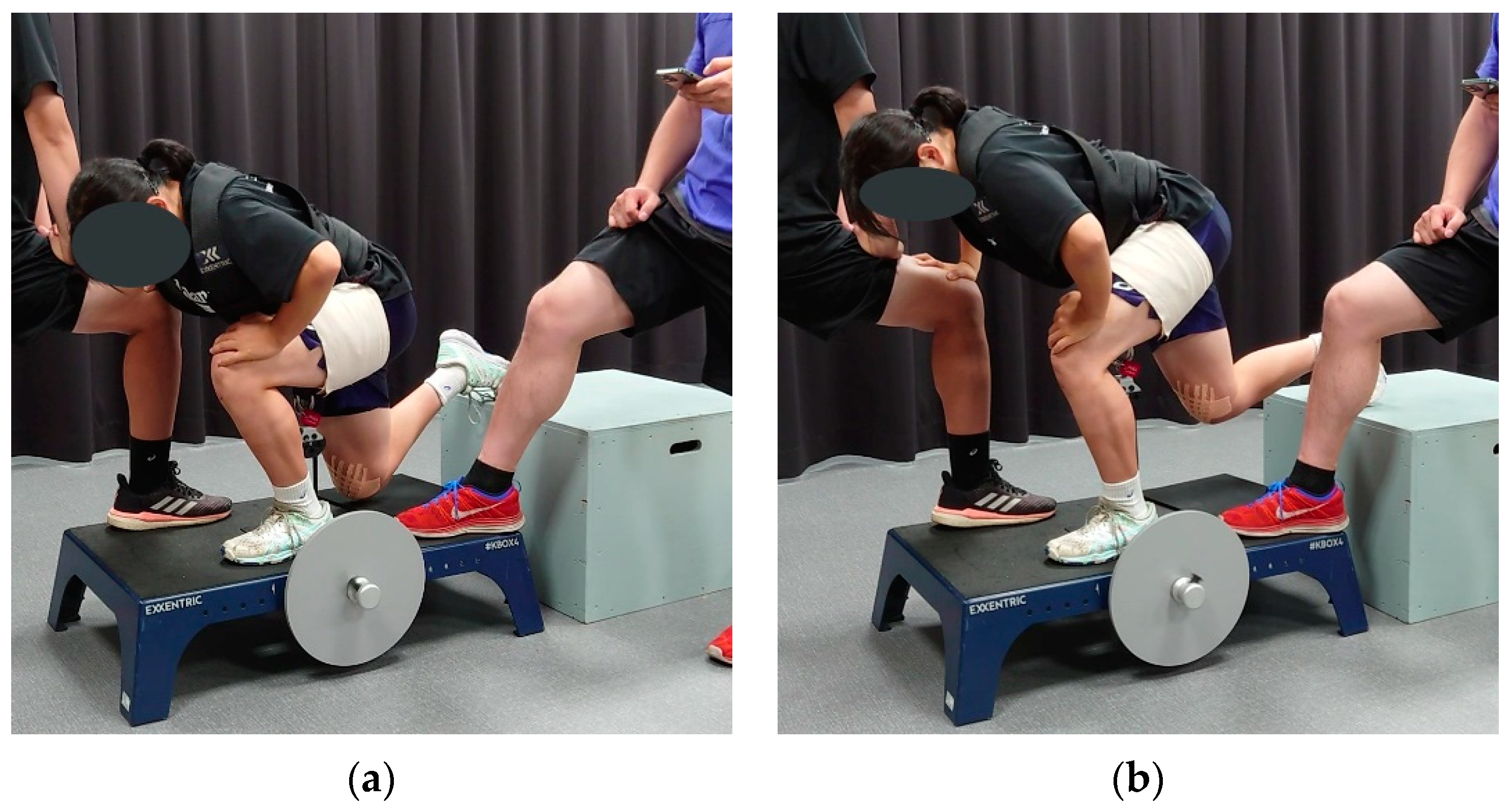
2.3. Flywheel Training
2.4. Quadriceps Measure Setup
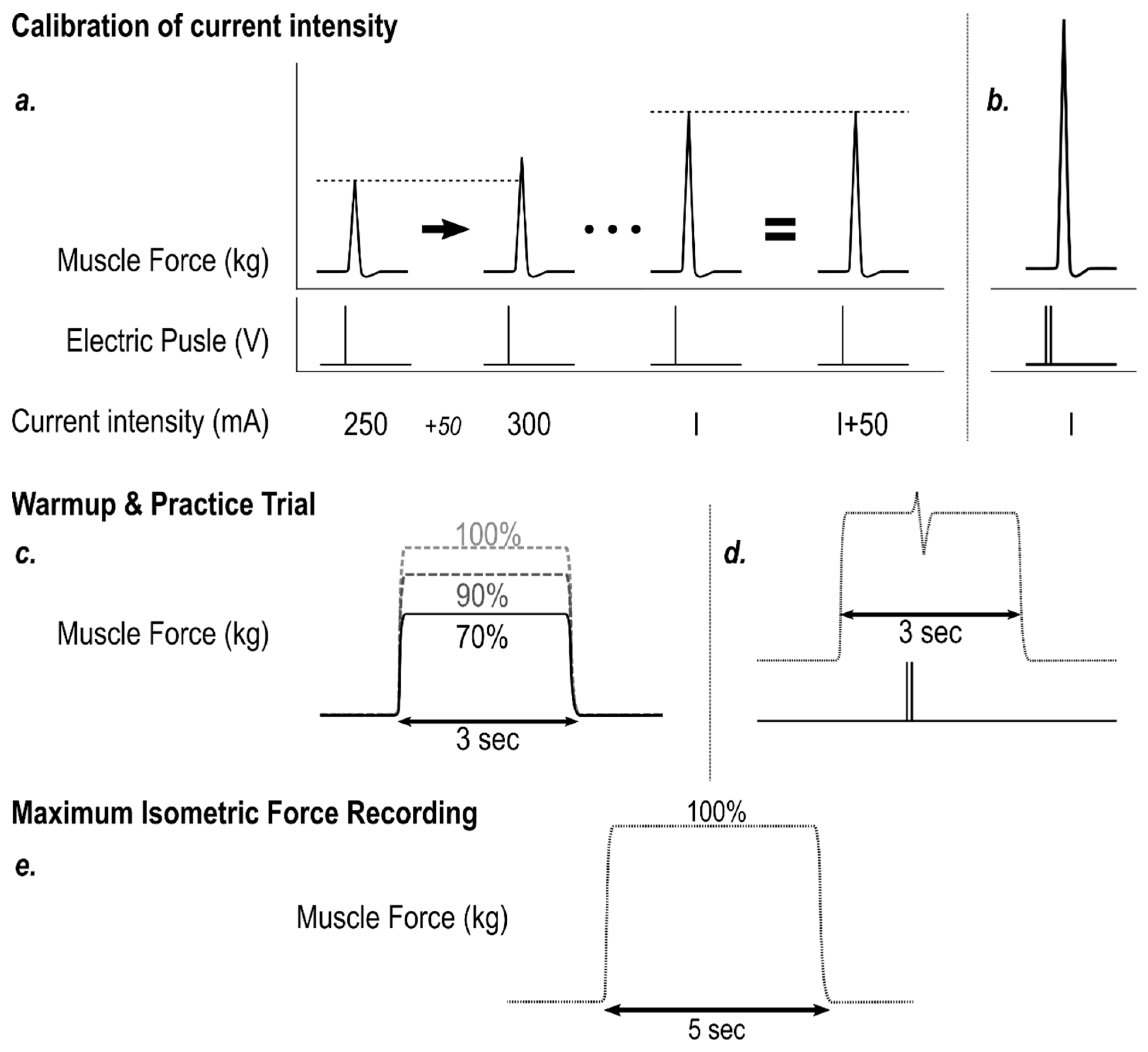
2.5. Electrostimulation Calibration & Warm-up
2.6. MVIC
2.7. RFD and MVIC
2.8. Central Activation Ratio
2.9. Analysis
3. Results
3.1. Execution of the Experiment
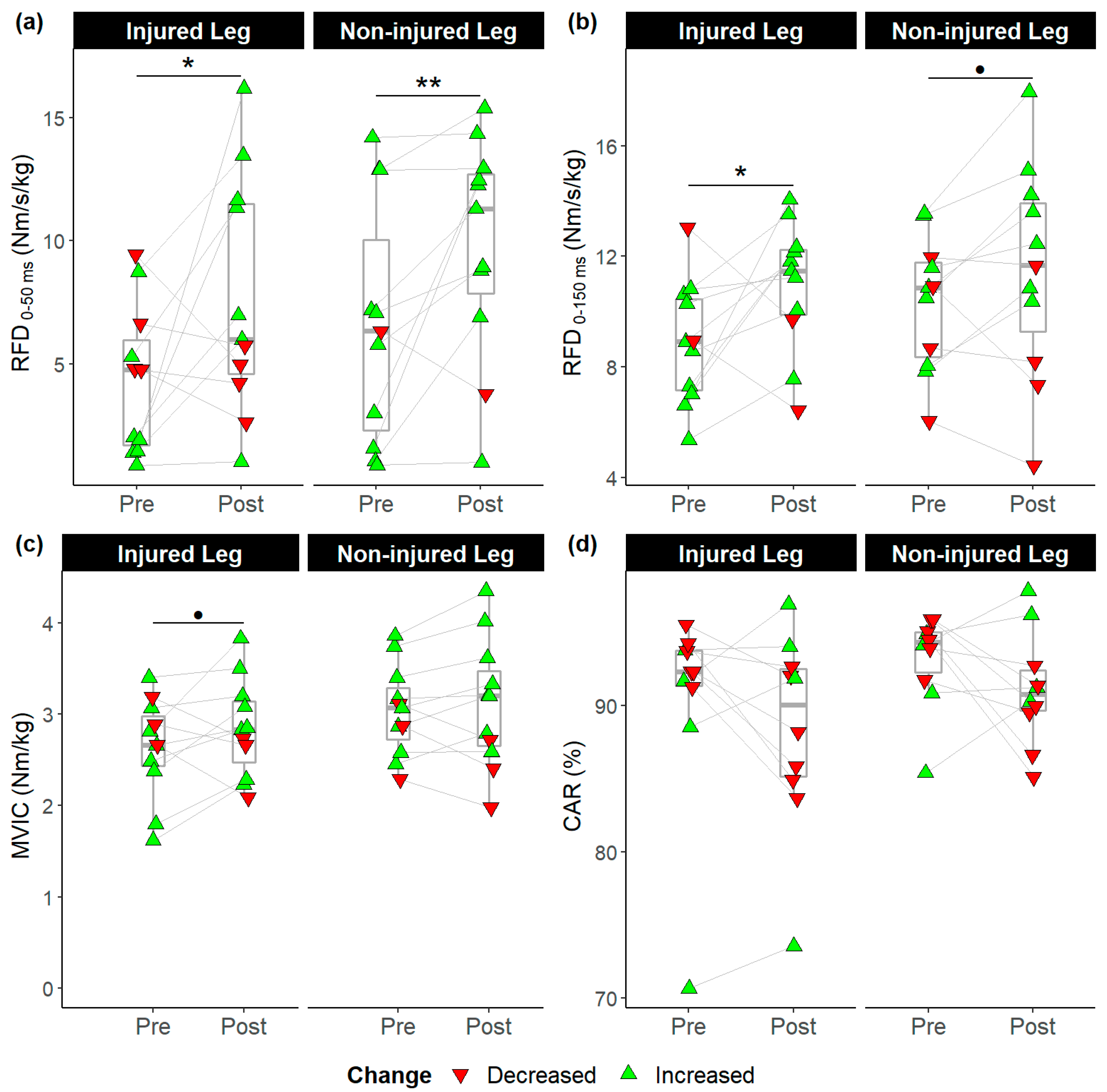
3.2. Pre-post Comparisons
3.3. Between-Leg Comparisons
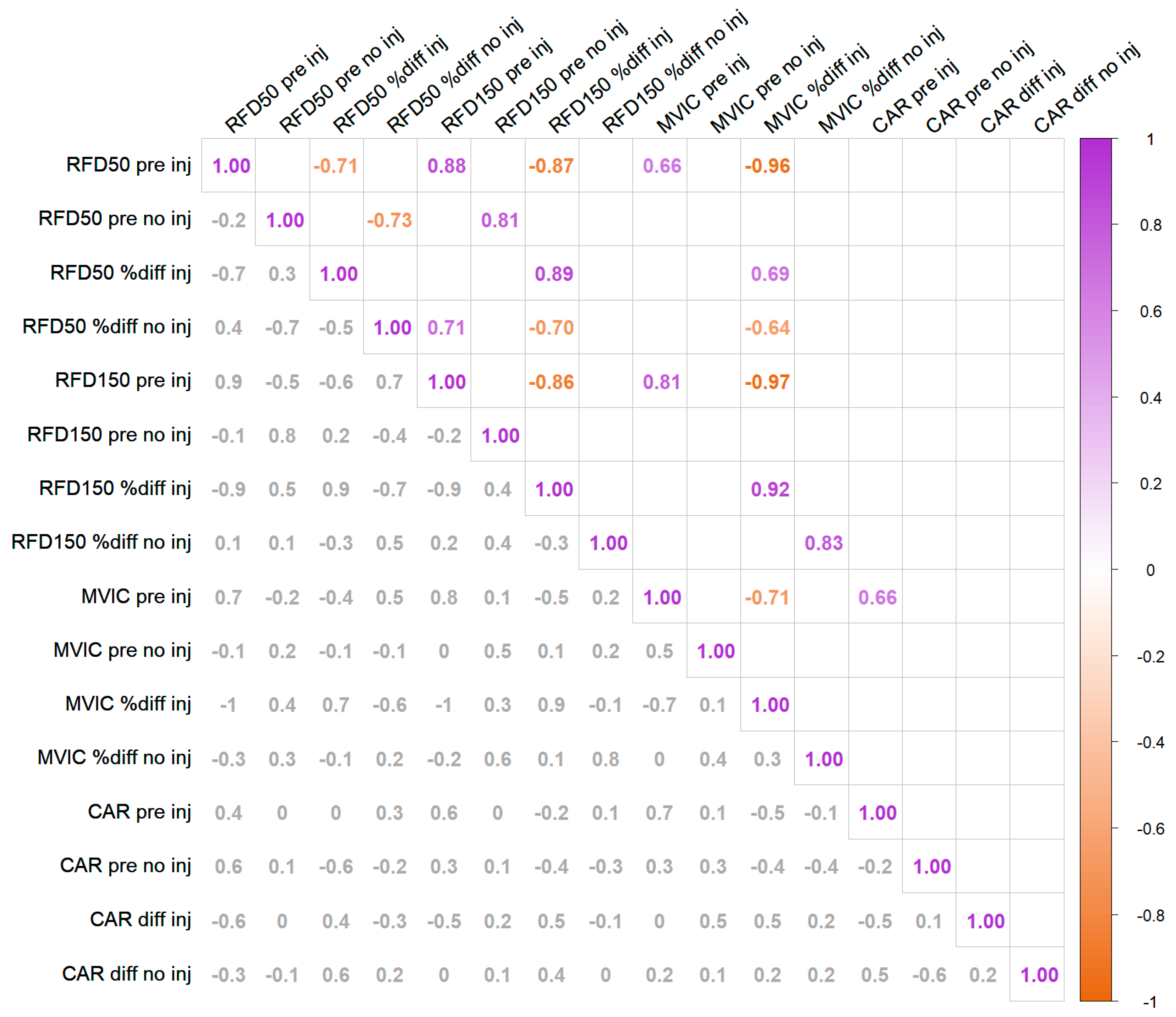
3.4. Baseline—Change Amplitude Correlation Anaylsis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonnery-Cottet, B.; Saithna, A.; Quelard, B.; Daggett, M.; Borade, A.; Ouanezar, H.; Thaunat, M.; Blakeney, W.G. Arthrogenic Muscle Inhibition after ACL Reconstruction: A Scoping Review of the Efficacy of Interventions. Br. J. Sport. Med. 2019, 53, 289–298. [Google Scholar] [CrossRef]
- Rice, D.A.; McNair, P.J. Quadriceps Arthrogenic Muscle Inhibition: Neural Mechanisms and Treatment Perspectives. Semin. Arthritis Rheum. 2010, 40, 250–266. [Google Scholar] [CrossRef]
- Krogsgaard, M.R.; Fischer-Rasmussen, T.; Dyhre-Poulsen, P. Absence of Sensory Function in the Reconstructed Anterior Cruciate Ligament. J. Electromyogr. Kinesiol. 2011, 21, 82–86. [Google Scholar] [CrossRef]
- Ochi, M.; Iwasa, J.; Uchio, Y.; Adachi, N.; Sumen, Y. The Regeneration of Sensory Neurons in the Reconstruction of the Anterior Cruciate Ligament. J. Bone Jt. Surg. 1999, 81, 902–906. [Google Scholar] [CrossRef]
- Freeman, M.A.; Wyke, B. Articular Contributions to Limb Muscle Reflexes. The Effects of Partial Neurectomy of the Knee-Joint on Postural Reflexes. Br. J. Surg. 1966, 53, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Sjölander, P.; Sojka, P. A Sensory Role for the Cruciate Ligaments. Clin. Orthop. Relat. Res. 1991, 268, 161–178. [Google Scholar]
- Konishi, Y.; Fukubayashi, T.; Takeshita, D. Possible Mechanism of Quadriceps Femoris Weakness in Patients with Ruptured Anterior Cruciate Ligament. Med. Sci. Sport. Exerc. 2002, 34, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Lepley, A.S.; Lepley, L.K. Mechanisms of Arthrogenic Muscle Inhibition. J. Sport Rehabil. 2021, 31, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Henderson, F.J.; Sasakabe, W.; Satoshi, K.; Shima, N.; Shimokochi, Y. Quadriceps Function and Athletic Performance in Highly Trained Female Athletes. J. Sport Rehabil. 2022, 1, 1–7. [Google Scholar] [CrossRef]
- O’Malley, E.; Richter, C.; King, E.; Strike, S.; Moran, K.; Franklyn-Miller, A.; Moran, R. Countermovement Jump and Isokinetic Dynamometry as Measures of Rehabilitation Status After Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2018, 53, 687–695. [Google Scholar] [CrossRef]
- Pua, Y.H.; Mentiplay, B.F.; Clark, R.A.; Ho, J.Y. Associations Among Quadriceps Strength and Rate of Torque Development 6 Weeks Post Anterior Cruciate Ligament Reconstruction and Future Hop and Vertical Jump Performance: A Prospective Cohort Study. J. Orthop. Sport. Phys. 2017, 47, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.H.; Blackburn, J.T.; Padua, D.A.; Stanley, L.E.; Harkey, M.S.; Luc-Harkey, B.A.; Pietrosimone, B. Quadriceps Neuromuscular Function and Jump-Landing Sagittal-Plane Knee Biomechanics After Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2018, 53, 135–143. [Google Scholar] [CrossRef]
- De la Fuente, C.; Stoelben, K.J.V.; Silvestre, R.; Yañez, R.; Cheyre, J.; Guadagnin, E.C.; Carpes, F.P. Steadiness Training Improves the Quadriceps Strength and Self-Reported Outcomes in Persistent Quadriceps Weakness Following Nine Months of Anterior Cruciate Ligament Reconstruction and Failed Conventional Physiotherapy. Clin. Biomech. 2022, 92, 105585. [Google Scholar] [CrossRef]
- Lepley, L.K.; Wojtys, E.M.; Palmieri-Smith, R.M. Combination of Eccentric Exercise and Neuromuscular Electrical Stimulation to Improve Quadriceps Function Post-ACL Reconstruction. Knee 2015, 22, 270–277. [Google Scholar] [CrossRef]
- Hedayatpour, N.; Falla, D. Physiological and Neural Adaptations to Eccentric Exercise: Mechanisms and Considerations for Training. BioMed. Res. Int. 2015, 2015, e193741. [Google Scholar] [CrossRef]
- Norrbrand, L.; Pozzo, M.; Tesch, P.A. Flywheel Resistance Training Calls for Greater Eccentric Muscle Activation than Weight Training. Eur. J. Appl. Physiol. 2010, 110, 997–1005. [Google Scholar] [CrossRef]
- Adam, A.; De Luca, C.J. Recruitment Order of Motor Units in Human Vastus Lateralis Muscle Is Maintained during Fatiguing Contractions. J. Neurophysiol. 2003, 90, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Henneman, E.; Somjen, G.; Carpenter, D.O. Excitability and Inhibitibility of Motoneurons of Different Sizes. J. Neurophysiol. 1965, 28, 599–620. [Google Scholar] [CrossRef]
- Beutler, A.I.; Cooper, L.W.; Kirkendall, D.T.; Garrett, W.E. Electromyographic Analysis of Single-Leg, Closed Chain Exercises: Implications for Rehabilitation After Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2002, 37, 13–18. [Google Scholar] [PubMed]
- Beynnon, B.D.; Fleming, B.C.; Johnson, R.J.; Nichols, C.E.; Renström, P.A.; Pope, M.H. Anterior Cruciate Ligament Strain Behavior During Rehabilitation Exercises In Vivo. Am. J. Sport. Med. 1995, 23, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Angelozzi, M.; Madama, M.; Corsica, C.; Calvisi, V.; Properzi, G.; McCaw, S.T.; Cacchio, A. Rate of Force Development as an Adjunctive Outcome Measure for Return-to-Sport Decisions after Anterior Cruciate Ligament Reconstruction. J. Orthop. Sport. Phys. 2012, 42, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Folland, J.P.; Buckthorpe, M.W.; Hannah, R. Human Capacity for Explosive Force Production: Neural and Contractile Determinants. Scand. J. Med. Sci. Sport. 2014, 24, 894–906. [Google Scholar] [CrossRef]
- Maffiuletti, N.A.; Aagaard, P.; Blazevich, A.J.; Folland, J.; Tillin, N.; Duchateau, J. Rate of Force Development: Physiological and Methodological Considerations. Eur. J. Appl. Physiol. 2016, 116, 1091–1116. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, S.P.; Halkjaer-Kristensen, J.; Dyhre-Poulsen, P. Neural Inhibition during Maximal Eccentric and Concentric Quadriceps Contraction: Effects of Resistance Training. J. Appl. Physiol. 2000, 89, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Increased Rate of Force Development and Neural Drive of Human Skeletal Muscle Following Resistance Training. J. Appl. Physiol. 2002, 93, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, C.J.; Kooistra, R.D.; Paalman, M.I.; de Haan, A. Initial Phase of Maximal Voluntary and Electrically Stimulated Knee Extension Torque Development at Different Knee Angles. J. Appl. Physiol. 2004, 97, 1693–1701. [Google Scholar] [CrossRef]
- Buckthorpe, M.W.; Hannah, R.; Pain, T.G.; Folland, J.P. Reliability of Neuromuscular Measurements during Explosive Isometric Contractions, with Special Reference to Electromyography Normalization Techniques. Muscle Nerve 2012, 46, 566–576. [Google Scholar] [CrossRef]
- Tillin, N.A.; Jimenez-Reyes, P.; Pain, M.T.G.; Folland, J.P. Neuromuscular Performance of Explosive Power Athletes versus Untrained Individuals. Med. Sci. Sport. Exerc. 2010, 42, 781–790. [Google Scholar] [CrossRef]
- Lepley, L.K.; Palmieri-Smith, R.M. Quadriceps Strength, Muscle Activation Failure, and Patient-Reported Function at the Time of Return to Activity in Patients Following Anterior Cruciate Ligament Reconstruction: A Cross-Sectional Study. J. Orthop. Sport. Phys. 2015, 45, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Kent-Braun, J.A.; Le Blanc, R. Quantitation of Central Activation Failure during Maximal Voluntary Contractions in Humans. Muscle Nerve 1996, 19, 861–869. [Google Scholar] [CrossRef]
- Park, J.; Hopkins, J.T. Within- and Between-Session Reliability of the Maximal Voluntary Knee Extension Torque and Activation. Int. J. Neurosci. 2012, 123, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-203-77158-7. [Google Scholar]
- de Ruiter, C.J.; Van Leeuwen, D.; Heijblom, A.; Bobbert, M.F.; de Haan, A. Fast Unilateral Isometric Knee Extension Torque Development and Bilateral Jump Height. Med. Sci. Sport. Exerc. 2006, 38, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P. Training-Induced Changes in Neural Function. Exerc. Sport Sci. Rev. 2003, 31, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mattes, K.; Wolff, S.; Alizadeh, S. Kinematic Stride Characteristics of Maximal Sprint Running of Elite Sprinters—Verification of the “Swing-Pull Technique”. J. Hum. Kinet. 2021, 77, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Folland, J.P.; Williams, A.G. The Adaptations to Strength Training: Morphological and Neurological Contributions to Increased Strength. Sport Med. 2007, 37, 145–168. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, C.J.; Vermeulen, G.; Toussaint, H.M.; de Haan, A. Isometric Knee-Extensor Torque Development and Jump Height in Volleyball Players. Med. Sci. Sport. Exerc. 2007, 39, 1336–1346. [Google Scholar] [CrossRef]
- Sale, D.G. Neural Adaptation to Resistance Training. Med. Sci. Sport. Exerc. 1988, 20, S135–S145. [Google Scholar] [CrossRef]
- Appleby, B.; Newton, R.U.; Cormie, P. Changes in Strength over a 2-Year Period in Professional Rugby Union Players. J. Strength Cond. Res. 2012, 26, 2538–2546. [Google Scholar] [CrossRef]
- Shimokochi, Y.; Shultz, S.J. Mechanisms of Noncontact Anterior Cruciate Ligament Injury. J. Athl. Train. 2008, 43, 396–408. [Google Scholar] [CrossRef]
- Shimokochi, Y.; Ide, D.; Kokubu, M.; Nakaoji, T. Relationships Among Performance of Lateral Cutting Maneuver From Lateral Sliding and Hip Extension and Abduction Motions, Ground Reaction Force, and Body Center of Mass Height. J. Strength Cond. Res. 2013, 27, 1851–1860. [Google Scholar] [CrossRef]
- Shimokochi, Y.; Ambegaonkar, J.P.; Meyer, E.G. Changing Sagittal-Plane Landing Styles to Modulate Impact and Tibiofemoral Force Magnitude and Directions Relative to the Tibia. J. Athl. Train. 2016, 51, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Shimokochi, Y.; Ambegaonkar, J.P.; Meyer, E.G.; Lee, S.Y.; Shultz, S.J. Changing Sagittal Plane Body Position during Single-Leg Landings Influences the Risk of Non-Contact Anterior Cruciate Ligament Injury. Knee Surg. Sport. Traumatol. Arthrosc. 2013, 21, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Smidt, G.L. Biomechanical Analysis of Knee Flexion and Extension. J. Biomech. 1973, 6, 79–92. [Google Scholar] [CrossRef]
- Neumann, D.A. Chapter 13 Knee. In Kinesiology of the Musculoskeletal System: Foundations for Physical Rehabilitation; Mosby Inc.: St. Louis, MO, USA, 2010; pp. 560–562. [Google Scholar]
- Sabido, R.; Hernández-Davó, J.L.; Pereyra-Gerber, G.T. Influence of Different Inertial Loads on Basic Training Variables During the Flywheel Squat Exercise. Int. J. Sport. Physiol. Perform. 2018, 13, 482–489. [Google Scholar] [CrossRef]
- Otzel, D.M.; Chow, J.W.; Tillman, M.D. Long-Term Deficits in Quadriceps Strength and Activation Following Anterior Cruciate Ligament Reconstruction. Phys. Ther. Sport 2015, 16, 22–28. [Google Scholar] [CrossRef]
- Bishop, P.; Cureton, K.; Collins, M. Sex Difference in Muscular Strength in Equally-Trained Men and Women. Ergonomics 1987, 30, 675–687. [Google Scholar] [CrossRef]
| Subject | Height (m) | Weight (kg) | Age (Years) | Dom. Leg | Injured Leg | Graft | Sport | Sex | Days from Surgery |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.72 | 60.3 | 21 | Left | Left | Hamstrings | Handball | Female | 405 |
| 2 | 1.75 | 93.1 | 22 | Left | Left | Quadriceps | Handball | Male | 858 |
| 3 | 1.63 | 63.5 | 21 | Left | Right | Hamstrings | Handball | Female | 354 |
| 4 | 1.61 | 57.8 | 19 | Left | Left | Hamstrings | Handball | Female | 374 |
| 5 | 1.64 | 58.3 | 20 | Right | Right | Hamstrings | Handball | Female | 346 |
| 6 | 1.71 | 66.1 | 20 | Left | Left | Hamstrings | Soccer | Male | 288 |
| 7 | 1.63 | 57.0 | 19 | Left | Left | BPTB | Volleyball | Female | 343 |
| 8 | 1.73 | 76.5 | 19 | Left | Right | Hamstrings | Handball | Female | 1053 |
| 9 | 1.83 | 66.6 | 28 | Left | Right | Hamstrings | Volleyball | Female | 783 |
| 10 | 1.86 | 73.5 | 19 | Left | Left | Hamstrings | Basketball | Male | 657 |
| 11 | 1.59 | 58.6 | 19 | Right | Left | Hamstrings | Handball | Female | 589 |
| Mean | 1.71 | 67.3 | 20.8 | 546.1 | |||||
| SD | 0.09 | 11.2 | 2.7 | 270.2 |
| KERRYPNX | t | df | p | MD (SD) | 95%CI | Cohen’s d | Magnitude | |
|---|---|---|---|---|---|---|---|---|
| RFD0–50 ms | ACLR * | 1.98 | 10 | 0.04 | 3.35 (5.61) | [0.28, +∞) | 0.8 [−0.2, 1.9] | Large |
| Pre-Post | Uninvolved * | 2.60 | 10 | 0.01 | 3.20 (4.08) | [0.97, +∞) | 0.7 [0.1, 1.3] | Medium |
| RFD0–150 ms | ACLR* | 2.15 | 10 | 0.03 | 2.08 (3.20) | [0.33, +∞) | 0.9 [−0.1, 2.0] | Large |
| Pre-Post | Uninvolved | 1.57 | 10 | 0.07 | 1.15 (2.44) | [−0.18, +∞) | 0.3 [−0.1, 0.7] | Small |
| MVIC | ACLR | 1.42 | 10 | 0.09 | 0.21 (0.49) | [−0.06, +∞) | 0.4 [−0.2, 1.0] | Small |
| Pre-Post | Uninvolved | 0.74 | 10 | 0.24 | 0.07 (0.32) | [−0.10, +∞) | 0.1 [−0.2, 0.4] | Trivial |
| MVIC | Pre * | 2.53 | 10 | 0.01 | 0.40 (0.53) | [0.12, +∞) | 0.8 [0.1, 1.5] | Large |
| Interlimb | Post | 1.92 | 10 | 0.04 | 0.27 (0.46) | [0.01, +∞) | 0.4 [−0.1, 0.9] | Small |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, F.J.; Konishi, Y.; Shima, N.; Shimokochi, Y. Effects of 8-Week Exhausting Deep Knee Flexion Flywheel Training on Persistent Quadriceps Weakness in Well-Trained Athletes Following Anterior Cruciate Ligament Reconstruction. Int. J. Environ. Res. Public Health 2022, 19, 13209. https://doi.org/10.3390/ijerph192013209
Henderson FJ, Konishi Y, Shima N, Shimokochi Y. Effects of 8-Week Exhausting Deep Knee Flexion Flywheel Training on Persistent Quadriceps Weakness in Well-Trained Athletes Following Anterior Cruciate Ligament Reconstruction. International Journal of Environmental Research and Public Health. 2022; 19(20):13209. https://doi.org/10.3390/ijerph192013209
Chicago/Turabian StyleHenderson, Frederick James, Yu Konishi, Norihiro Shima, and Yohei Shimokochi. 2022. "Effects of 8-Week Exhausting Deep Knee Flexion Flywheel Training on Persistent Quadriceps Weakness in Well-Trained Athletes Following Anterior Cruciate Ligament Reconstruction" International Journal of Environmental Research and Public Health 19, no. 20: 13209. https://doi.org/10.3390/ijerph192013209
APA StyleHenderson, F. J., Konishi, Y., Shima, N., & Shimokochi, Y. (2022). Effects of 8-Week Exhausting Deep Knee Flexion Flywheel Training on Persistent Quadriceps Weakness in Well-Trained Athletes Following Anterior Cruciate Ligament Reconstruction. International Journal of Environmental Research and Public Health, 19(20), 13209. https://doi.org/10.3390/ijerph192013209








