The Relationship between Cognitive Performance and Quality of Life in Elite Athletes after Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
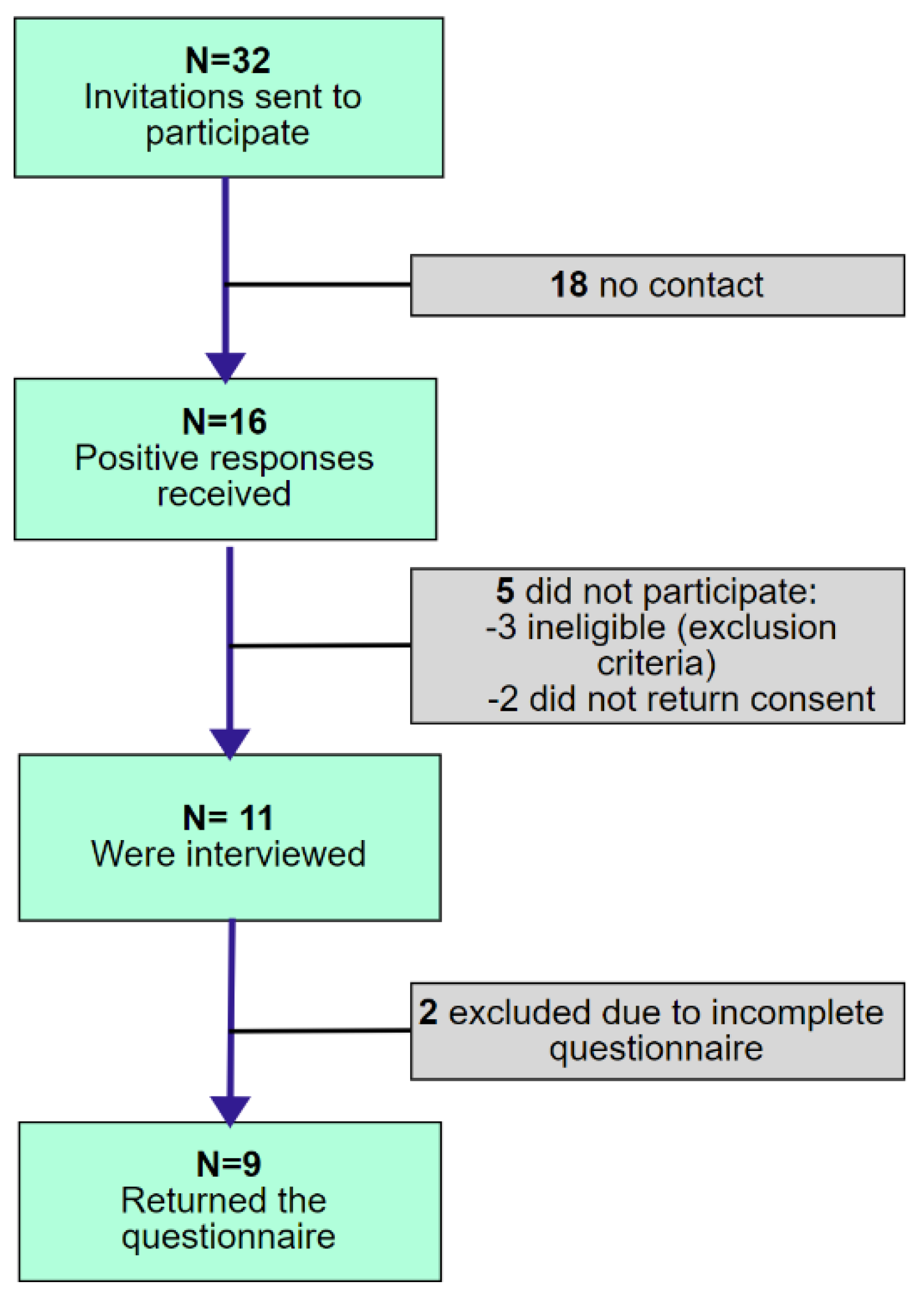
2.1. Participants
2.2. Procedure
2.2.1. Demographics and Injury Characteristics
2.2.2. Quality of Life
2.2.3. Cognitive Performance
COWAT
Digit Span Test
Stroop Color–Word Test
2.3. Data Analysis
3. Results
3.1. Quality of Life
3.2. Cognitive Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidoff, G.N.; Roth, E.J.; Richards, J.S. Cognitive Deficits in Spinal Cord Injury: Epidemiology and Outcome. Arch. Phys. Med. Rehabil. 1992, 73, 275–284. [Google Scholar] [PubMed]
- Jegede, A.B.; Rosado-Rivera, D.; Bauman, W.A.; Cardozo, C.P.; Sano, M.; Moyer, J.M.; Brooks, M.; Wecht, J.M. Cognitive Performance in Hypotensive Persons with Spinal Cord Injury. Clin. Auton. Res. 2010, 20, 3–9. [Google Scholar] [CrossRef][Green Version]
- Chiaravalloti, N.D.; Weber, E.; Wylie, G.; Dyson-Hudson, T.; Wecht, J.M. Patterns of Cognitive Deficits in Persons with Spinal Cord Injury as Compared with Both Age-Matched and Older Individuals without Spinal Cord Injury. J. Spinal Cord Med. 2020, 43, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Guest, R.; Tran, Y.; Middleton, J. Cognitive Impairment and Mood States after Spinal Cord Injury. J. Neurotrauma 2017, 34, 1156–1163. [Google Scholar] [CrossRef]
- Molina, B.; Segura, A.; Serrano, J.P.; Alonso, F.J.; Molina, L.; Pérez-Borrego, Y.A.; Ugarte, M.I.; Oliviero, A. Cognitive Performance of People with Traumatic Spinal Cord Injury: A Cross-Sectional Study Comparing People with Subacute and Chronic Injuries. Spinal Cord 2018, 56, 796–805. [Google Scholar] [CrossRef]
- Dowler, R.N.; Harrington, D.L.; Haaland, K.Y.; Swanda, R.M.; Fee, F.; Fiedler, K. Profiles of Cognitive Functioning in Chronic Spinal Cord Injury and the Role of Moderating Variables. J. Int. Neuropsychol. Soc. JINS 1997, 3, 464–472. [Google Scholar] [CrossRef]
- North, N.T. The Psychological Effects of Spinal Cord Injury: A Review. Spinal Cord 1999, 37, 671–679. [Google Scholar] [CrossRef]
- Bonekat, H.W.; Andersen, G.; Squires, J. Obstructive Disordered Breathing during Sleep in Patients with Spinal Cord Injury. Paraplegia 1990, 28, 392–398. [Google Scholar] [CrossRef]
- Sajkov, D.; Marshall, R.; Walker, P.; Mykytyn, I.; McEvoy, R.D.; Wale, J.; Flavell, H.; Thornton, A.T.; Antic, R. Sleep Apnoea Related Hypoxia Is Associated with Cognitive Disturbances in Patients with Tetraplegia. Spinal Cord 1998, 36, 231–239. [Google Scholar] [CrossRef]
- Elliott, T.R.; Frank, R.G. Depression Following Spinal Cord Injury. Arch. Phys. Med. Rehabil. 1996, 77, 816–823. [Google Scholar] [CrossRef]
- Hadjipavlou, G.; Cortese, A.; Ramaswamy, B. Spinal Cord Injury and Chronic Pain. BJA Educ. 2016, 16, mkv073. [Google Scholar] [CrossRef]
- Domżał, T.M. Ból Przewlekły-Problemy Kliniczne i Terapeutyczne. Pol. Przegląd Neurol. 2008, 4, 1–8. [Google Scholar]
- Wu, J.; Zhao, Z.; Kumar, A.; Lipinski, M.M.; Loane, D.J.; Stoica, B.A.; Faden, A.I. Endoplasmic Reticulum Stress and Disrupted Neurogenesis in the Brain Are Associated with Cognitive Impairment and Depressive-Like Behavior after Spinal Cord Injury. J. Neurotrauma 2016, 33, 1919–1935. [Google Scholar] [CrossRef] [PubMed]
- Molina-Gallego, B.; Gómez-Cantarino, S.; Ugarte-Gurrutxaga, M.I.; Molina-Gallego, L.; Mordillo-Mateos, L. Neuropsychological Study in Patients with Spinal Cord Injuries. Healthc. Basel Switz. 2021, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Pasipanodya, E.C.; Dirlikov, B.; Castillo, K.; Shem, K.L. Cognitive Profiles Among Individuals With Spinal Cord Injuries: Predictors and Relations With Psychological Well-Being. Arch. Phys. Med. Rehabil. 2021, 102, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tognini, S.; Pasqualetti, G.; Calsolaro, V.; Polini, A.; Monzani, F. Cognitive Function and Quality of Life in Mild Thyroid Hormone Deficiency. Recent Pat. Endocr. Metab. Immune Drug Discov. 2014, 8, 124–134. [Google Scholar] [CrossRef]
- Wang, N.; Huang, H.-L.; Zhou, H.; Yu, C.-Y. Cognitive Impairment and Quality of Life in Patients with Migraine-Associated Vertigo. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4913–4917. [Google Scholar]
- Correa, D.D.; Hess, L.M. Cognitive Function and Quality of Life in Ovarian Cancer. Gynecol. Oncol. 2012, 124, 404–409. [Google Scholar] [CrossRef]
- Lanzillo, R.; Chiodi, A.; Carotenuto, A.; Magri, V.; Napolitano, A.; Liuzzi, R.; Costabile, T.; Rainone, N.; Freda, M.F.; Valerio, P.; et al. Quality of Life and Cognitive Functions in Early Onset Multiple Sclerosis. Eur. J. Paediatr. Neurol. EJPN 2016, 20, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Buanes, E.A.; Gramstad, A.; Søvig, K.K.; Hufthammer, K.O.; Flaatten, H.; Husby, T.; Langørgen, J.; Heltne, J.-K. Cognitive Function and Health-Related Quality of Life Four Years after Cardiac Arrest. Resuscitation 2015, 89, 13–18. [Google Scholar] [CrossRef]
- Ueoka, Y.; Tomotake, M.; Tanaka, T.; Kaneda, Y.; Taniguchi, K.; Nakataki, M.; Numata, S.; Tayoshi, S.; Yamauchi, K.; Sumitani, S.; et al. Quality of Life and Cognitive Dysfunction in People with Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, M.; Brown, J.C.; Lambert, M.I.; Mechelen, W.; Verhagen, E. Quality of life among individuals with rugby-related spinal cord injuries in South Africa: A descriptive crosssectional study. BMJ Open 2018, 8, e020890. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chiu, W.-T. Comparisons of the Brief Form of the World Health Organization Quality of Life and Short Form-36 for Persons with Spinal Cord Injuries. Am. J. Phys. Med. Rehabil. 2007, 86, 104–113. [Google Scholar] [CrossRef]
- Jang, Y.; Hsieh, C.-L.; Wang, Y.-H.; Wu, Y.-H. A Validity Study of the WHOQOL-BREF Assessment in Persons with Traumatic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2004, 85, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- The Whoqol Group. The World Health Organization Quality of Life Assessment (WHOQOL): Development and General Psychometric Properties. Soc. Sci. Med. (1982) 1998, 46, 1569–1585. [Google Scholar] [CrossRef]
- World Health Organization. Division of Mental Health and Prevention of Substance Abuse WHOQOL: Measuring Quality of Life; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Ponichtera-Kasprzykowska, M.; Sobow, T. Adaptation and Usage of the Verbal Fuency Test in the World. Psychiatr. Psychol. Klin. 2014, 14, 178–187. [Google Scholar] [CrossRef]
- Baldo, J.V.; Schwartz, S.; Wilkins, D.; Dronkers, N.F. Role of Frontal versus Temporal Cortex in Verbal Fluency as Revealed by Voxel-Based Lesion Symptom Mapping. J. Int. Neuropsychol. Soc. JINS 2006, 12, 896–900. [Google Scholar] [CrossRef]
- Ross, T.P.; Calhoun, E.; Cox, T.; Wenner, C.; Kono, W.; Pleasant, M. The Reliability and Validity of Qualitative Scores for the Controlled Oral Word Association Test. Arch. Clin. Neuropsychol. 2007, 22, 475–488. [Google Scholar] [CrossRef]
- Zimmermann, N.; Cardoso, C.d.O.; Trentini, C.M.; Grassi-Oliveira, R.; Fonseca, R.P. Brazilian Preliminary Norms and Investigation of Age and Education Effects on the Modified Wisconsin Card Sorting Test, Stroop Color and Word Test and Digit Span Test in Adults. Dement. Neuropsychol. 2015, 9, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.L.; Krause, J.S.; Focht, K.L. A Longitudinal Study of Depression in Survivors of Spinal Cord Injury. Spinal Cord 2012, 50, 72–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Byra, S. Basic Hope and Posttraumatic Growth in People with Traumatic Paraplegia—The Mediating Effect of Acceptance of Disability. Spinal Cord 2019, 57, 301–307. [Google Scholar] [CrossRef]
- Byra, S.; Mróz, J.; Kaleta, K. Forgiveness and Acceptance of Disability in People with Traumatic Spinal Cord Injury-the Mediating Role of Disability Appraisal. A Cross-Sectional Study. Spinal Cord 2020, 58, 1317–1324. [Google Scholar] [CrossRef]
- de Roon-Cassini, T.A.; de St Aubin, E.; Valvano, A.; Hastings, J.; Horn, P. Psychological Well-Being after Spinal Cord Injury: Perception of Loss and Meaning Making. Rehabil. Psychol. 2009, 54, 306–314. [Google Scholar] [CrossRef]
- Carrard, V.; Kunz, S.; Peter, C. Mental Health, Quality of Life, Self-Efficacy, and Social Support of Individuals Living with Spinal Cord Injury in Switzerland Compared to That of the General Population. Spinal Cord 2021, 59, 398–409. [Google Scholar] [CrossRef]
- Hampton, N.Z. Disability Status, Perceived Health, Social Support, Self-Efficacy, and Quality of Life among People with Spinal Cord Injury in the People’s Republic of China. Int. J. Rehabil. Res. Int. Z. Rehabil. Rev. Int. Rech. Readaptation 2001, 24, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.; Muller, R.; Cieza, A.; Geyh, S. Psychological Resources in Spinal Cord Injury: A Systematic Literature Review. Spinal Cord 2011, 50, 188–201. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; Kozak, J.; Rees, L. Normative Data Stratified by Age and Education for Two Measures of Verbal Fluency: FAS and Animal Naming. Arch. Clin. Neuropsychol. 1999, 14, 167–177. [Google Scholar] [CrossRef]
- GrÉGoire, J.; Van Der Linden, M. Effect of Age on Forward and Backward Digit Spans. Aging Neuropsychol. Cogn. 1997, 4, 140–149. [Google Scholar] [CrossRef]
- Walsh, E.I.; Smith, L.; Northey, J.; Rattray, B.; Cherbuin, N. Towards an Understanding of the Physical Activity-BDNF-Cognition Triumvirate: A Review of Associations and Dosage. Ageing Res. Rev. 2020, 60, 101044. [Google Scholar] [CrossRef]
- Kopp, A.; Jekauc, D. The Influence of Emotional Intelligence on Performance in Competitive Sports: A Meta-Analytical Investigation. Sports Basel Switz. 2018, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Goraczko, A.; Zurek, G.; Lachowicz, M.; Zurek, A. Purpose in Life of Elite Athletes after Spinal Cord Injury. Int. J. Environ. Res. Public. Health 2021, 18, 5563. [Google Scholar] [CrossRef] [PubMed]
| Participant | Age | Gender | Nationality | Marital Status | Discipline before SCI | Sport after SCI |
|---|---|---|---|---|---|---|
| P1 | 41 | Male | British | Divorced | BMX dirt jumps | No |
| P2 | 29 | Male | Austrian | Single | Ski jumping | Rugby, skiing |
| P3 | 24 | Female | Polish | Informal relation | Karate | Wheelchair dancing |
| P4 | 37 | Male | British | Informal relation | Rugby | No |
| P5 | 55 | Male | American | Married | Mountain bike racing | No |
| P6 | 45 | Female | Canadian | Married | Mountain biking | Wheelchair basketball |
| P7 | 31 | Male | British | Informal relation | Motocross | Car race |
| P8 | 40 | Male | Polish | Informal relation | Judo | Canoe |
| P9 | 47 | Male | Polish | Single | Speedway | Hand cycling |
| Participant | SCI Level | Years since Injury | Pain | Brain Injury/Whiplash | Hypopnea/Apnea | Medicines (Number) |
|---|---|---|---|---|---|---|
| P1 | C3/4 | 14 | 7 | B | Yes | 8 |
| P2 | C6/7 | 5 | 7 | W | No | 1 |
| P3 | Th11/12 | 6 | 3 | No | No | 0 |
| P4 | C4/5 | 16 | 0 | W | Yes | 1 |
| P5 | C6/7 | 4 | 6 | No | No | 3 |
| P6 | Th12/L1 | 14 | 1 | W | No | 1 |
| P7 | Th6 | 15 | 0 | B | No | 2 |
| P8 | Th11 | 17 | 3 | No | No | 0 |
| P9 | L1/2 | 15 | 7 | No | No | 0 |
| Scale | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | Mean ± SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WHOQOL | Q1 | 3 * | 4 | 4 | 5 | 2 * | 5 | 5 | 4 | 5 | 4.1 ± 1.1 |
| Q2 | 1 * | 5 | 4 | 3 | 2 * | 5 | 5 | 4 | 5 | 3.8 ± 1.5 | |
| D1 | 14 | 18 | 11 * | 15 | 11 * | 19 | 20 | 16 | 14 | 15.3 ± 3.2 | |
| D2 | 13 * | 19 | 15 | 15 | 7 * | 19 | 17 | 15 | 20 | 15.6 ± 4.0 | |
| D3 | 13 * | 20 | 12 * | 17 | 9 * | 19 | 17 | 16 | 20 | 15.9 ± 3.8 | |
| D4 | 16 | 17 | 16 | 17 | 13 * | 20 | 20 | 13 * | 20 | 16.9 ± 2.8 | |
| Scale | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | Mean ± SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| COWAT | F | 6 * | 12 | 9 | 11 | 11 | 9 | 10 | 12 | 6 * | 10.0 ± 2.0 |
| A | 6 * | 8 | 8 | 9 | 10 | 15 | 11 | 18 | 6 * | 10.0 ± 4.0 | |
| S | 6 * | 11 | 17 | 7 | 9 | 7 | 13 | 19 | 9 | 11.0 ± 4.6 | |
| All | 18 * | 31 | 34 | 27 | 30 | 31 | 34 | 49 | 21 * | 31.0 ± 8.8 | |
| Digit Span | DForw | 7 | 7 | 6 * | 3 * | 7 | 7 | 7 | 8 | 5 | 6.0 ± 2.0 |
| DBack | 4 | 6 | 4 | 3 * | 4 | 4 | 2 * | 6 | 2 * | 4.0 ± 1.0 | |
| Stroop color– word test | Word correct answers | 92 | 90 | 86 | 54 * | 77 | 97 | 80 | 127 | 82 | 87.0 ± 19.0 |
| Color correct answers | 54 | 58 | 42 * | 42 * | 60 | 76 | 71 | 75 | 70 | 61.0 ± 13.0 | |
| Word and color correct answers | 36 | 53 | 46 | 36 | 31 * | 51 | 60 | 53 | 52 | 46.0 ± 10.0 | |
| COWAT | Digit Span | Stroop | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | A | S | All | DForw | DBack | Word | Color | Word–Color | ||
| WHOQoL | Q1 | −0.18 | 0.17 | −0.05 | 0.07 | −0.44 | −0.60 | −0.16 | 0.29 | 0.46 |
| Q2 | 0.03 | 0.17 | 0.34 | 0.37 | 0.00 | −0.17 | 0.17 | 0.53 | 0.79 | |
| D1 | 0.28 | 0.51 | 0.08 | 0.37 | 0.36 | −0.02 | 0.27 | 0.59 | 0.73 | |
| D2 | −0.14 | −0.07 | 0.10 | 0.08 | −0.21 | −0.23 | 0.17 | 0.40 | 0.67 | |
| D3 | −0.01 | −0.12 | −0.11 | −0.12 | −0.19 | −0.17 | 0.12 | 0.32 | 0.58 | |
| D4 | −0.39 | −0.11 | −0.25 | −0.16 | −0.39 | −0.62 | −0.14 | 0.28 | 0.44 | |
| Pain | −0.21 | −0.67 | −0.13 | −0.46 | 0.10 | 0.33 | 0.27 | −0.15 | −0.12 | |
| Medicines | −0.09 | −0.07 | −0.57 | −0.43 | 0.23 | −0.09 | −0.24 | −0.10 | −0.37 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goraczko, A.; Zurek, A.; Lachowicz, M.; Kujawa, K.; Zurek, G. The Relationship between Cognitive Performance and Quality of Life in Elite Athletes after Spinal Cord Injury. Int. J. Environ. Res. Public Health 2022, 19, 948. https://doi.org/10.3390/ijerph19020948
Goraczko A, Zurek A, Lachowicz M, Kujawa K, Zurek G. The Relationship between Cognitive Performance and Quality of Life in Elite Athletes after Spinal Cord Injury. International Journal of Environmental Research and Public Health. 2022; 19(2):948. https://doi.org/10.3390/ijerph19020948
Chicago/Turabian StyleGoraczko, Agata, Alina Zurek, Maciej Lachowicz, Katarzyna Kujawa, and Grzegorz Zurek. 2022. "The Relationship between Cognitive Performance and Quality of Life in Elite Athletes after Spinal Cord Injury" International Journal of Environmental Research and Public Health 19, no. 2: 948. https://doi.org/10.3390/ijerph19020948
APA StyleGoraczko, A., Zurek, A., Lachowicz, M., Kujawa, K., & Zurek, G. (2022). The Relationship between Cognitive Performance and Quality of Life in Elite Athletes after Spinal Cord Injury. International Journal of Environmental Research and Public Health, 19(2), 948. https://doi.org/10.3390/ijerph19020948







