Neurological Outpatients Prefer EEG Home-Monitoring over Inpatient Monitoring—An Analysis Based on the UTAUT Model
Abstract
1. Introduction
2. Methods
2.1. Procedure
2.2. Recruitment
2.3. Measurement
3. Results
3.1. Preference Analysis
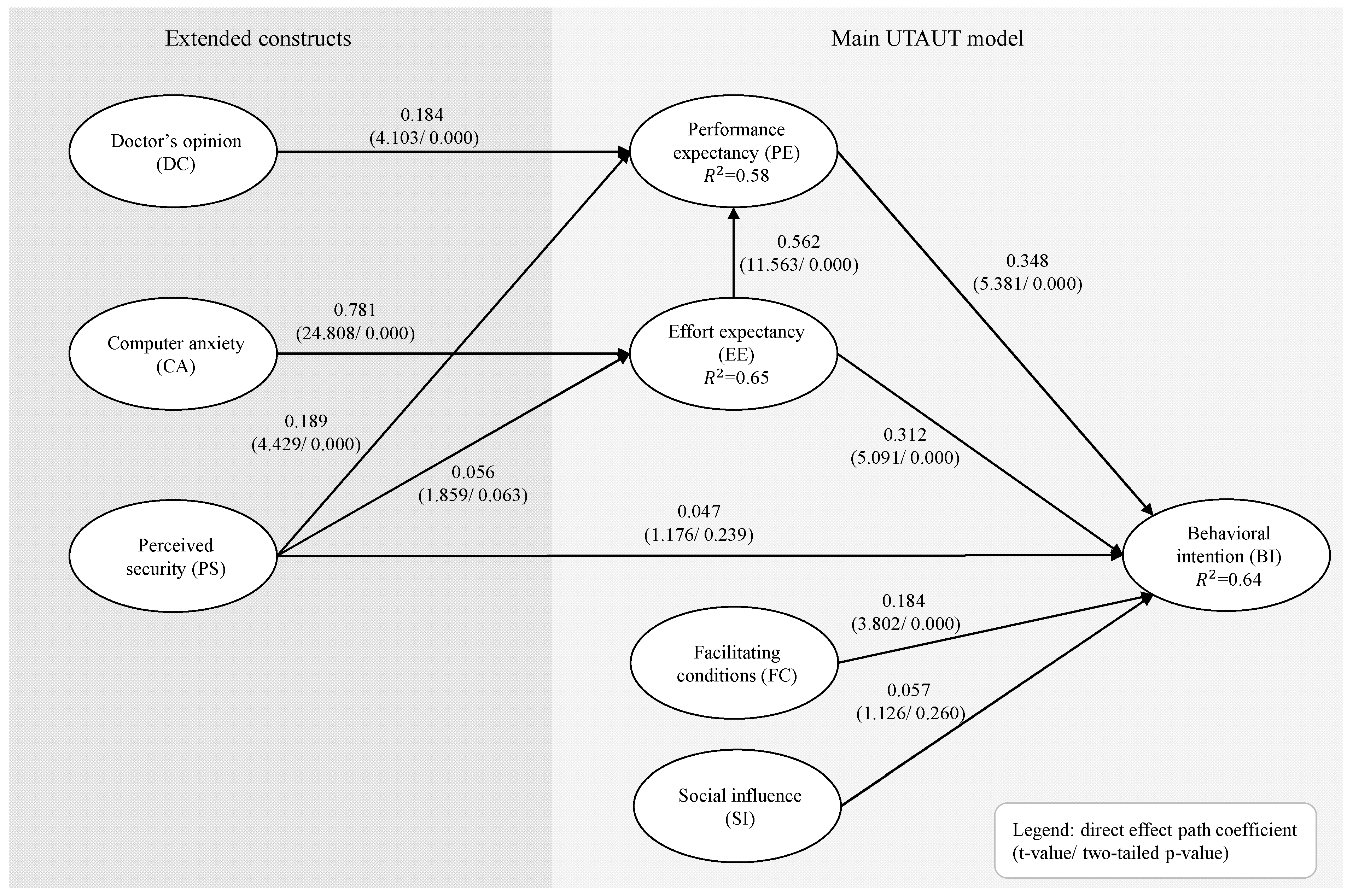
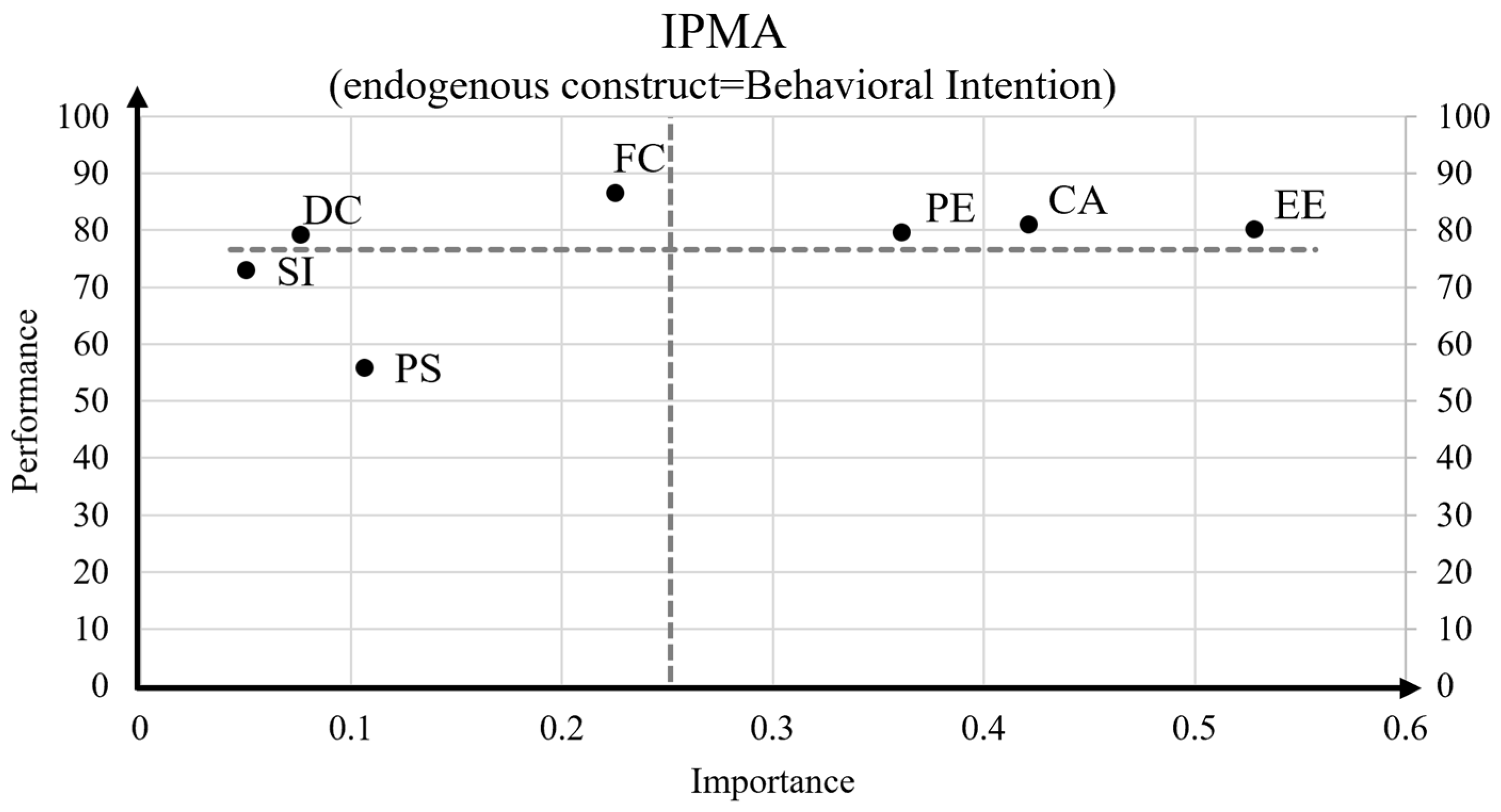
3.2. Drivers of BI
3.3. Impact of BI Score, Health Measurement, Age, and Gender on Preference
4. Discussion
4.1. Theoretical Implications
4.2. Practical Implications
4.3. Limitations and Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Survey Scenario
Please Imagine the Following Situation
Appendix B. Presentation of Inpatient Monitoring and Home-Monitoring Options
Appendix B.1. Option 1: EEG Inpatient Monitoring
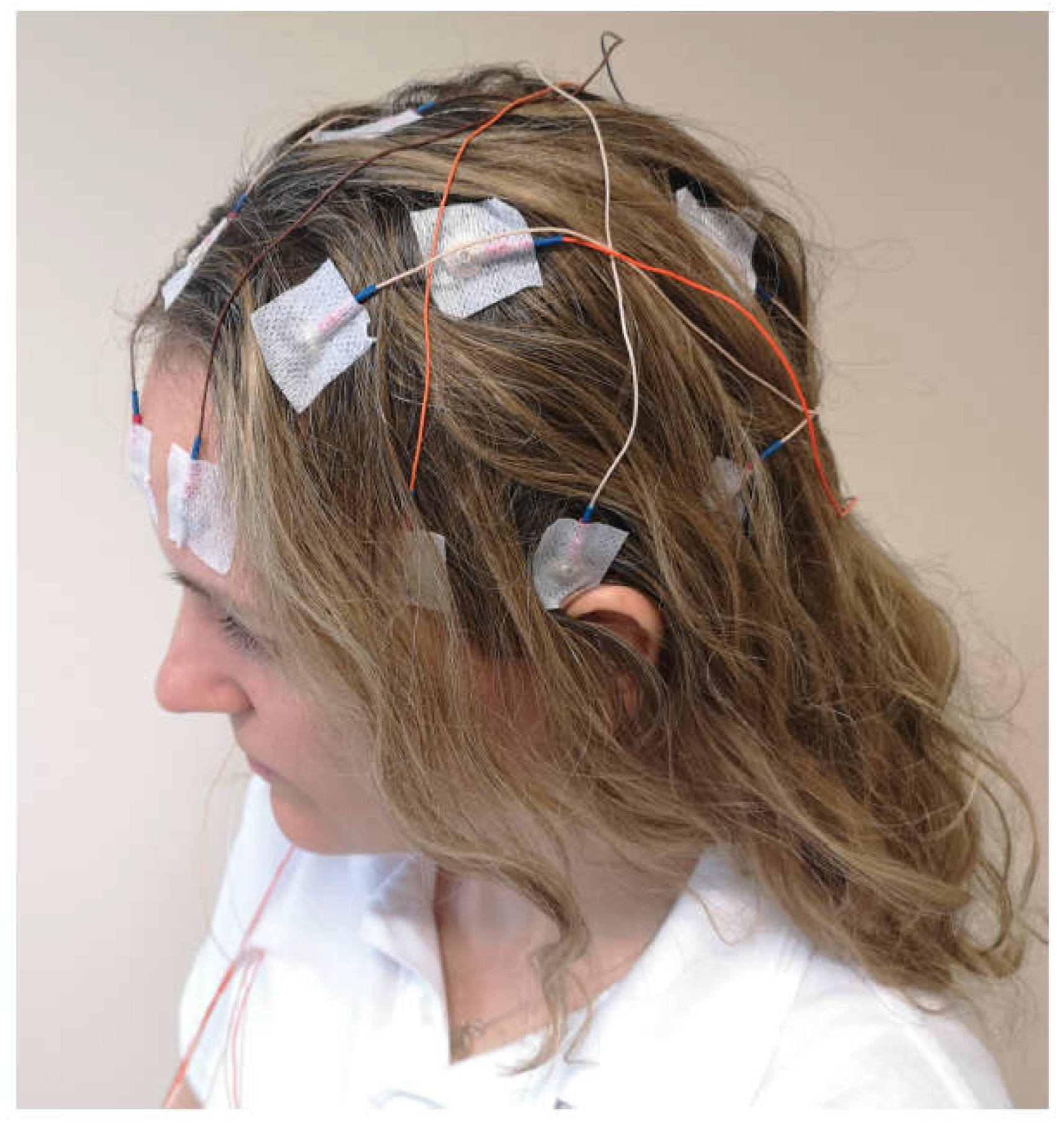
Appendix B.2. Option 2: EEG Home-Monitoring

Appendix C. Measurement Information for the Extended UTAUT Model
| Construct | Item | Loading | English Wording | German Wording |
|---|---|---|---|---|
| Performance Expectancy (PE)–the degree to which an individual believes that using EEG home-monitoring will help him or her increase their health performance/quality | ||||
| AVE = 0.702 α = 0.894 C.R. = 0.922 | PE1 | 0.816 | I find that using EEG home-monitoring would be helpful in monitoring my health. | Ich denke, dass die Durchführung eines EEG-Home-Monitorings bei der Diagnostik meines Krankheitsbildes und der Überwachung meiner Gesundheit hilfreich ist. |
| PE2 | 0.811 | I find that using EEG home-monitoring would make me feel safer in my daily life. | Ich denke, dass ich mich durch die Durchführung eines EEG-Home-Monitorings sicherer fühle, was meine Gesundheit betrifft. | |
| PE3 | 0.838 | EEG home-monitoring could enhance the level of convenience in accessing medical care services. | Die Möglichkeit, eine EEG-Untersuchung zu Hause durchführen zu können (EEG-Home-Monitoring), könnte den Zugang zur medizinischen Versorgung erleichtern. | |
| PE4 | 0.829 | EEG home-monitoring could enhance the quality of my life. | Das EEG-Home-Monitoring als Untersuchung in der Häuslichkeit könnte meine Lebensqualität verbessern. | |
| PE5 | 0.894 | Overall, I find that EEG home-monitoring would be highly useful. | Insgesamt finde ich das EEG-Home-Monitoring sehr nützlich. | |
| Effort Expectancy (EE)–the degree of ease associated with the use of EEG home-monitoring | ||||
| AVE = 0.819 α = 0.926 C.R. = 0.948 | EE1 | 0.896 | I find that using EEG home-monitoring would be simple. | Ich finde, dass die Durchführung des EEG-Home-Monitorings einfach wäre. |
| EE2 | 0.928 | I find that using EEG home-monitoring would be easy to learn. | Ich finde, dass die Durchführung des EEG-Home-Monitorings leicht zu erlernen wäre. | |
| EE3 | 0.920 | I find that EEG home-monitoring would be easily understandable and clear for me. | Ich finde, dass das EEG-Home-Monitoring für mich leicht verständlich und klar wäre. | |
| EE4 | 0.876 | Overall, I find that using EEG home-monitoring would be convenient. | Insgesamt finde ich die Verwendung des EEG-Home-Monitorings praktisch. | |
| Social Influence (SI)–influence of peers and colleagues’ opinions | ||||
| AVE = 0.808 α = 0.881 C.R. = 0.927 | SI1 | 0.870 | Peers and colleagues would support me in using EEG home-monitoring. | Gleichaltrige und Kollegen würden mich bei der Entscheidung, das EEG-Home-Monitoring durchzuführen, unterstützen. |
| SI2 | 0.915 | People who influence my behavior would support my use of EEG home-monitoring. | Menschen, die mich beeinflussen, würden mich bei der Entscheidung, das EEG-Home-Monitoring durchzuführen, unterstützen. | |
| SI3 | 0.911 | People who are important to me would support my use of EEG home-monitoring. | Menschen, die mir wichtig sind, würden mich bei der Entscheidung, das EEG-Home-Monitoring zu durchzuführen, unterstützen. | |
| Facilitating Conditions (FC)–technical support for using EEG home-monitoring | ||||
| AVE = 0.741 α = 0.824 C.R. = 0.895 | FC1 | 0.870 | I believe that guidance will be available to me when deciding whether to use EEG home-monitoring. | Ich gehe davon aus, dass ich bei meinem Neurologen ein Aufklärungsgespräch erhalten werde, wenn ich entscheiden muss, ob ich das EEG-Home-Monitoring durchführen möchte. |
| FC2 | 0.912 | I believe that specialized instructions concerning the use of EEG home-monitoring will be available to me. | Ich gehe davon aus, dass mir spezielle Anweisungen zur Verwendung des EEG-Home- Monitorings zur Verfügung stehen. | |
| FC3 | 0.796 | I believe that specific persons (or a group) will be available for assistance with EEG home-monitoring difficulties (e.g., nursing service or a call center). | Unabhängig von der Einweisung durch den Pflegedienst gehe ich davon aus, dass ich während der Nutzung des EEG-Home- Monitorings Unterstützung bei Anwendungsschwierigkeiten erhalten werde (z. B. durch den Pflegedienst oder ein Callcenter). | |
| Computer Anxiety (CA)–anxiety concerning the use of EEG home-monitoring option | ||||
| AVE = 0.674 α = 0.838 C.R. = 0.892 | CA1 | 0.824 | Anyone can learn to use a mobile EEG cap if they are patient and motivated. | Jeder kann lernen, eine mobile EEG-Haube zu verwenden, wenn er geduldig und motiviert ist. |
| CA2 | 0.855 | I do not hesitate to use a mobile EEG cap for fear of making mistakes. | Ich zögere nicht, eine mobile EEG-Haube zu verwenden, weil ich keine Angst habe, Fehler zu machen. | |
| CA3 | 0.855 | If given the opportunity, I would like to learn about and use the mobile EEG cap. | Wenn ich die Gelegenheit dazu hätte, würde ich gerne die mobile EEG-Haube kennenlernen und nutzen. | |
| CA4 | 0.744 | I feel that computers are necessary tools in both educational and work settings. | Ich bin der Meinung, dass Computertechnik in der Medizin notwendiges Werkzeug ist. | |
| Perceived Security (PS)–the degree to which using information technology enables the administration of personal health information | ||||
| AVE = 0.869 α = 0.950 C.R. = 0.964 | PS1 | 0.920 | I would feel secure sending personal health information using the Internet and computers. | Ich würde mich sicher fühlen, persönliche Gesundheitsinformationen über das Internet zu senden. |
| PS2 | 0.916 | The Internet offers a secure means through which to send sensitive personal information. | Das Internet ist ein sicheres Medium, um vertrauliche persönliche Informationen zu senden. | |
| PS3 | 0.950 | I would feel totally safe providing sensitive personal information about myself over the Internet. | Ich würde mich absolut sicher fühlen, sensible persönliche Informationen über das Internet bereitzustellen. | |
| PS4 | 0.943 | Overall, using the EEG cap and an Internet connection is a safe way to transmit sensitive personal health information. | Insgesamt ist die Verwendung der EEG-Haube und einer Internetverbindung eine sichere Möglichkeit, vertrauliche persönliche Gesundheitsinformationen zu übertragen. | |
| Doctor’s Opinion (DC)–doctor’s expert power influence | ||||
| AVE = 0.683 α = 0.919 C.R. = 0.937 | DC1 | 0.877 | I trust my doctor’s judgment. | Ich vertraue dem Urteil meines Arztes. |
| DC2 | 0.907 | The doctor’s expertise makes him/her more likely to be right. | Aufgrund des medizinischen Fachwissens des Arztes ist es wahrscheinlicher, dass er/sie recht hat. | |
| DC3 | 0.906 | The doctor has a lot of experience and usually knows best. | Der Arzt hat viel Erfahrung und weiß es normalerweise am besten. | |
| DC4 | 0.871 | The doctor’s knowledge usually makes him/her right. | Durch sein Wissen hat der Arzt für gewöhnlich recht. | |
| DC5 | 0.867 | I trust my doctor’s judgment about the use of EEG home-monitoring. | Ich vertraue dem Urteil meines Arztes, was den Einsatz des EEG-Home-Monitorings betrifft. | |
| DC6 | 0.566 | In the case of deciding to use EEG home-monitoring, I don’t know as much about what is required as the doctor does. | Wenn ich mich für das EEG-Home-Monitoring entscheide, kenne ich mich damit nicht so gut aus wie der Arzt. | |
| DC7 | 0.734 | Doctors are intelligent. | Ärzte sind klug. | |
| Behavioral Intention to Use (BI)–the degree to which an individual intends to use EEG home-monitoring | ||||
| AVE = 0.869 α = 0.950 C.R. = 0.964 | BI1 | 0.913 | Assuming there was a medical need to perform EEG home-monitoring, I would use it. | Angenommen es bestünde die medizinische Notwendigkeit, das EEG-Home-Monitoring durchzuführen, würde ich es verwenden. |
| BI2 | 0.928 | I assume that in the future I would regularly use EEG home-monitoring if it was medically necessary. | Ich gehe davon aus, dass ich bei Notwendigkeit in Zukunft regelmäßig das EEG-Home-Monitoring verwenden würde. | |
| BI3 | 0.937 | I intend to use EEG home-monitoring in the future if medical necessity exists. | Ich beabsichtige, in Zukunft das EEG-Home-Monitoring zu verwenden, falls die Notwendigkeit besteht. | |
| BI4 | 0.950 | Providing I had access to EEG home-monitoring, I would use the services when needed. | Wenn ich Zugang zur Verwendung des EEG-Home-Monitorings hätte, würde ich die Dienste bei Notwendigkeit nutzen. | |
Construct | Behavioral Intention (BI) | Computer Anxiety (CA) | Doctor’s Opinion (DC) | Effort Expectancy (EE) | Facilitating Conditions (FC) | Performance Expectancy (PE) | Perceived Security (PS) | Social Influence (SI) |
|---|---|---|---|---|---|---|---|---|
| Behavioral intention (BI) | 0.932 | [0.799; 0.917] | [0.444; 0.638] | [0.706; 0.825] | [0.581; 0.758] | [0.714; 0.847] | [0.322; 0.489] | [0.488; 0.683] |
| Computer anxiety (CA) | 0.771 | 0.821 | [0.401; 0.637] | [0.836; 0.961] | [0.705; 0.856] | [0.718; 0.882] | [0.362; 0.533] | [0.591; 0.791] |
| Doctor’s opinion (DC) | 0.520 | 0.467 | 0.826 | [0.345; 0.560] | [0.471; 0.708] | [0.421; 0.623] | [0.245; 0.425] | [0.356; 0.552] |
| Effort expectancy (EE) | 0.722 | 0.803 | 0.432 | 0.905 | [0.582; 0.755] | [0.705; 0.843] | [0.309; 0.476] | [0.557; 0.731] |
| Facilitating conditions (FC) | 0.602 | 0.647 | 0.534 | 0.595 | 0.861 | [0.537; 0.729] | [0.192; 0.367] | [0.460; 0.657] |
| Performance expectancy (PE) | 0.725 | 0.702 | 0.486 | 0.712 | 0.553 | 0.838 | [0.410; 0.573] | [0.521; 0.717] |
| Perceived security (PS) | 0.394 | 0.410 | 0.319 | 0.376 | 0.255 | 0.461 | 0.932 | [0.331; 0.524] |
| Social influence (SI) | 0.542 | 0.602 | 0.417 | 0.586 | 0.479 | 0.561 | 0.398 | 0.899 |
| Variance Inflation Factors (VIF) | Behavioral Intention (BI) | Effort Expectancy (EE) | Performance Expectancy (PE) |
|---|---|---|---|
| Computer anxiety (CA) | 1.203 | ||
| Doctor’s opinion (DC) | 1.274 | ||
| Effort expectancy (EE) | 2.470 | 1.333 | |
| Facilitating conditions (FC) | 1.678 | ||
| Performance expectancy (PE) | 2.405 | ||
| Perceived security (PS) | 1.322 | 1.203 | 1.207 |
| Social influence (SI) | 1.721 |
Appendix D. Items Wording and Scale Quality Assessment of 36-Short Form Health Survey
| English Wording | German Wording |
| Physical functioning α = 0.946 (0 = yes, limited a lot; 50 = yes, limited a little; 100 = no, not limited at all) | |
| The following items are about activities you might carry out during a typical day. Does your health now limit you in these activities? If so, how much? | Im Folgenden sind einige Tätigkeiten beschrieben, die Sie vielleicht an einem normalen Tag ausüben. Sind Sie durch Ihren derzeitigen Gesundheitszustand bei diesen Tätigkeiten eingeschränkt? Wenn ja, wie stark? |
| vigorous activities, such as running, lifting heavy objects, participating in strenuous sports | anstrengende Tätigkeiten, z.B. schnell laufen |
| moderate activities, such as moving a table, pushing a vacuum cleaner, bowling, or playing golf | mittelschwere Tätigkeiten, z.B. einen Tisch verschieben, staubsaugen, kegeln, Golf spielen |
| lifting or carrying groceries | Einkaufstaschen heben oder tragen |
| climbing several flights of stairs | mehrere Treppenabsätze steigen |
| climbing one flight of stairs | einen Treppenabsatz steigen |
| bending, kneeling, or stooping | sich beugen, knien, bücken |
| walking more than a mile | mehr als 1 Kilometer zu Fuß gehen |
| walking several blocks | mehrere Straßenkreuzungen weit zu Fuß gehen |
| walking one block | eine Straßenkreuzung weit zu Fuß gehen |
| bathing or dressing yourself | sich baden oder anziehen |
| Physical health α = 0.888 (0 = yes, 100 = no) | |
| During the past 4 weeks, have you experienced any of the following problems with your work or other regular daily activities as a result of your physical health? | Hatten Sie in den vergangenen 4 Wochen aufgrund Ihrer körperlichen Gesundheit irgendwelche Schwierigkeiten bei der Arbeit oder anderen alltäglichen Tätigkeiten im Beruf bzw. zu Hause? |
| cut down the amount of time you spent on work or other activities | Ich konnte nicht so lange wie üblich tätig sein. |
| accomplished less than you would like | Ich habe weniger geschafft als ich wollte. |
| were limited in the kind of work or other activities | Ich konnte nur bestimmte Dinge tun. |
| had difficulty performing the work or other activities (for example, it took extra effort) | Ich hatte Schwierigkeiten bei der Ausführung. |
| Emotional problems α = 0.909 (0 = yes, 100 = no) | |
| During the past 4 weeks, have you experienced any of the following problems with your work or other regular daily activities as a result of any emotional problems (such as feeling depressed or anxious)? | Hatten Sie in den vergangenen 4 Wochen aufgrund seelischer Probleme irgendwelche Schwierigkeiten bei der Arbeit oder anderen alltäglichen Tätigkeiten im Beruf bzw. zu Hause (z.B., weil Sie sich niedergeschlagen oder ängstlich fühlten)? |
| cut down the amount of time you spent on work or other activities | Ich konnte nicht so lange wie üblich tätig sein |
| accomplished less than you would like | Ich habe weniger geschafft als ich wollte. |
| didn’t complete work or other activities as carefully as usual | Ich konnte nicht so sorgfältig wie üblich arbeiten. |
| Energy/fatigue α = 0.873 (100 = all of the time, 80 = most of the time, 60 = a good portion of the time, 40 = some of the time, 20 = a little of the time, 0 = none of the time) | |
| These questions are about how you feel and how things have been with you during the past four weeks. For each question, please give the one answer that comes closest to the way you have been feeling. How much of the time during the past four weeks... | In diesen Fragen geht es darum, wie Sie sich fühlen und wie es Ihnen in den vergangenen 4 Wochen gegangen ist. Wie oft waren Sie in den vergangenen 4 Wochen… |
| did you feel full of pep? | ...voller Schwung? |
| did you have a lot of energy? | ...voller Energie? |
| did you feel worn out? (R) | ...erschöpft? (R) |
| did you feel tired? (R) | ...müde? (R) |
| Emotional well-being α = 0.882 (100 = none of the time, 80 = a little of the time, 60 = some of the time, 40 = a good portion of the time, 20 = most of the time, 0 = all of the time) | |
| These questions are about how you feel and how things have been with you during the past four weeks. For each question, please give the one answer that comes closest to the way you have been feeling. How much of the time during the past four weeks... | In diesen Fragen geht es darum, wie Sie sich fühlen und wie es Ihnen in den vergangenen 4 Wochen gegangen ist. Wie oft waren Sie in den vergangenen 4 Wochen… |
| have you felt like a very nervous person? | ...sehr nervös? |
| have you felt so down in the dumps that nothing could cheer you up? | so niedergeschlagen, dass Sie nichts aufheitern konnte? |
| have you felt calm and peaceful? (R) | ...ruhig und gelassen? (R) |
| have you felt downhearted and blue? | ...entmutigt und traurig? |
| have you been a happy person? (R) | ... glücklich? (R) |
| Social functioning α = 0.895 (Item 1: 100 = not at all, 75 = a little bit, 50 = moderately, 25 = quite a bit, 0 = extremely. Item 2: 0 = all of the time, 25 = most of the time, 50 = some of the time, 75 = a little bit of the time, 100 = none of the time) | |
| During the past four weeks, to what extent have your physical health or emotional problems interfered with your normal social activities with family, friends, neighbors, or groups? | Wie sehr haben Ihre körperliche Gesundheit oder seelischen Probleme in den vergangenen 4 Wochen Ihre normalen Kontakte zu Familienangehörigen, Freunden, Nachbarn oder zum Bekanntenkreis beeinträchtigt? |
| During the past four weeks, how much of the time has your physical health or emotional problems interfered with your social activities (like visiting friends, relatives, etc.)? (R) | Wie häufig haben Ihre körperliche Gesundheit oder seelischen Probleme in den vergangenen 4 Wochen Ihre Kontakte zu anderen Menschen (Besuche bei Freunden, Verwandten usw.) beeinträchtigt? |
| Painα = 0.903 (Item 1: 100 = none, 80 = very mild, 60 = mild, 40 = moderate, 20 = severe, 0 = very severe. Item 2: 100 = not at all, 75 = a little bit, 50 = moderately, 25 = quite a bit, 0 = extremely) | |
| How much bodily pain have you had during the past four weeks? | Wie stark waren Ihre Schmerzen in den vergangenen 4 Wochen? |
| During the past four weeks, how much did pain interfere with your normal work (including both work outside the home and housework)? | Inwieweit haben die Schmerzen Sie in den vergangenen 4 Wochen bei der Ausübung Ihrer Alltagstätigkeiten zu Hause und im Beruf behindert? |
| General health α = 0.812 | |
| (Item 1: 100 = excellent, 75 = very good, 50 = good, 25 = fair, 0 = poor. Item 2–5: 100 = definitely true, 75 = mostly true, 50 = don’t know, 25 = mostly false, 0 = definitely false) | |
| In general, would you say that your health is: | Wie würden Sie Ihren Gesundheitszustand im Allgemeinen beschreiben? |
| I seem to get sick a little easier than other people (R) | Ich scheine etwas leichter als andere krank zu werden. |
| I am as healthy as anybody I know | Ich bin genauso gesund wie alle anderen, die ich kenne. |
| I expect my health to get worse (R) | Ich erwarte, dass meine Gesundheit nachlässt. |
| My health is excellent | Ich erfreue mich ausgezeichneter Gesundheit. |
| Health change (100 = Much better now than one year ago, 75 = Somewhat better now than one year ago, 50 = Approximately the same, 25 = Somewhat worse now than one year ago, 0 = Much worse now than one year ago) | |
| Compared to one year ago, how would you rate your health in general now? | Im Vergleich zum vergangenen Jahr, wie würden Sie Ihren derzeitigen Gesundheitszustand beschreiben? |
Appendix E. Detailed Information about Regression Analysis
| Model 1 | B | Std. Error | Beta | t | p | CI | |
| (Constant) | 5.147 | 0.086 | 60.097 | 0.000 | 4.979 | 5.316 | |
| BI score | 0.973 | 0.086 | 0.485 | 11.364 | 0.000 | 0.805 | 1.142 |
| Model 2 | B | Std. Error | Beta | t | p | CI | |
| (Constant) | 5.398 | 0.408 | 13.223 | 0.000 | 4.596 | 6.201 | |
| BI score | 1.015 | 0.087 | 0.506 | 11.605 | 0.000 | 0.843 | 1.187 |
| Physical functioning | −0.002 | 0.005 | −0.019 | −0.294 | 0.769 | −0.012 | 0.009 |
| Physical health | 0.004 | 0.004 | 0.082 | 1.124 | 0.262 | −0.003 | 0.012 |
| Emotional problems | 0.004 | 0.003 | 0.078 | 1.184 | 0.237 | −0.003 | 0.011 |
| Energy /fatigue | −0.003 | 0.007 | −0.035 | −0.450 | 0.653 | −0.017 | 0.011 |
| Emotional well-being | −0.008 | 0.008 | −0.078 | −0.920 | 0.358 | −0.024 | 0.009 |
| Social functioning | −0.010 | 0.005 | −0.135 | −1.935 | 0.054 | −0.021 | 0.000 |
| Pain | 0.002 | 0.005 | 0.022 | 0.316 | 0.752 | −0.009 | 0.012 |
| General health | 0.009 | 0.006 | 0.092 | 1.350 | 0.178 | −0.004 | 0.022 |
| Health change | 0.003 | 0.005 | 0.026 | 0.556 | 0.579 | −0.007 | 0.013 |
| Model 3 | B | Std. Error | Beta | t | p | CI | |
| (Constant) | 5.636 | 0.631 | 8.935 | 0.000 | 4.396 | 6.877 | |
| BI score | 1.012 | 0.087 | 0.505 | 11.575 | 0.000 | 0.840 | 1.184 |
| Physical functioning | −0.003 | 0.006 | −0.032 | −0.483 | 0.629 | −0.014 | 0.008 |
| Physical health | 0.004 | 0.004 | 0.068 | 0.929 | 0.354 | −0.004 | 0.011 |
| Emotional problems | 0.005 | 0.003 | 0.088 | 1.332 | 0.184 | −0.002 | 0.011 |
| Energy/fatigue | −0.001 | 0.007 | −0.010 | −0.122 | 0.903 | −0.015 | 0.013 |
| Emotional well-being | −0.006 | 0.008 | −0.066 | −0.775 | 0.439 | −0.023 | 0.010 |
| Social functioning | −0.010 | 0.005 | −0.134 | −1.919 | 0.056 | −0.021 | 0.000 |
| Pain | 0.003 | 0.005 | 0.033 | 0.473 | 0.636 | −0.008 | 0.013 |
| General health | 0.006 | 0.007 | 0.059 | 0.850 | 0.396 | −0.007 | 0.019 |
| Health change | 0.002 | 0.005 | 0.017 | 0.363 | 0.716 | −0.008 | 0.012 |
| Age | −0.009 | 0.007 | −0.064 | −1.337 | 0.182 | −0.022 | 0.004 |
| Gender | 0.177 | 0.174 | 0.044 | 1.014 | 0.311 | −0.166 | 0.520 |
| Model 4 | B | Std. Error | Beta | t | p | CI | |
| (Constant) | 5391 | 0.634 | 8500 | 0 | 4144 | 6637 | |
| BI score | 1007 | 0.087 | 0.503 | 11,563 | 0 | 0.835 | 1178 |
| Physical functioning | −0.004 | 0.005 | −0.046 | −0.705 | 0.481 | −0.015 | 0.007 |
| Physical health | 0.004 | 0.004 | 0.077 | 1054 | 0.292 | −0.004 | 0.012 |
| Emotional problems | 0.005 | 0.003 | 0.092 | 1392 | 0.165 | −0.002 | 0.012 |
| Energy/fatigue | −0.002 | 0.007 | −0.021 | −0.274 | 0.784 | −0.016 | 0.012 |
| Emotional well-being | −0.006 | 0.008 | −0.061 | −0.726 | 0.469 | −0.022 | 0.01 |
| Social functioning | −0.01 | 0.005 | −0.126 | −1828 | 0.068 | −0.02 | 0.001 |
| Pain | 0.003 | 0.005 | 0.038 | 0.553 | 0.581 | −0.007 | 0.013 |
| General health | 0.005 | 0.007 | 0.056 | 0.811 | 0.418 | −0.008 | 0.018 |
| Health change | 0.002 | 0.005 | 0.016 | 0.343 | 0.732 | −0.008 | 0.012 |
| Age | −0.008 | 0.007 | −0.061 | −1277 | 0.202 | −0.021 | 0.005 |
| Gender | 0.166 | 0.174 | 0.041 | 0.952 | 0.342 | −0.176 | 0.508 |
| Participant type | 0.331 | 0.299 | 0.048 | 1107 | 0.269 | −0.257 | 0.919 |
| Video | 0.466 | 0.172 | 0.116 | 2714 | 0.007 | 0.128 | 0.803 |
References
- Sorg, H.; Ehlers, J.P.; Sorg, C.G.G. Digitalization in Medicine: Are German Medical Students Well Prepared for the Future? Int. J. Environ. Res. Public Health 2022, 19, 8308. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Herrmann, M.; Ehlers, J.P.; Mondritzki, T.; Hensel, K.O.; Truebel, H.; Boehme, P. Perception of the Progressing Digitization and Transformation of the German Health Care System Among Experts and the Public: Mixed Methods Study. JMIR Public Health Surveill. 2019, 5, e14689. [Google Scholar] [CrossRef] [PubMed]
- Knörr, V.; Dini, L.; Gunkel, S.; Hoffmann, J.; Mause, L.; Ohnhäuser, T.; Stöcker, A.; Scholten, N. Use of telemedicine in the outpatient sector during the COVID-19 pandemic: A cross-sectional survey of German physicians. BMC Prim. Care 2022, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Gerke, S.; Shachar, C.; Chai, P.R.; Cohen, I.G. Regulatory, safety, and privacy concerns of home monitoring technologies during COVID-19. Nat. Med. 2020, 26, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C.; Skoll, D.; Saxon, L.A. Home Monitoring of Cardiac Devices in the Era of COVID-19. Curr. Cardiol. Rep. 2020, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Neurol, H.C. (Ed.) Chapter 9—Normal EEG Variants; Elsevier: Amsterdam, the Netherlands, 2019; ISBN 9780444640321. [Google Scholar]
- Beniczky, S.; Schomer, D.L. Electroencephalography: Basic biophysical and technological aspects important for clinical applications. Epileptic Disord. 2020, 22, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Craciun, L.; Gardella, E.; Alving, J.; Terney, D.; Mindruta, I.; Zarubova, J.; Beniczky, S. How long shall we record electroencephalography? Acta Neurol. Scand. 2014, 129, e9–e11. [Google Scholar] [CrossRef]
- Sinha, S.R.; Sullivan, L.; Sabau, D.; San-Juan, D.; Dombrowski, K.E.; Halford, J.J.; Hani, A.J.; Drislane, F.W.; Stecker, M.M. American Clinical Neurophysiology Society Guideline 1: Minimum Technical Requirements for Performing Clinical Electroencephalography. J. Clin. Neurophysiol. 2016, 33, 303–307. [Google Scholar] [CrossRef]
- Salinsky, M.; Kanter, R.; Dasheiff, R.M. Effectiveness of Multiple EEGs in Supporting the Diagnosis of Epilepsy: An Operational Curve. Epilepsia 1987, 28, 331–334. [Google Scholar] [CrossRef]
- Foley, C.M.; Legido, A.; Miles, D.K.; Chandler, D.A.; Grover, W.D. Long-term computer-assisted outpatient electroencephalogram monitoring in children and adolescents. J. Child Neurol. 2000, 15, 49–55. [Google Scholar] [CrossRef]
- Dash, D.; Hernandez-Ronquillo, L.; Moien-Afshari, F.; Tellez-Zenteno, J.F. Ambulatory EEG: A cost-effective alternative to inpatient video-EEG in adult patients. Epileptic Disord. 2012, 14, 290–297. [Google Scholar] [CrossRef]
- Faulkner, H.J.; Arima, H.; Mohamed, A. The utility of prolonged outpatient ambulatory EEG. Seizure 2012, 21, 491–495. [Google Scholar] [CrossRef]
- Burkholder, D.B.; Britton, J.W.; Rajasekaran, V.; Fabris, R.R.; Cherian, P.J.; Kelly-Williams, K.M.; So, E.L.; Nickels, K.C.; Wong-Kisiel, L.C.; Lagerlund, T.D.; et al. Routine vs extended outpatient EEG for the detection of interictal epileptiform discharges. Neurology 2016, 86, 1524–1530. [Google Scholar] [CrossRef]
- Siddiqi, M.; Ahmed, S.N. No Further Yield of Ambulatory EEG for Epileptiform Discharges Beyond 13 Hours. Neurodiagnostic J. 2017, 57, 211–223. [Google Scholar] [CrossRef]
- Kuo, J.; Lee-Messer, C.; Le, S. Optimal recording duration of ambulatory EEG (aEEG). Epilepsy Res. 2019, 149, 9–12. [Google Scholar] [CrossRef]
- Tutkavul, K.; Çetinkaya, Y. Optimum recording time of routine electroencephalogram for adults with epilepsy. Turk. J. Med. Sci. 2019, 49, 635–638. [Google Scholar] [CrossRef]
- Jamal Omidi, S.; Hampson, J.P.; Lhatoo, S.D. Long-term Home Video EEG for Recording Clinical Events. J. Clin. Neurophysiol. 2021, 38, 92–100. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Klinische Neurophysiologie. 8. Empfehlungen für EEG-Langzeitableitungen. Available online: https://dgkn.de/fuer-experten/eeg/empfehlungen-hilfsmittel (accessed on 10 May 2022).
- Slater, J.D.; Eaddy, M.; Butts, C.M.; Meltser, I.; Murty, S. The real-world economic impact of home-based video electroencephalography: The payer perspective. J. Med. Econ. 2019, 22, 1030–1040. [Google Scholar] [CrossRef]
- Ives, J.; Woods, J. 4-Channel 24 hour cassette recorder for long-term EEG monitoring of ambulatory patients. Electroencephalogr. Clin. Neurophysiol. 1975, 39, 88–92. [Google Scholar] [CrossRef]
- Ebersole, J.S. Ambulatory cassette EEG in epilepsy diagnosis. Yale J. Biol. Med. 1987, 60, 85–91. [Google Scholar]
- Bridgers, S.L.; Ebersole, J.S. The clinical utility of ambulatory cassette EEG. Neurology 1985, 35, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.S.; Bridgers, S.L. Direct comparison of 3 and 8 channel ambulatory cassette EEG with intensive inpatient monitoring. Neurology 1985, 35, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.L.; Galezowska, J.; Leroy, R.; North, R. The results of computer-assisted ambulatory 16-channel EEG. Electroenephalogr. Clin. Neurophysiol. 1994, 91, 229–231. [Google Scholar] [CrossRef]
- Morris, G.L. The clinical utility of computer-assisted ambulatory 16 channel EEG. J. Med. Eng. Technol. 1997, 21, 47–52. [Google Scholar] [CrossRef]
- Liporace, J.; Tatum IV, W.; Lee Morris III, G.; French, J. Clinical utility of sleep-deprived versus computer-assisted ambulatory 16-channel EEG in epilepsy patients: A multi-center study. Epilepsy Res. 1998, 32, 357–362. [Google Scholar] [CrossRef]
- Askamp, J.; van Putten, M.J.A.M. Mobile EEG in epilepsy. Int. J. Psychophysiol. 2014, 91, 30–35. [Google Scholar] [CrossRef]
- Lopez-Gordo, M.A.; Sanchez-Morillo, D.; Pelayo Valle, F. Dry EEG Electrodes. Sensors 2014, 14, 12847–12870. [Google Scholar] [CrossRef]
- Lau-Zhu, A.; Lau, M.P.H.; McLoughlin, G. Mobile EEG in research on neurodevelopmental disorders: Opportunities and challenges. Dev. Cogn. Neurosci. 2019, 36, 100635. [Google Scholar] [CrossRef]
- Fiedler, P.; Pedrosa, P.; Griebel, S.; Fonseca, C.; Vaz, F.; Supriyanto, E.; Zanow, F.; Haueisen, J. Novel Multipin Electrode Cap System for Dry Electroencephalography. Brain Topogr. 2015, 28, 647–656. [Google Scholar] [CrossRef]
- Marini, F.; Lee, C.; Wagner, J.; Makeig, S.; Gola, M. A comparative evaluation of signal quality between a research-grade and a wireless dry-electrode mobile EEG system. J. Neural Eng. 2019, 16, 54001. [Google Scholar] [CrossRef]
- Heijs, J.J.A.; Havelaar, R.J.; Fiedler, P.; van Wezel, R.J.A.; Heida, T. Validation of Soft Multipin Dry EEG Electrodes. Sensors 2021, 21, 6827. [Google Scholar] [CrossRef]
- Fiedler, P.; Fonseca, C.; Supriyanto, E.; Zanow, F.; Haueisen, J. A high-density 256-channel cap for dry electroencephalography. Hum. Brain Mapp. 2022, 43, 1295–1308. [Google Scholar] [CrossRef]
- Sauleau, P.; Despatin, J.; Cheng, X.; Lemesle, M.; Touzery-de Villepin, A.; N’Guyen The Tich, S.; Kubis, N. National French survey on tele-transmission of EEG recordings: More than a simple technological challenge. Neurophysiol. Clin. 2016, 46, 109–118. [Google Scholar] [CrossRef]
- Rosenow, F.; Audebert, H.J.; Hamer, H.M.; Hinrichs, H.; Keßler-Uberti, S.; Kluge, T.; Noachtar, S.; Remi, J.; Sotoodeh, A.; Strzelczyk, A.; et al. Tele-EEG: Current Applications, Challenges, and Technical Solutions. Klin. Neurophysiol. 2018, 49, 208–215. [Google Scholar] [CrossRef]
- Neumann, T.; Baum, A.K.; Baum, U.; Deike, R.; Feistner, H.; Hinrichs, H.; Stokes, J.; Robra, B.-P. Diagnostic and therapeutic yield of a patient-controlled portable EEG device with dry electrodes for home-monitoring neurological outpatients-rationale and protocol of the HOMEONE pilot study. Pilot Feasibility Stud. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Neumann, T.; Baum, A.K.; Baum, U.; Deike, R.; Feistner, H.; Scholz, M.; Hinrichs, H.; Robra, B.-P. Assessment of the technical usability and efficacy of a new portable dry-electrode EEG recorder: First results of the HOMEONE study. Clin. Neurophysiol. 2019, 130, 2076–2087. [Google Scholar] [CrossRef]
- Baum, U.; Baum, A.-K.; Deike, R.; Feistner, H.; Markgraf, B.; Hinrichs, H.; Robra, B.-P.; Neumann, T. Feasibility assessment of patient-controlled EEG home-monitoring: More results from the HOMEONE study. Clin. Neurophysiol. 2022, 140, 12–20. [Google Scholar] [CrossRef]
- Baum, U.; Baum, A.-K.; Deike, R.; Feistner, H.; Scholz, M.; Markgraf, B.; Hinrichs, H.; Robra, B.-P.; Neumann, T. Eignung eines mobilen Trockenelektroden-EEG-Gerätes im Rahmen der Epilepsiediagnostik. Klin. Neurophysiol. 2020, 51, 156–160. [Google Scholar] [CrossRef]
- Hinrichs, H.; Scholz, M.; Baum, A.K.; Kam, J.W.Y.; Knight, R.T.; Heinze, H.-J. Comparison between a wireless dry electrode EEG system with a conventional wired wet electrode EEG system for clinical applications. Sci. Rep. 2020, 10, 5218. [Google Scholar] [CrossRef]
- Venkatesh, V.; Morris, M.G.; Davis, G.B.; Davis, F.D. User Acceptance of Information Technology: Toward a Unified View. MIS Q. 2003, 27, 425–478. [Google Scholar] [CrossRef]
- Davis, F.D. Perceived Usefulness, Perceived Ease of Use, and User Acceptance of Information Technology. MIS Q. 1989, 13, 319. [Google Scholar] [CrossRef]
- AlQudah, A.A.; Al-Emran, M.; Shaalan, K. Technology Acceptance in Healthcare: A Systematic Review. Appl. Sci. 2021, 11, 10537. [Google Scholar] [CrossRef]
- Yap, Y.-Y.; Tan, S.-H.; Choon, S.-W. Elderly’s intention to use technologies: A systematic literature review. Heliyon 2022, 8, e08765. [Google Scholar] [CrossRef]
- Cimperman, M.; Brenčič, M.M.; Trkman, P. Analyzing older users’ home telehealth services acceptance behavior-applying an Extended UTAUT model. Int. J. Med. Inform. 2016, 90, 22–31. [Google Scholar] [CrossRef]
- Hoque, R.; Sorwar, G. Understanding factors influencing the adoption of mHealth by the elderly: An extension of the UTAUT model. Int. J. Med. Inform. 2017, 101, 75–84. [Google Scholar] [CrossRef]
- Duarte, P.; Pinho, J.C. A mixed methods UTAUT2-based approach to assess mobile health adoption. J. Bus. Res. 2019, 102, 140–150. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, X.; Zhao, G.; Li, C.; Shi, J. Adoption of mobile health services using the unified theory of acceptance and use of technology model: Self-efficacy and privacy concerns. Front. Psychol. 2022, 13, 944976. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Luo, S.; Xie, Y.; Liu, F.; Li, X.; Zhou, Z. Factors Influencing Patients’ Intentions to Use Diabetes Management Apps Based on an Extended Unified Theory of Acceptance and Use of Technology Model: Web-Based Survey (Preprint); National Institutes of Health: Bethesda, MD, USA, 2019. [Google Scholar]
- Talukder, M.S.; Sorwar, G.; Bao, Y.; Ahmed, J.U.; Palash, M.A.S. Predicting antecedents of wearable healthcare technology acceptance by elderly: A combined SEM-Neural Network approach. Technol. Forecast. Soc. Chang. 2020, 150, 119793. [Google Scholar] [CrossRef]
- Wang, H.; Da, T.; Yu, N.; Qu, X. Understanding consumer acceptance of healthcare wearable devices: An integrated model of UTAUT and TTF. Int. J. Med. Inform. 2020, 139, 104156. [Google Scholar] [CrossRef]
- Monika Bullinger, I.K. Fragebogen zum Allgemeinen Gesundheitszustand SF 36; Hogrefe-Verlag für Psychologie, GmbH & Co. KG.: Göttingen, Germany, 2011. [Google Scholar]
- Hair, J.F.; Sarstedt, M.; Ringle, C.M.; Mena, J.A. An assessment of the use of partial least squares structural equation modeling in marketing research. J. Acad. Mark. Sci. 2012, 40, 414–433. [Google Scholar] [CrossRef]
- Hair, J.F.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M. A Primer on Partial Least Squares Structural Equation Modeling (PLS-SEM), 3rd ed.; SAGE PUBLICATIONS: Thousand Oaks, CA, USA, 2021; ISBN 9781544396408. [Google Scholar]
- Sarstedt, M.; Hair, J.F.; Ringle, C.M.; Thiele, K.O.; Gudergan, S.P. Estimation issues with PLS and CBSEM: Where the bias lies! J. Bus. Res. 2016, 69, 3998–4010. [Google Scholar] [CrossRef]
- Arfi, W.B.; Nasr, I.B.; Kondrateva, G.; Hikkerova, L. The role of trust in intention to use the IoT in eHealth: Application of the modified UTAUT in a consumer context. Technol. Forecast. Soc. Chang. 2021, 167, 120688. [Google Scholar] [CrossRef]
- Luyten, J.; Marneffe, W. Examining the acceptance of an integrated Electronic Health Records system: Insights from a repeated cross-sectional design. Int. J. Med. Inform. 2021, 150, 104450. [Google Scholar] [CrossRef] [PubMed]
- Zobair, K.M.; Sanzogni, L.; Houghton, L.; Islam, M.Z. Forecasting care seekers satisfaction with telemedicine using machine learning and structural equation modeling. PLoS ONE 2021, 16, e0257300. [Google Scholar] [CrossRef]
- Serrano, K.M.; Mendes, G.H.S.; Lizarelli, F.L.; Ganga, G.M.D. Assessing the telemedicine acceptance for adults in Brazil. Int. J. Health Care Qual. Assur. 2020. ahead-of-print. [Google Scholar] [CrossRef]
- Sarstedt, M.; Ringle, C.M.; Henseler, J.; Hair, J.F. On the Emancipation of PLS-SEM: A Commentary on Rigdon (2012). Long Range Plan. 2014, 47, 154–160. [Google Scholar] [CrossRef]
- Rigdon, E.E. Rethinking Partial Least Squares Path Modeling: In Praise of Simple Methods. Long Range Plan. 2012, 45, 341–358. [Google Scholar] [CrossRef]
- Ringle, C.M.; Wende, S.; Becker, J.M. SmartPLS 3 [Computer Software]; SmartPLS GmbH: Boenningstedt, Germany, 2015. [Google Scholar]
- Sarstedt, M.; Henseler, J.; Ringle, C.M. Multigroup Analysis in Partial Least Squares (PLS) Path Modeling: Alternative Methods and Empirical Results. In Measurement and Research Methods in International Marketing: Advances in International Marketing Vol 22; Schwaiger, M., Taylor, C.R., Sarstedt, M., Eds.; Emerald Group Publishing Ltd.: Bradford, UK, 2011; pp. 195–218. ISBN 978-1-78052-094-0. [Google Scholar]
- Hock, C.; Ringle, C.M.; Sarstedt, M. Management of multi-purpose stadiums: Importance and performance measurement of service interfaces. IJSTM 2010, 14, 188. [Google Scholar] [CrossRef]
- Shmueli, G.; Ray, S.; Velasquez Estrada, J.M.; Chatla, S.B. The elephant in the room: Predictive performance of PLS models. J. Bus. Res. 2016, 69, 4552–4564. [Google Scholar] [CrossRef]
- Shmueli, G.; Sarstedt, M.; Hair, J.F.; Cheah, J.-H.; Ting, H.; Vaithilingam, S.; Ringle, C.M. Predictive model assessment in PLS-SEM: Guidelines for using PLSpredict. EJM 2019, 53, 2322–2347. [Google Scholar] [CrossRef]
- Danks, N.P. The Piggy in the Middle. SIGMIS Database 2021, 52, 24–42. [Google Scholar] [CrossRef]
- Ray, S.; Danks, N.P.; Valdez, A.C. R Package Seminr: Domain-Specific Language for Building and Estimating Structural Equation Models; Elsevier: Amsterdam, the Netherlands, 2022. [Google Scholar]
- Hair, J.F.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M.; Danks, N.P.; Ray, S. Partial Least Squares Structural Equation Modeling (PLS-SEM) Using R.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-80518-0. [Google Scholar]
- Kijsanayotin, B.; Pannarunothai, S.; Speedie, S.M. Factors influencing health information technology adoption in Thailand’s community health centers: Applying the UTAUT model. Int. J. Med. Inform. 2009, 78, 404–416. [Google Scholar] [CrossRef]
- Calvin, K.L.; Ben-Tzion, K. The Patient Technology Acceptance Model (PTAM) for Homecare Patients with Chronic Illness; Sage CA: Los Angeles, CA, USA, 2006. [Google Scholar]
- Rho, M.J.; Kim, H.S.; Chung, K.; Choi, I.Y. Factors influencing the acceptance of telemedicine for diabetes management. Clust. Comput 2015, 18, 321–331. [Google Scholar] [CrossRef]
- Lewis, W.; Agarwal, R.; Sambamurthy, V. Sources of Influence on Beliefs about Information Technology Use: An Empirical Study of Knowledge Workers. MIS Q. 2003, 27, 657–678. [Google Scholar] [CrossRef]
- Baum, U.; Baum, A.-K.; Deike, R.; Feistner, H.; Scholz, M.; Markgraf, B.; Robra, B.-P.; Neumann, T. Das EEG-Home-Monitoring als alternatives Versorgungskonzept [Meeting Abstract]. 2020. Available online: https://www.egms.de/static/en/meetings/dkvf2020/20dkvf190.shtml (accessed on 20 August 2022).
- Fornell, C.; Larcker, D.F. Evaluating Structural Equation Models with Unobservable Variables and Measurement Error. J. Mark. Res. 1981, 18, 39–50. [Google Scholar] [CrossRef]
- Henseler, J.; Ringle, C.M.; Sarstedt, M. A new criterion for assessing discriminant validity in variance-based structural equation modeling. J. Acad. Mark. Sci. 2015, 43, 115–135. [Google Scholar] [CrossRef]
| Characteristic | Patient/ Video (n = 15) | Patient/ No-Video (n = 25) | Non-Patient/Video (n = 200) | Non-Patient/No-Video (n = 181) | Full Sample (n = 421) |
|---|---|---|---|---|---|
| age (F(3, 417) = 0.923; p = 0.430) | |||||
| mean (SD) | 44.33 (13.75) | 51.76 (16.96) | 48.73 (13.87) | 49.61 (15.16) | 49.13 (14.62) |
| gender (Fisher’s exact p = 0.080) | |||||
| male | 9 (60.0%) | 17 (68.0%) | 103 (51.5%) | 102 (56.4%) | 231 (54.9%) |
| female | 6 (40%) | 7 (28.0%) | 97 (48.5%) | 79 (43.6%) | 189 (44.9%) |
| divers | 0 (0.0%) | 1 (4.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) |
| Graduation * ((15) = 15.91; p = 0.388) | |||||
| main school | 1 (6.7%) | 1 (4.2%) | 23 (11.5%) | 19 (10.5%) | 44 (10.5%) |
| secondary school | 1 (6.7%) | 7 (29.2%) | 23 (11.5%) | 17 (9.4%) | 48 (11.4%) |
| middle school | 3 (20.0%) | 2 (8.3%) | 58 (29.0%) | 53 (29.3%) | 116 (27.6%) |
| university | 10 (66.7%) | 14 (58.3%) | 93 (46.5%) | 90 (49.7%) | 207 (49.3%) |
| none | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 1 (0.2%) |
| prefer not to say | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) | 2 (1.1%) | 4 (1.0%) |
| Vocational qualification ((27) = 32.25; p = 0.223) | |||||
| apprenticeship | 7 (46.7%) | 8 (32.0%) | 90 (45.0%) | 83 (45.9%) | 188 (44.7%) |
| professional school degree | 1 (6.7%) | 2 (8.0%) | 30 (15.0%) | 27 (14.9%) | 60 (14.3%) |
| professional school degree (incl. administrative and engineer college degree) | 1 (6.7%) | 1 (4.0%) | 9 (4.5%) | 9 (5.0%) | 20 (4.8%) |
| college degree | 3 (30.0%) | 2 (8.0%) | 13 (6.5%) | 7 (3.9%) | 25 (5.9%) |
| Bachelor’s degree | 0 (0.0%) | 0 (0.0%) | 17 (8.5%) | 8 (4.4%) | 25 (5.9%) |
| Master’s degree | 0 (0.0%) | 1 (4.0%) | 10 (5.0%) | 9 (5.0%) | 20 (4.8%) |
| diploma | 1 (6.7%) | 6 (24.0%) | 15 (7.5%) | 18 (9.9%) | 40 (9.5%) |
| promotion | 0 (0.0%) | 0 (0.0%) | 3 (1.5%) | 3 (1.7%) | 6 (1.4%) |
| none | 0 (0.0%) | 3 (12.0%) | 5 (2.5%) | 10 (5.5%) | 18 (4.3%) |
| prefer not to say | 2 (13.3%) | 2 (8.0%) | 8 (4.0%) | 7 (3.9%) | 19 (4.5%) |
| Path | Total Effect (t-Value/p-Value/[95% CI]) | Direct Effect (t-Value/p-Value/[95% CI]) | Indirect Effect (t-Value/p-Value/[95% CI]) | |||
|---|---|---|---|---|---|---|
| DC → BI | 0.065 (3.016/0.003/[0.028; 0.110]) | - | 0.065 (3.016/0.003/[0.028; 0.110]) | |||
| CA → BI | 0.397 (8.533/0.000/[0.308; 0.489]) | - | 0.397 (8.533/0.000/[0.308; 0.489]) | |||
| PS → BI | 0.142 (3.632/0.000/[0.066; 0.217]) | 0.047 (1.176/0.239/ [−0.031; 0.125]) | 0.095 (3.872/0.000/[0.050; 0.147]) | |||
| via EE 0.018 (1.737/0.082/ [−0.001; 0.039]) | via PE 0.066 (3.278/0.001/[0.030; 0.109]) | |||||
| PE → BI | 0.348 (5.381/0.000/[0.223; 0.472]) | 0.348 (5.381/0.000/[0.223; 0.472]) | - | |||
| EE → BI | 0.508 (9.549/0.000/[0.403; 0.611]) | 0.312 (5.091/0.000/[0.189; 0.431]) | 0.196 (4.921/0.000/[0.122; 0.276]) | |||
| FC → BI | 0.184 (3.802/0.000/[0.090; 0.278]) | 0.184 (3.802/0.000/[0.090; 0.278]) | - | |||
| SI → BI | 0.057 (1.126/0.260/ [−0.041; 0.160]) | 0.057 (1.126/0.260/ [−0.041; 0.160]) | - | |||
| EE → PE | 0.562 (11.563/0.000/[0.461; 0.655]) | 0.562 (11.563/0.000/[0.461; 0.655]) | - | |||
| CA → EE | 0.781 (24.808/0.000/[0.714; 0.837]) | 0.781 (24.808/0.000/[0.714; 0.837]) | - | |||
| PS → EE | 0.056 (1.859/0.063/ [−0.004; 0.116]) | 0.056 (1.859/0.063/ [−0.004; 0.116]) | - | |||
| PS → PE | 0.221 (5.296/0.000/ [0.140; 0.304]) | 0.189 (4.429/0.000/ [0.106; 0.273]) | 0.032 (1.800/0.072/ [−0.002; 0.067]) | |||
| DC → PE | 0.184 (4.103/0.000/[0.100; 0.272]) | 0.184 (4.103/0.000/[0.100; 0.272]) | - | |||
| CA → PE | 0.440 (9.884/0.000/[0.351; 0.525]) | - | 0.440 (9.884/0.000/[0.351; 0.525]) | |||
| Item | PLS-SEM RMSE | Linear Model RMSE |
|---|---|---|
| BI1 | 0.877 | 0.853 |
| BI2 | 0.971 | 1.002 |
| BI3 | 0.902 | 0.886 |
| BI4 | 0.863 | 0.823 |
| PE1 | 0.915 | 0.890 |
| PE2 | 1.281 | 1.304 |
| PE3 | 1.092 | 1.135 |
| PE4 | 1.235 | 1.200 |
| PE5 | 1.037 | 1.027 |
| EE1 | 0.989 | 0.965 |
| EE2 | 0.877 | 0.864 |
| EE3 | 0.886 | 0.937 |
| EE4 | 0.883 | 0.914 |
| Dependent Variable = Home Monitoring Preference | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|
| b (t/p) | b (t/p) | b (t/p) | b (t/p) | ||
| Intercept | 5.147 (60.097/0.000) | 5.398 (13.223/0.000) | 5.693 (8.935/0.000) | 5.391 (8.500/0.000) | |
| Main variable | Behavioral intention score | 0.973 (11.364/0.000) | 1.015 (11.605/0.000) | 1.012 (11.575/0.000) | 1.007 (11.563/0.000) |
| Health | Physical functioning | −0.002 (−0.294/0.769) | −0.003 (−0.483/0.629) | −0.004 (−0.705/0.481) | |
| Physical health | 0.004 (1.124/0.262) | 0.004 (0.929 /0.354) | 0.004 (1.054/0.292) | ||
| Emotional problems | 0.004 (1.184/0.237) | 0.005 (1.332/0.184) | 0.005 (1.392/0.165) | ||
| Energy/fatigue | −0.003 (−0.450/0.653) | −0.001 (−0.122/0.903) | −0.002 (−0.274/0.784) | ||
| Emotional well-being | −0.008 (−0.920/0.358) | −0.006 (−0.775/0.439) | −0.006 (−0.726/0.469) | ||
| Social functioning | −0.010 (−1.935/0.054) | −0.010 (−1.919/0.056) | −0.010 (−1.828/0.068) | ||
| Pain | 0.002 (0.361/0.752) | 0.003 (0.473/0.636) | 0.003 (0.553/0.581) | ||
| General health | 0.009 (1.350/0.178) | 0.006 (0.850/0.396) | 0.005 (0.811/0.418) | ||
| Health change | 0.003 (0.556/0.579) | 0.002 (0.363/0.716) | 0.002 (0.343/0.732) | ||
| Demographics | Age | −0.009 (−1.337/0.182) | −0.008 (-1.277/0.202) | ||
| Gender (0 = male, 1 = female) | 0.177 (1.014/0.311) | 0.166 (0.952/0.342) | |||
| Conditions | Participant type | 0.331 (1.107/0.269) | |||
| Video (0 = no, 1 = yes) | 0.466 (2.714/0.007) | ||||
| Summary | Observations | 421 | 421 | 420 | 420 |
| 0.236 | 0.259 | 0.264 | 0.278 | ||
| Adjusted | 0.234 | 0.240 | 0.242 | 0.253 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baum, U.; Kühn, F.; Lichters, M.; Baum, A.-K.; Deike, R.; Hinrichs, H.; Neumann, T. Neurological Outpatients Prefer EEG Home-Monitoring over Inpatient Monitoring—An Analysis Based on the UTAUT Model. Int. J. Environ. Res. Public Health 2022, 19, 13202. https://doi.org/10.3390/ijerph192013202
Baum U, Kühn F, Lichters M, Baum A-K, Deike R, Hinrichs H, Neumann T. Neurological Outpatients Prefer EEG Home-Monitoring over Inpatient Monitoring—An Analysis Based on the UTAUT Model. International Journal of Environmental Research and Public Health. 2022; 19(20):13202. https://doi.org/10.3390/ijerph192013202
Chicago/Turabian StyleBaum, Ulrike, Frauke Kühn, Marcel Lichters, Anne-Katrin Baum, Renate Deike, Hermann Hinrichs, and Thomas Neumann. 2022. "Neurological Outpatients Prefer EEG Home-Monitoring over Inpatient Monitoring—An Analysis Based on the UTAUT Model" International Journal of Environmental Research and Public Health 19, no. 20: 13202. https://doi.org/10.3390/ijerph192013202
APA StyleBaum, U., Kühn, F., Lichters, M., Baum, A.-K., Deike, R., Hinrichs, H., & Neumann, T. (2022). Neurological Outpatients Prefer EEG Home-Monitoring over Inpatient Monitoring—An Analysis Based on the UTAUT Model. International Journal of Environmental Research and Public Health, 19(20), 13202. https://doi.org/10.3390/ijerph192013202







