Severe Primary Open-Angle Glaucoma and Agricultural Profession: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Judgment Criteria
2.3. Data Collection
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Main Endpoint
3.3. Secondary Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Sommer, A. Glaucoma risk factors observed in the Baltimore Eye Survey. Curr. Opin. Ophthalmol. 1996, 7, 93–98. [Google Scholar] [CrossRef]
- Hollands, H.; Johnson, D.; Hollands, S.; Simel, D.L.; Jinapriya, D.; Sharma, S. Do findings on routine examination identify patients at risk for primary open-angle glaucoma? The rational clinical examination systematic review. JAMA 2013, 309, 2035–2042. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H. Obstructive sleep apnea/hypopnea syndrome increases glaucoma risk: Evidence from a meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 297–303. [Google Scholar]
- Carbonaro, F.; Hysi, P.G.; Fahy, S.J.; Nag, A.; Hammond, C.J. Optic disc planimetry; corneal hysteresis; central corneal thickness; and intraocular pressure as risk factors for glaucoma. Am. J. Ophthalmol. 2014, 157, 441–446. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Tsantes, A.; Peponis, V. Epidemiological association between cigarette smoking and primary open-angle glaucoma: A meta-analysis. Public Health 2004, 118, 256–261. [Google Scholar] [CrossRef]
- Jain, V.; Jain, M.; Abdull, M.M.; Bastawrous, A. The association between cigarette smoking and primary open-angle glaucoma: A systematic review. Int. Ophthalmol. 2017, 37, 291–301. [Google Scholar] [CrossRef]
- Yu, M.; Chen, B.; Gong, B.; Shuai, P.; Wu, Z.-Z.; Lin, W. Association of n3 and n6 polyunsaturated fatty acids in red blood cell membrane and plasma with severity of normal tension glaucoma. Int. J. Ophthalmol. 2015, 8, 476–483. [Google Scholar]
- Chemaly, A.; Arnould, L.; Seydou, A.; Gabrielle, P.H.; Baudin, F.; Acar, N.; Creuzot-Garcher, C. Plasma fatty acids and primary open-angle glaucoma in the elderly: The Montrachet population-based study. BMC Ophthalmol. 2021, 21, 146. [Google Scholar] [CrossRef]
- Denoyer, A. Physiopathogénie de la Neuropathie Optique Glaucomateuse. Available online: https://www.em-consulte.com/em/SFO/2014/html/file_100019.html (accessed on 12 June 2021).
- Lee, K.; Yang, H.; Kim, J.Y.; Seong, G.J.; Kim, C.Y.; Bae, H.W. Risk Factors Associated with Structural Progression in Normal-Tension Glaucoma: Intraocular Pressure; Systemic Blood Pressure; and Myopia. Investig. Ophthalmol. Vis. Sci. 2020, 61, 35. [Google Scholar] [CrossRef]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: A review of the literature. Int. Ophthalmol. 2019, 39, 259–271. [Google Scholar] [CrossRef]
- Kab, S.; Spinosi, J.; Chaperon, L.; Dugravot, A.; Singh-Manoux, A.; Moisan, F.; Elbaz, A. Agricultural activities and the incidence of Parkinson’s disease in the general French population. Eur. J. Epidemiol. 2017, 32, 203–216. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.S.; Uddin, M.J.; Mamum-Or-Rashid, A.N.M.; Pang, M.-G.; Rhim, H. Emerging risk of environmental factors: Insight mechanisms of Alzheimer’s diseases. Environ. Sci. Pollut. Res. 2020, 27, 44659–44672. [Google Scholar] [CrossRef]
- Elbaz, A.; Dufouil, C.; Alpérovitch, A. Interaction between genes and environment in neurodegenerative diseases. Comptes Rendus Biol. 2007, 330, 318–328. [Google Scholar] [CrossRef]
- Renard, J.P.; Rouland, J.F.; Bron, A.; Sellem, E.; Nordmann, J.P.; Baudouin, C.; Denis, P.; Villain, M.; Chaine, G.; Colin, J.; et al. Nutritional; lifestyle and environmental factors in ocular hypertension and primary open-angle glaucoma: An exploratory case-control study. Acta Ophthalmol. 2013, 91, 505–513. [Google Scholar] [CrossRef]
- Agarwal, H.C.; Gulati, V.; Sihota, R. Visual field assessment in glaucoma: Comparative evaluation of manual kinetic Goldmann perimetry and automated static perimetry. Indian J. Ophthalmol. 2000, 48, 301–306. [Google Scholar]
- Kim, J.W.; Ko, J.; Woo, Y.J.; Bae, H.W.; Yoon, J.S. Prevalence of Ocular Hypertension and Glaucoma as Well as Associated Factors in Graves’ Orbitopathy. J. Glaucoma 2018, 27, 464–469. [Google Scholar] [CrossRef]
- Cockerham, K.P.; Pal, C.; Jani, B.; Wolter, A.; Kennerdell, J.S. The prevalence and implications of ocular hypertension and glaucoma in thyroid-associated orbitopathy. Ophthalmology 1997, 104, 914–917. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Lai, T.Y.Y.; Lai, C.-C.; Cheung, C.M.G. Updates of pathologic myopia. Prog. Retin. Eye Res. 2016, 52, 156–187. [Google Scholar] [CrossRef]
- Aptel, F.; Aryal-Charles, N.; Giraud, J.M.; El Chehab, H.; Delbarre, M.; Chiquet, C.; Romanet, J.-P.; Renard, J.-P. Progression of visual field in patients with primary open-angle glaucoma-ProgF study 1. Acta Ophthalmol. 2015, 93, e615–e620. [Google Scholar] [CrossRef] [Green Version]
- Mills, R.P.; Budenz, D.L.; Lee, P.P.; Noecker, R.J.; Walt, J.G.; Siegartel, L.R.; Evans, S.J.; Doyle, J.J. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am. J. Ophthalmol. 2006, 141, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Sample, P.A.; Pascual, J.P.; Zangwill, L.M.; Girkin, C.A.; Liebmann, J.M.; Weinreb, R.N.; Racette, L. Comparison of visual field severity classification systems for glaucoma. J. Glaucoma 2012, 21, 551–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toulouse, E.; Lafont, B.; Granier, S.; Mcgurk, G.; Bazin, J.-E. French legal approach to patient consent in clinical research. Anaesth. Crit. Care Pain Med. 2020, 39, 883–885. [Google Scholar] [CrossRef]
- Légifrance. Article R1121-2 du Code de la Santé Publique. Available online: https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000034696957 (accessed on 2 September 2021).
- De Goeij, M.C.M.; van Diepen, M.; Jager, K.J.; Tripepi, G.; Zoccali, C.; Dekker, F.W. Multiple imputation: Dealing with missing data. Nephrol. Dial. Transplant. 2013, 28, 2415–2420. [Google Scholar] [CrossRef] [Green Version]
- Yücel, Y.H.; Zhang, Q.; Weinreb, R.N.; Kaufman, P.L.; Gupta, N. Effects of retinal ganglion cell loss on magno-; parvo-; koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog. Retin. Eye Res. 2003, 22, 465–481. [Google Scholar] [CrossRef]
- Mancino, R.; Martucci, A.; Cesareo, M.; Giannini, C.; Corasaniti, M.T.; Bagetta, G.; Nucci, C. Glaucoma and Alzheimer Disease: One Age-Related Neurodegenerative Disease of the Brain. Curr. Neuropharmacol. 2018, 16, 971–977. [Google Scholar] [CrossRef]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; López-Cuenca, I.; Rojas, P.; Triviño, A.; Ramírez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease; Parkinson; and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.-T.; Wang, Z.-Z.; Yuan, Y.-H.; Sun, H.-M.; Chen, N.-H.; Zhang, Y. Update on the association between alpha-synuclein and tau with mitochondrial dysfunction: Implications for Parkinson’s disease. Eur. J. Neurosci. 2021, 53, 2946–2959. [Google Scholar] [CrossRef] [PubMed]
- Chorostecki, J.; Seraji-Bozorgzad, N.; Shah, A.; Bao, F.; Bao, G.; George, E.; Gorden, V.; Caon, C.; Frohman, E.; Bhatti, M.T.; et al. Characterization of retinal architecture in Parkinson’s disease. J. Neurol. Sci. 2015, 355, 44–48. [Google Scholar] [CrossRef]
- Légifrance. Décret N° 2020-1125 du 10 Septembre 2020 Révisant et Complétant les Tableaux de Maladies Professionnelles Annexés au Livre VII du Code Rural et de la Pêche Maritime. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000042325497 (accessed on 11 July 2021).
- Liu, B.; Gao, H.-M.; Hong, J.-S. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: Role of neuroinflammation. Environ. Health Perspect. 2003, 111, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Uversky, V.N.; Li, J.; Fink, A.L. Pesticides directly accelerate the rate of α-synuclein fibril formation: A possible factor in Parkinson’s disease. FEBS Lett. 2001, 500, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Klintworth, H.; Garden, G.; Xia, Z. Rotenone and Paraquat do not Directly Activate Microglia or Induce Inflammatory Cytokine Release. Neurosci. Lett. 2009, 462, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Surguchov, A.; McMahan, B.; Masliah, E.; Surgucheva, I. Synucleins in ocular tissues. J. Neurosci. Res. 2001, 65, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Surgucheva, I.; Weisman, A.D.; Goldberg, J.L.; Shnyra, A.; Surguchov, A. Gamma-synuclein as a marker of retinal ganglion cells. Mol. Vis. 2008, 14, 1540–1548. [Google Scholar] [PubMed]
- Surgucheva, I.; Shestopalov, V.I.; Surguchov, A. Effect of gamma-synuclein silencing on apoptotic pathways in retinal ganglion cells. J. Biol. Chem. 2008, 283, 36377–36385. [Google Scholar] [CrossRef] [Green Version]
- Surgucheva, I.; McMahan, B.; Ahmed, F.; Tomarev, S.; Wax, M.B.; Surguchov, A. Synucleins in glaucoma: Implication of gamma-synuclein in glaucomatous alterations in the optic nerve. J. Neurosci. Res. 2002, 68, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Laborde, A. Impact of pesticide exposure in childhood. Rev. Environ. Health 2020, 35, 221–227. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Brouwer, M.; Kromhout, H.; Vermeulen, R.; Duyzer, J.; Kramer, H.; Hazeu, G.; de Snoo, G.; Huss, A. Assessment of residential environmental exposure to pesticides from agricultural fields in The Netherlands. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 173–181. [Google Scholar] [CrossRef]
- Légifrance. Arrêté du 27 Décembre 2019 Relatif aux Mesures de Protection des Personnes Lors de L’Utilisation de Produits Phytopharmaceutiques et Modifiant L’Arrêté du 4 Mai 2017 Relatif à la Mise Sur le Marché et à L’Utilisation Des Produits Phytopharmaceutiques et de Leurs Adjuvants Visés à L’Article L. 253-1 du Code Rural et de la Pêche Maritime. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000039686039/ (accessed on 31 August 2021).
- Spinosi, J.; Févotte, J.; Le Programme MATPHYTO. Matrices Cultures-Expositions aux Produits Phytosanitaires. Available online: https://www.santepubliquefrance.fr/determinants-de-sante/exposition-a-des-substances-chimiques/pesticides/le-programme-matphyto.-matrices-cultures-expositions-aux-produits-phytosanitaires (accessed on 18 July 2021).
- Ng, W.S.; Agarwal, P.K.; Sidiki, S.; McKay, L.; Townend, J.; Azuara-Blanco, A. The effect of socio-economic deprivation on severity of glaucoma at presentation. Br. J. Ophthalmol. 2010, 94, 85–87. [Google Scholar] [CrossRef] [Green Version]
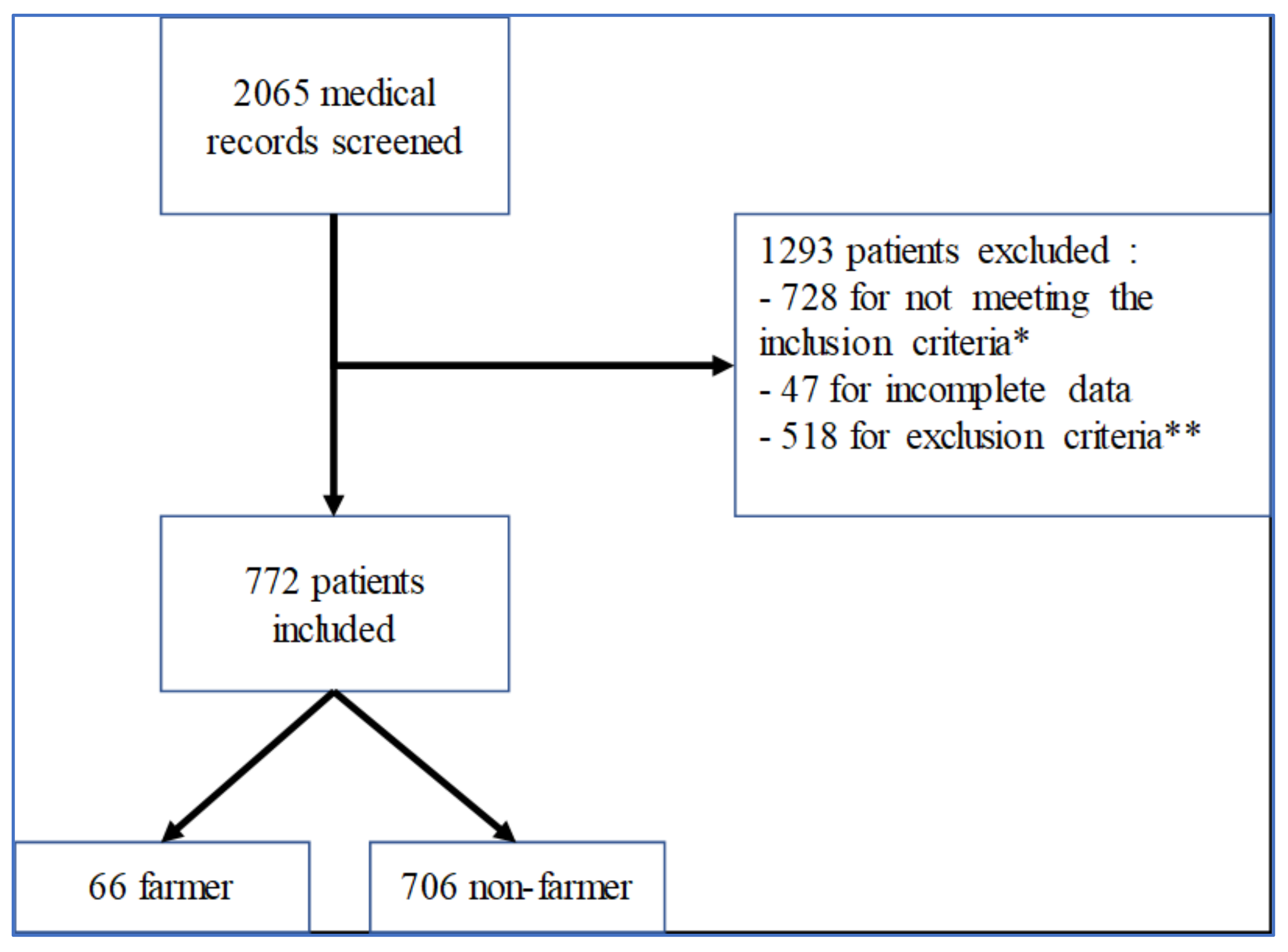
| Overall N = 772 Subjects | Farmer N = 66 | Nonfarmer N = 706 | p-Value | |
|---|---|---|---|---|
| Age (years): means ± SD | 69.7 ± 10.8 | 71.7 ± 11.2 | 69.6 ± 10.8 | 0.12 |
| Gender | 0.32 | |||
| Male | 388 (50.3) | 37 (56.1) | 351 (49.7) | |
| Female | 384 (49.7) | 29 (43.9) | 355 (50.3) | |
| Intraocular pressure (mmHg): median (IQR) | 16 (14–19) | 16 (15–20) | 16 (14–19) | 0.05 |
| Selected (most affected) eye | 0.44 | |||
| Right | 351 (45.5) | 27 (40.9) | 324 (45.9) | |
| Left | 421 (54.5) | 39 (59.1) | 382 (54.1) | |
| Corneal thickness (μm): means ± SD | 533.1 ± 42.2 | 536.8 ± 34.6 | 532.7 ± 42.9 | 0.38 |
| Spherical equivalent (diopters): median (IQR) | 0.25 (0.00–0.75) | 0.25 (0.00–0.75) | 0.25 (0.00–0.75) | 0.72 |
| OSAS | 78 (10.1) | 6 (9.1) | 72 (10.02) | 0.77 |
| Diabetes | 159 (20.6) | 11 (16.9) | 148 (21.0) | 0.44 |
| Duration of follow-up (months): median (IQR) | 43 (23–66) | 49 (25–69) | 42 (23–66) | 0.33 |
| Farmers N = 66 | Nonfarmers N = 706 | OR (95% CI) | p-Value | Adjusted OR † (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Primary endpoint | ||||||
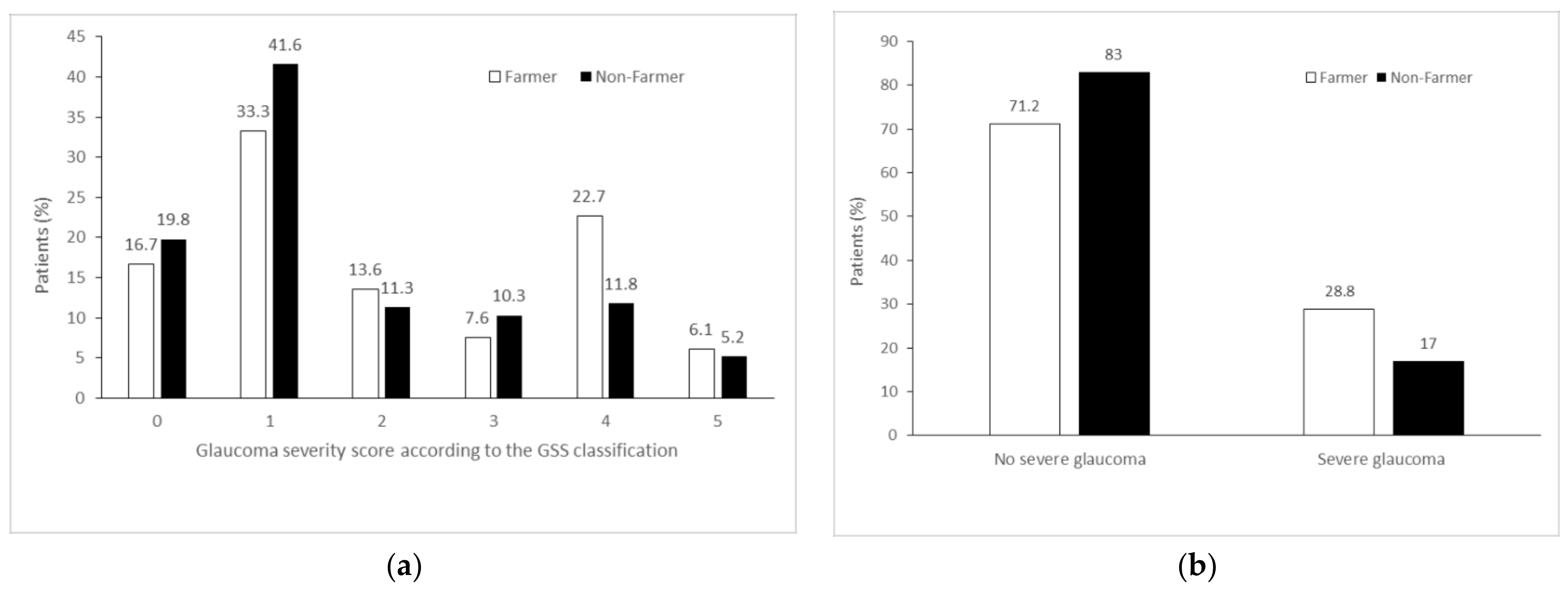
| Severe glaucoma 1 | 19 (28.8) | 120 (17.0) | 1.97 (1.12–3.48) | 0.02 | 1.87 (1.05–3.34) | 0.03 |
| Secondary endpoints | ||||||
| Surgery | 11 (16.7) | 55 (7.8) | 2.37 (1.17–4.78) | 0.02 | 2.28 (1.12–4.64) | 0.08 |
| RNFL thickness (μm): means ± SD | 71.2 ± 20.7 | 73.7 ± 20.6 | 0.99 (0.98–1.01) | 0.34 | 1.00 (0.98–1.01) | 0.52 |
| Treatment: median (IQR) | 2 (1–3) | 2 (1–3) | 1.16 (0.93–1.44) | 0.20 | 1.14 (0.91–1.43) | 0.26 |
| VFI (%): means ± SD | 73.1 ± 32.4 | 79.0 ± 28.9 | 0.99 (0.99–1.00) | 0.18 | 0.99 (0.99–1.00) | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosselin, M.; Bouazzi, L.; Ferreira de Moura, T.; Arndt, C.; Thorigny, M.; Sanchez, S.; Denoyer, A. Severe Primary Open-Angle Glaucoma and Agricultural Profession: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 926. https://doi.org/10.3390/ijerph19020926
Grosselin M, Bouazzi L, Ferreira de Moura T, Arndt C, Thorigny M, Sanchez S, Denoyer A. Severe Primary Open-Angle Glaucoma and Agricultural Profession: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(2):926. https://doi.org/10.3390/ijerph19020926
Chicago/Turabian StyleGrosselin, Mathilde, Leila Bouazzi, Thomas Ferreira de Moura, Carl Arndt, Maxime Thorigny, Stéphane Sanchez, and Alexandre Denoyer. 2022. "Severe Primary Open-Angle Glaucoma and Agricultural Profession: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 19, no. 2: 926. https://doi.org/10.3390/ijerph19020926
APA StyleGrosselin, M., Bouazzi, L., Ferreira de Moura, T., Arndt, C., Thorigny, M., Sanchez, S., & Denoyer, A. (2022). Severe Primary Open-Angle Glaucoma and Agricultural Profession: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 19(2), 926. https://doi.org/10.3390/ijerph19020926






