Emerging Zoonotic Infections, Social Processes and Their Measurement and Enhanced Surveillance to Improve Zoonotic Epidemic Responses: A “Big Events” Perspective
Abstract
:1. Introduction
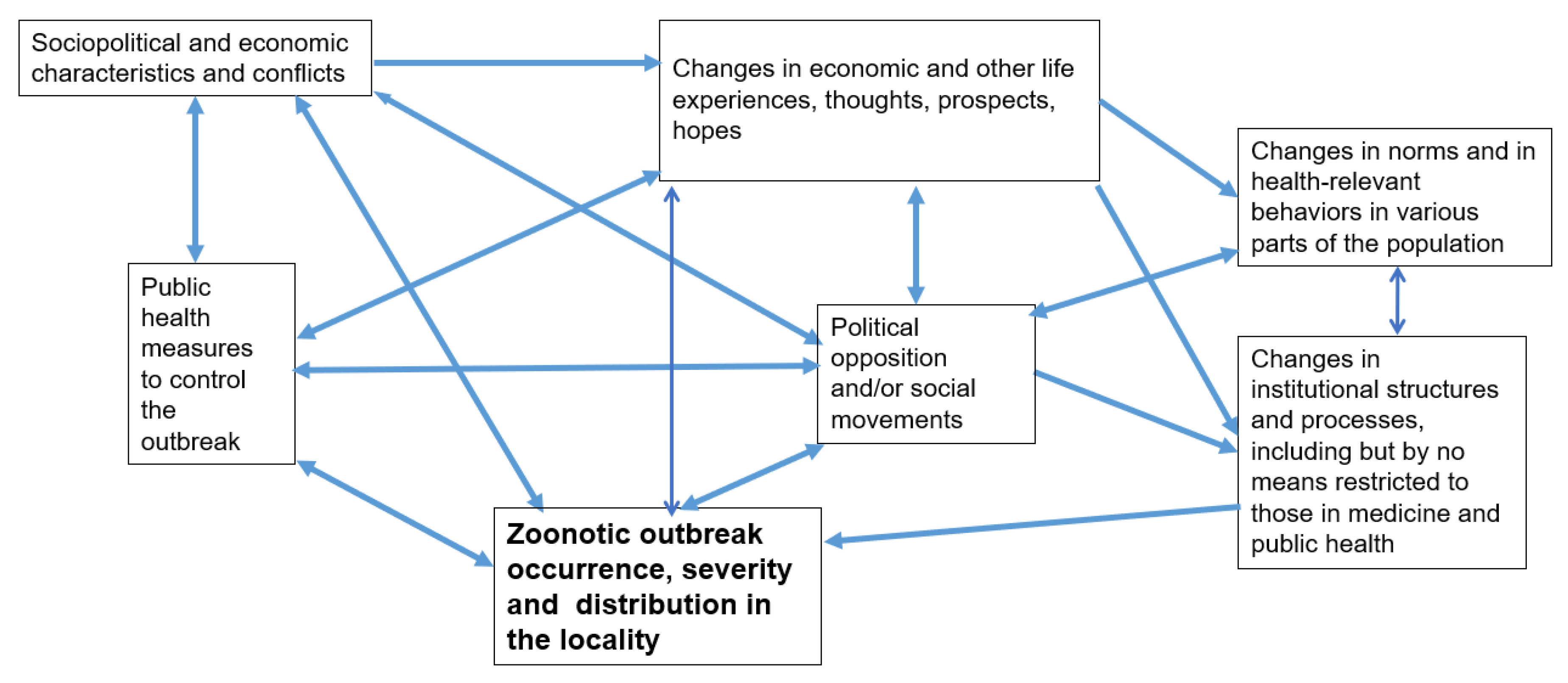
2. A General Framework of Response to a Zoonotic Outbreak
3. Example: The Covid-19 Outbreak in the US in a Big Events Framework
4. Social Readiness Monitoring and Other Forms of Preparation and Learning
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wallace, R. Dead Epidemiologists: On the Origins of COVID-19; Monthly Review Press: New York, NY, USA, 2020. [Google Scholar]
- Baylis, M. Potential impact of climate change on emerging vector-borne and other infections in the UK. Environ. Health 2017, 16, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Short, E.E.; Caminade, C.; Thomas, B.N. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect. Dis. Res. Treat. 2017, 10, 1178633617732296. [Google Scholar] [CrossRef] [Green Version]
- Bartlow, A.W.; Manore, C.; Xu, C.; Kaufeld, K.A.; Del Valle, S.; Ziemann, A.; Fairchild, G.; Fair, J.M. Forecasting Zoonotic Infectious Disease Response to Climate Change: Mosquito Vectors and a Changing Environment. Vet. Sci. 2019, 6, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobson, A.P.; Pimm, S.L.; Hannah, L.; Kaufman, L.; Ahumada, J.A.; Ando, A.W.; Bernstein, A.; Busch, J.; Daszak, P.; Engelmann, J.; et al. Ecology and economics for pandemic prevention. Science 2020, 369, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Davis, M. Monster at Our Door: The Global Threat of Avian Flu. BMJ 2005, 331, 1275. [Google Scholar]
- Friedman, S.R. Environmental change and infectious diseases in the Mediterranean region and the world: An interpretive dialectical analysis. Euro-Mediterr. J. Environ. Integr. 2021, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Malm, A. Corona, Climate, Chronic Emergency: War Communism in the Twenty-First Century; Verso Books: London, UK, 2020. [Google Scholar]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernecke, B.; Millar, D.A.; Walters, M.; Ganswindt, A.; Dziba, L.; Wright, C.Y. Preventing the next pandemic’—A 2020 UNEP Frontiers Series Report on zoonotic diseases with reflections for South Africa. S. Afr. J. Sci. 2020, 116, 1–4. [Google Scholar] [CrossRef]
- Wallace, R. Big Farms Make Big Flu: Dispatches on Infectious Disease, Agribusiness, and the Nature of Science; Monthly Review Press: New York, NY, USA, 2016. [Google Scholar]
- Wallace, R.; Liebman, A.; Chaves, L.F.; Wallace, R. COVID-19 and the Circuits of Capital: New York to China and Back. Mon. Rev. 2020, 72, 1–15. [Google Scholar] [CrossRef]
- Weiss, R.A.; McMichael, A.J. Social and environmental risk factors in the emergence of infectious diseases. Nat. Med. 2004, 10 (Suppl. 12), S70–S76. [Google Scholar] [CrossRef]
- Friedman, S.R.; Mateu-Gelabert, P.; Nikolopoulos, G.K.; Cerdá, M.; Rossi, D.; Jordan, A.E.; Townsend, T.; Khan, M.R.; Perlman, D.C. Big Events theory and measures may help explain emerging long-term effects of current crises. Glob. Public Heal. 2021, 16, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.R.; Rossi, D.; Braine, N. Theorizing “Big Events” as a potential risk environment for drug use, drug-related harm and HIV epidemic outbreaks. Int. J. Drug Policy 2009, 20, 283–291. [Google Scholar] [CrossRef]
- Rhodes, T.; Lowndes, C.; Judd, A.; Mikhailova, L.A.; Sarang, A.; Rylkov, A.; Tichonov, M.; Lewis, K.; Ulyanova, N.; Alpatova, T.; et al. Explosive spread and high prevalence of HIV infection among injecting drug users in Togliatti City, Russia. AIDS 2002, 16, F25–F31. [Google Scholar] [CrossRef] [PubMed]
- Aral, S.O.; Lawrence, J.S.S. The Ecology of Sex Work and Drug Use in Saratov Oblast, Russia. Sex. Transm. Dis. 2002, 29, 798–805. [Google Scholar] [CrossRef] [Green Version]
- Strathdee, S.A.; Stachowiak, J.A.; Todd, C.S.; Al-Delaimy, W.K.; Wiebel, W.; Hankins, C.; Patterson, T.L. Complex emergencies, HIV, and substance use: No “big easy” solution. Subst. Use Misuse 2006, 41, 1637–1651. [Google Scholar] [CrossRef]
- Lagerspetz, M.; Moskalewicz, J. Drugs in the Postsocialist Transitions of Estonia, Latvia, Lithuania and Poland. Eur. Addict. Res. 2002, 8, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.; Rossi, D.; Phaswana-Mafuya, N. Globalization and Interacting Large-Scale Processes and How They May Affect the HIV/AIDS Epidemic. In Globalizing Theory on HIV/AIDS; Routledge, Inc.: New York, NY, USA, 2009; pp. 491–500. [Google Scholar]
- Friedman, S.R.R. Gary, The need for dialectical models as shown in the response to the HIV/AIDS epidemic. Int. J. Sociol. Soc. Policy 2002, 22, 177–200. [Google Scholar] [CrossRef]
- Jenkins, R.; Klein, J.; Parker, C. Mental health in post-communist countries. BMJ 2005, 331, 173–174. [Google Scholar] [CrossRef] [Green Version]
- Nikolopoulos, G.K.; Sypsa, V.; Bonovas, S.; Paraskevis, D.; Malliori-Minerva, M.; Hatzakis, A.; Friedman, S.R. Big Events in Greece and HIV Infection Among People Who Inject Drugs. Subst. Use Misuse 2015, 50, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Vasylyeva, T.I.; Liulchuk, M.; Friedman, S.R.; Sazonova, I.; Faria, N.R.; Katzourakis, A.; Babii, N.; Scherbinska, A.; Thézé, J.; Pybus, O.G.; et al. Molecular epidemiology reveals the role of war in the spread of HIV in Ukraine. Proc. Natl. Acad. Sci. USA 2018, 115, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Pouget, E.R.; Sandoval, M.; Nikolopoulos, G.K.; Friedman, S.R. Immediate Impact of Hurricane Sandy on People Who Inject Drugs in New York City. Subst. Use Misuse 2015, 50, 878–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, S.R.; Rossi, D. Some Musings About Big Events and the Past and Future of Drug Use and of HIV and Other Epidemics. Subst. Use Misuse 2015, 50, 899–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, S.R.; Sandoval, M.; Mateu-Gelabert, P.; Rossi, D.; Gwadz, M.; Dombrowski, K.; Smyrnov, P.; Vasylyeva, T.; Pouget, E.R.; Perlman, D. Theory, measurement and hard times: Some issues for HIV/AIDS research. AIDS Behav. 2013, 17, 1915–1925. [Google Scholar] [CrossRef] [Green Version]
- Friedman, S.R.; Kippax, S.C.; Phaswana-Mafuya, N.; Rossi, D.; Newman, C.E. Emerging future issues in HIV/AIDS social research. AIDS 2006, 20, 959–965. [Google Scholar] [CrossRef]
- Friedman, S.R.; Pouget, E.R.; Sandoval, M.; Nikolopoulos, G.K.; Mateu-Gelabert, P.; Rossi, D.; Auerbach, J.D. New Measures for Research on Men Who Have Sex with Men and for At-Risk Heterosexuals: Tools to Study Links Between Structural Interventions or Large-Scale Social Change and HIV Risk Behaviors, Service Use, and Infection. AIDS Behav. 2020, 24, 257–273. [Google Scholar] [CrossRef]
- Pouget, E.R.; Sandoval, M.; Nikolopoulos, G.K.; Mateu-Gelabert, P.; Rossi, D.; Smyrnov, P.; Jones, Y.; Friedman, S.R. Developing Measures of Pathways that May Link Macro Social/Structural Changes with HIV Epidemiology. AIDS Behav. 2016, 20, 1808–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, S.R.; Pouget, E.R.; Sandoval, M.; Rossi, D.; Mateu-Gelabert, P.; Nikolopoulos, G.K.; Schneider, J.A.; Smyrnov, P.; Stall, R.D. Interpersonal Attacks on the Dignity of Members of HIV Key Populations: A Descriptive and Exploratory Study. AIDS Behav. 2017, 21, 2561–2578. [Google Scholar] [CrossRef]
- Friedman, S.R.; Pouget, E.R.; Sandoval, M.; Jones, Y.; Nikolopoulos, G.K.; Mateu-Gelabert, P. Measuring Altruistic and Solidaristic Orientations Toward Others Among People Who Inject Drugs. J. Addict. Dis. 2015, 34, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Friedman, S.R.; Pouget, E.R.; Sandoval, M.; Jones, Y.; Mateu-Gelabert, P. Formal and Informal Organizational Activities of People Who Inject Drugs in New York City: Description and Correlates. J. Addict. Dis. 2015, 34, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunting, A.M.; Frank, D.; Arshonsky, J.; Bragg, M.A.; Friedman, S.R.; Krawczyk, N. Socially-supportive norms and mutual aid of people who use opioids: An analysis of Reddit during the initial COVID-19 pandemic. Drug Alcohol Depend. 2021, 222, 108672. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Wolfe, N.D.; Dunavan, C.P.; Diamond, J. Origins of major human infectious diseases. Nature 2007, 447, 279–283. [Google Scholar] [CrossRef]
- Hahn, B.H.; Shaw, G.M.; De, K.M.; Cock; Sharp, P.M. AIDS as a zoonosis: Scientific and public health implications. Science 2000, 287, 607–614. [Google Scholar] [CrossRef] [Green Version]
- McNeill, W.H.; McNeill, W. Plagues and Peoples; Anchor: New York, NY, USA, 1998. [Google Scholar]
- Harper, K. The Fate of Rome; Princeton University Press: Princeton, NJ, USA, 2017. [Google Scholar]
- Anievas, A.; Nişancıoğlu, K. How the West Came to Rule: The Geopolitical Origins of Capitalism; Pluto Press: London, UK, 2015. [Google Scholar]
- Crosby, A.W. Ecological Imperialism: The Biological Expansion of Europe, 900–1900; Cambridge University Press: New York, NY, USA, 2004. [Google Scholar]
- Tudela, F. El encuentro entre dos mundos: Impacto ambiental de la conquista. In Nueva Sociedad; NUEVA SOCIEDAD: Ciudad de Buenos Aires, Argentina, 1992; Volume 122, pp. 198–209. [Google Scholar]
- Abu-Lughod, J.L. Before European Hegemony: The World System A.D. 1250–1350; Oxford University Press: New York, NY, USA, 1989; p. 443. [Google Scholar]
- Barrett, P.; Chen, S. Social repercussions of pandemics. International Monetary Fund. 2021. Available online: https://www.imf.org/en/Publications/WP/Issues/2021/01/29/Social-Repercussions-of-Pandemics-50041 (accessed on 23 December 2021).
- Bristow, N. American Pandemic: The Lost Worlds of the 1918 Influenza Epidemic; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Martini, M.; Gazzaniga, V.; Bragazzi, N.L.; Barberis, I. The Spanish Influenza Pandemic: A lesson from history 100 years after 1918. J. Prev. Med. Hyg. 2019, 60, E64. [Google Scholar]
- Da Silva, P.G.; Mesquita, J.R.; Nascimento, M.d.S.J.; Ferreira, V.A.M. Viral, host and environmental factors that favor anthropozoonotic spillover of coronaviruses: An opinionated review, focusing on SARS-CoV, MERS-CoV and SARS-CoV-2. Sci. Total Environ. 2021, 750, 141483. [Google Scholar] [CrossRef] [PubMed]
- Adimora, A.A.; Auerbach, J.D. Structural interventions for HIV prevention in the United States. J. Acquir. Immune Defic. Syndr. 2010, 55, S132–S135. [Google Scholar] [CrossRef] [Green Version]
- Baker, P.; Rogers, K.; Enrich, D.; Haberman, M. Trump’s aggressive advocacy of malaria drug for treating coronavirus divides medical community. New York Times, 6 April 2021. Available online: https://www.nytimes.com/2020/04/06/us/politics/coronavirus-trump-malaria-drug.html (accessed on 10 January 2022).
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious disease in an era of global change. Nat. Rev. Genet. 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Xagoraraki, I. A water-focused one-health approach for early detection and prevention of viral outbreaks. One Health 2019, 7, 100094. [Google Scholar] [CrossRef]
- Solomon, S. Water: The Epic Struggle for Wealth, Power, and Civilizatio; Harper: New York, NY, USA, 2010. [Google Scholar]
- Partelow, S. Private Oceans: The Enclosure and Marketisation of the Seas by Fiona Mccormack, London, Pluto Press, 2017, 184 Pp.£ 24.99 (Paperback), ISBN: 978-0-7453-9910. J. Marit. Res. 2019, 21, 155–158. [Google Scholar] [CrossRef]
- Kennedy, A.; Resnick, D. Governing a Crisis and Crises of Governance: The Political Dimensions of COVID-19; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Lönnroth, K.; Jaramillo, E.; Williams, B.; Dye, C.; Raviglione, M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Soc. Sci. Med. 2009, 68, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Morris, A. Reflections on social movement theory: Criticisms and proposals. Contemp. Sociol. 2000, 29, 445–454. [Google Scholar] [CrossRef]
- O’Connor, C. The Conspiracy Consortium Examining Discussions of COVID-19 Among Right-Wing Extremist Telegram Channels; Institute for Strategic Dialogue (ISD): London, UK, 2021. [Google Scholar]
- Umair Majid, J.T.; Wasim, A.; Truong, M. Anti-Mask Protests and Racism: What Is the Link? Longwoods Insights 2020. Available online: https://www.longwoods.com/content/26390/anti-mask-protests-and-racism-what-is-the-link- (accessed on 18 December 2021).
- Holder, M.; Jones, J.; Masterson, T. The Early Impact of Covid-19 on Job Losses among Black Women in the United States. Fem. Econ. 2021, 27, 103–116. [Google Scholar] [CrossRef]
- Dias, F.A.; Chance, J.; Buchanan, A. The motherhood penalty and The fatherhood premium in employment during covid-19: Evidence from The united states. Res. Soc. Strat. Mobil. 2020, 69, 100542. [Google Scholar] [CrossRef]
- Saenz, R.; Sparks, C. The Inequities of Job Loss and Recovery Amid the COVID-19 Pandemic. 2020. Available online: https://doi.org/10.34051/p/2021.3 (accessed on 23 December 2021).
- Fearnley, L. Virulent Zones: Animal Disease and Global Health at China’s Pandemic Epicenter; Duke University Press: Durham, NC, USA, 2020. [Google Scholar]
- Galea, S. The Contagion Next Time; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Krieger, N. Ecosocial Theory, Embodied Truths, and the People’s Health; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Scientists under attack and weird viruses—The week in infographics. Nature 2021. Available online: https://doi.org/10.1038/d41586-021-02817-8 (accessed on 1 January 2022). [CrossRef]
- Piret, J.; Boivin, G. Pandemics throughout history. Front. Microbiol. 2020, 11, 631736. [Google Scholar] [CrossRef]
- Saitone, T.L.; Schaefer, K.A.; Scheitrum, D.P. COVID-19 Morbidity and Mortality in U.S. Meatpacking Counties. Food Policy 2021, 101, 102072. [Google Scholar] [CrossRef]
- Taylor, C.A.; Boulos, C.; Almond, D. Livestock plants and COVID-19 transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 31706–31715. [Google Scholar] [CrossRef]
- Lawson, R.A.; Murphy, R.H.; Williamson, C.R. The relationship between income, economic freedom, and BMI. Public Health 2016, 134, 18–25. [Google Scholar] [CrossRef]
- Vogli, R.D.; Kouvonen, A.; Elovainio, M.; Marmot, M. Economic globalization, inequality and body mass index: A cross-national analysis of 127 countries. Crit. Public Health 2014, 24, 7–21. [Google Scholar] [CrossRef]
- Ewald, P.W. Evolution of Infectious Disease; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Ewald, P.W. Host-parasite relations, vectors, and the evolution of disease severity. Annu. Rev. Ecol. Syst. 1983, 14, 465–485. [Google Scholar] [CrossRef]
- Friedman, S.R.; Rossi, D. Dialectical theory and the study of HIV/AIDS and other epidemics. Dialect. Anthr. 2011, 35, 403–427. [Google Scholar] [CrossRef] [Green Version]
- Goodman, J.R. An evolving crisis. New Sci. 2020, 246, 41–45. [Google Scholar] [CrossRef]
- Weinstein, L. Street Corner Secrets: Sex, Work, and Migration in the City of Mumbai; SAGE Publications: Los Angeles, CA, USA, 2016. [Google Scholar]
- Beheyt, P. Contribution a l’etude des hepatites en Afrique. L’hepatite epidemique et l’hepatite par inoculation. Ann. Soc. Bel. Med. Trop. 1953, 33, 297–340. [Google Scholar]
- Levine, M.P.; Kimmel, M. Gay Macho: The Life and Death of the Homosexual Clone; NYU Press: New York, NY, USA, 1998. [Google Scholar]
- Wadman, M. Antivaccine forces gaining online. Science 2020, 368, 699. [Google Scholar] [CrossRef]
- Mervosh, S.; Lu, D.; Swales, V. See which states and cities have told residents to stay at home. New York Times, 20 April 2020. Available online: https://www.nytimes.com/interactive/2020/us/coronavirus-stay-at-home-order.html (accessed on 31 March 2020).
- Kimberly Kindy, T.M.A.R.H. The Trump administration approved faster line speeds at chicken plants. Those facilities are more likely to have covid-19 cases. Washington Post 2021. Available online: https://www.washingtonpost.com/politics/trump-chicken-covid-coronavirus-biden/2021/01/03/ea8902b0-3a39-11eb-98c4-25dc9f4987e8_story.html (accessed on 3 January 2021).
- Liptak, A. Splitting 5 to 4, Supreme Court Backs Religious Challenge to Cuomo’s Virus Shutdown Order. New York Times, 26 November 2020. Available online: https://www.nytimes.com/2020/11/26/us/supreme-court-coronavirus-religion-new-york.html (accessed on 1 January 2022).
- Blevins, J.L.; Edgerton, E.; Jason, D.P.; Lee, J.J. Shouting Into the Wind: Medical Science versus “B.S.” in the Twitter Maelstrom of Politics and Misinformation About Hydroxychloroquine. Soc. Media + Soc. 2021, 7. [Google Scholar] [CrossRef]
- Shin, S.H.; Ji, H.; Lim, H. Heterogeneity in preventive behaviors during COVID-19: Health risk, economic insecurity, and slanted information. Soc. Sci. Med. 2021, 278, 113944. [Google Scholar] [CrossRef] [PubMed]
- Pennycook, G.; McPhetres, J.; Bago, B.; Rand, D. Beliefs about COVID-19 in Canada, the UK, and the USA: A novel test of political polarization and motivated reasoning. PsyArXiv 2021. [Google Scholar] [CrossRef]
- Himelein-Wachowiak, M.; Giorgi, S.; Devoto, A.; Rahman, M.; Ungar, L.; Schwartz, H.A.; Epstein, D.H.; Leggio, L.; Curtis, B. Bots and Misinformation Spread on Social Media: Implications for COVID-19. J. Med. Internet Res. 2021, 23, e26933. [Google Scholar] [CrossRef]
- Al Khaja, K.A.; AlKhaja, A.K.; Sequeira, R.P. Drug information, misinformation, and disinformation on social media: A content analysis study. J. Public Health Policy 2018, 39, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, W.; Kaiser, J.; Kupferschmidt, K.; Malakoff, D.; Servick, K. The United States leads in coronavirus cases, but not pandemic response. Science News, 1 April 2020. [Google Scholar]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef]
- Ivory, M.B.A.D. Why Public Health Faces a Crisis Across the U.S. New York Times, 18 October 2021. Available online: https://www.nytimes.com/2021/10/18/us/coronavirus-public-health.html (accessed on 23 December 2021).
- Coronavirus: Armed protesters enter Michigan statehouse. BBC, 1 May 2020. Available online: https://www.bbc.com/news/world-us-canada-52496514 (accessed on 23 December 2021).
- Sokolow, A. Hundreds protest in front of the State House over vaccine, mask mandates. Boston Herald, 17 September 2021. Available online: https://www.bostonherald.com/2021/09/17/hundreds-protest-in-front-of-the-state-house-over-vaccine-mask-mandates/ (accessed on 23 December 2021).
- Kushner, G.S.; Wallace, G.S.; Pepinsky Thomas, B. Partisanship, health behavior, and policy attitudes in the early stages of the COVID-19 pandemic. PLoS ONE 2021, 16, e0249596. [Google Scholar]
- Kates, J.; Orgera, K. The red/blue divide in COVID-19 vaccination rates is growing. Kaiser Family Foundation, 14 September 2021. Available online: https://www.kff.org/policy-watch/the-red-blue-divide-in-covid-19-vaccination-rates/ (accessed on 23 December 2021).
- Parker, K.; Menasce Horowitz, J.; Minkin, R. How the Coronavirus Outbreak Has–and Hasn’t–Changed the Way Americans Work. Pew Res. Cent. 2020. Available online: https://www.pewresearch.org/social-trends/2020/12/09/how-the-coronavirus-outbreak-has-and-hasnt-changed-the-way-americans-work/ (accessed on 10 January 2022).
- Lukas, H.; Xu, C.; Yu, Y.; Gao, W. Emerging telemedicine tools for remote COVID-19 diagnosis, monitoring, and management. ACS Nano 2020, 14, 16180–16193. [Google Scholar] [CrossRef]
- Hincapié, M.A.; Gallego, J.C.; Gempeler, A.; Piñeros, J.A.; Nasner, D.; Escobar, M.F. Implementation and Usefulness of Telemedicine During the COVID-19 Pandemic: A Scoping Review. J. Prim. Care Community Heal. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Stockdill, B.C. ACT UP (Aids Coalition to Unleash Power). In The Wiley-Blackwell Encyclopedia of Social and Political Movements; Wiley: Malden, MA, USA, 2013; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9780470674871 (accessed on 10 January 2022).
- Chappell, B. Supreme Court declares same-sex marriage legal in all 50 states. National Public Radio, 26 June 2015. Available online: https://www.npr.org/sections/thetwo-way/2015/06/26/417717613/supreme-court-rules-all-states-must-allow-same-sex-marriages (accessed on 23 December 2021).
- Epstein, S. Impure Science: Aids, Activism, and the Politics of Knowledge; University of California Press: Berkeley, CA, USA, 1996; p. 466. [Google Scholar]
- National Association of County and City Health Officials (NACCHO). Proposed Limits on Public Health Authority: Dangerous for Public Health. 2021. Available online: https://www.naccho.org/uploads/downloadable-resources/Proposed-Limits-on-Public-Health-Authority-Dangerous-for-Public-Health-FINAL-5.24.21pm.pdf (accessed on 10 January 2022).
- Hadjikou, A.; Pavlopoulou, I.D.; Pantavou, K.; Georgiou, A.N.; Williams, L.D.; Christaki, E.; Voskarides, K.; Lavranos, G.; Lamnisos, D.; Pouget, E.R.; et al. Drug Injection-Related Norms and High-Risk Behaviors of People Who Inject Drugs in Athens, Greece. AIDS Res. Hum. Retroviruses 2021, 37, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Paraskevis, D.; Paraschiv, S.; Sypsa, V.; Nikolopoulos, G.; Tsiara, C.; Magiorkinis, G.; Psichogiou, M.; Flampouris, A.; Mardarescu, M.; Niculescu, I.; et al. Enhanced HIV-1 surveillance using molecular epidemiology to study and monitor HIV-1 outbreaks among intravenous drug users (IDUs) in Athens and Bucharest. Infect. Genet. Evol. 2015, 35, 109–121. [Google Scholar] [CrossRef]
- Roussos, S.; Paraskevis, D.; Psichogiou, M.; Kostaki, E.G.; Flountzi, E.; Angelopoulos, T.; Chaikalis, S.; Papadopoulou, M.; Pavlopoulou, I.D.; Malliori, M. Ongoing HIV transmission following a large outbreak among people with long term injecting drug use in Athens, Greece (2014-2020). medRxiv 2021. [Google Scholar] [CrossRef]
- Lobato-Cordero, A.; Quentin, E.; Lobato-Cordero, G. Spatiotemporal Analysis of Influenza Morbidity and Its Association with Climatic and Housing Conditions in Ecuador. J. Environ. Public Health 2019, 2019, 6741202-10. [Google Scholar] [CrossRef]
- Clark, M.; Riben, P.; Nowgesic, E. The association of housing density, isolation and tuberculosis in Canadian First Nations communities. Int. J. Epidemiol. 2002, 31, 940–945. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Garcia, D. Zip code-level risk factors for tuberculosis: Neighborhood environment and residential segregation in New Jersey, 1985-1992. Am. J. Public Health 2001, 91, 734–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Tawfiq, J.A.; Memish, Z.A. Drivers of MERS-CoV transmission: What do we know? Expert Rev. Respir. Med. 2016, 10, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehgal, A.R.; Himmelstein, D.U.; Woolhandler, S. Feasibility of separate rooms for home isolation and quarantine for COVID-19 in the United States. Ann. Intern. Med. 2021, 174, 127–129. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedman, S.R.; Jordan, A.E.; Perlman, D.C.; Nikolopoulos, G.K.; Mateu-Gelabert, P. Emerging Zoonotic Infections, Social Processes and Their Measurement and Enhanced Surveillance to Improve Zoonotic Epidemic Responses: A “Big Events” Perspective. Int. J. Environ. Res. Public Health 2022, 19, 995. https://doi.org/10.3390/ijerph19020995
Friedman SR, Jordan AE, Perlman DC, Nikolopoulos GK, Mateu-Gelabert P. Emerging Zoonotic Infections, Social Processes and Their Measurement and Enhanced Surveillance to Improve Zoonotic Epidemic Responses: A “Big Events” Perspective. International Journal of Environmental Research and Public Health. 2022; 19(2):995. https://doi.org/10.3390/ijerph19020995
Chicago/Turabian StyleFriedman, Samuel R., Ashly E. Jordan, David C. Perlman, Georgios K. Nikolopoulos, and Pedro Mateu-Gelabert. 2022. "Emerging Zoonotic Infections, Social Processes and Their Measurement and Enhanced Surveillance to Improve Zoonotic Epidemic Responses: A “Big Events” Perspective" International Journal of Environmental Research and Public Health 19, no. 2: 995. https://doi.org/10.3390/ijerph19020995
APA StyleFriedman, S. R., Jordan, A. E., Perlman, D. C., Nikolopoulos, G. K., & Mateu-Gelabert, P. (2022). Emerging Zoonotic Infections, Social Processes and Their Measurement and Enhanced Surveillance to Improve Zoonotic Epidemic Responses: A “Big Events” Perspective. International Journal of Environmental Research and Public Health, 19(2), 995. https://doi.org/10.3390/ijerph19020995







