Analysis of Experiences in Preventing COVID-19 in Hemodialysis Centers of the North of Poland before the Era of Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Study Subjects and Methods
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. Lancet 2020, 395, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Puchalska-Reglińska, E.; Dziewiatowski, K.; Szabat, K.; Katarzyński, P.; Zakrzewski, M.; Moys, K.; Lange, M.; Szczęśniewicz, A.; Lipka, M.; Dębska-Ślizień, A. COVID-19—Clinical course in a chronically hemodialysed patients—Case report and literature review. Forum Nefrol. 2020, 13, 142–148. [Google Scholar]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Kartsios, C.; Stefanidis, I. Disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007, 20, 440–451. [Google Scholar] [CrossRef]
- Francis, A.; Baigent, C.; Ikizler, T.A.; Cockwell, P.; Jha, V. The urgent need to vaccinate dialysis patients against severe acute respiratory syndrome coronavirus 2: A call to action. Kidney Int. 2021, 99, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Hilbrands, L.B. CKD is a key risk factor for COVID-19 mortality. Nat. Rev. Nephrol. 2020, 16, 705–706. [Google Scholar] [CrossRef]
- Puchalska-Reglińska, E.; Debska-Slizien, A.; Biedunkiewicz, B.; Tylicki, P.; Polewska, K.; Rutkowski, B.; Gellert, R.; Tylicki, L. Extremely high mortality in COVID-19 hemodialyzed patients before the anti-SARS-CoV-2 vaccination era. Large database from the North of Poland. Pol. Arch. Intern. Med. 2021, 131, 643–648. [Google Scholar] [CrossRef]
- Alberici, F.; Delbarba, E.; Manenti, C.; Econimo, L.; Valerio, F.; Pola, A.; Maffei, C.; Possenti, S.; Lucca, B.; Cortinovis, R.; et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020, 98, 20–26. [Google Scholar] [CrossRef]
- Basile, C.; Combe, C.; Pizzarelli, F.; Covic, A.; Davenport, A.; Kanbay, M.; Kirmizis, D.; Schneditz, D.; van der Sande, F.; Mitra, S. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol. Dial. Transpl. 2020, 35, 737–741. [Google Scholar] [CrossRef]
- Debska-Slizien, A.; Rutkowski, B.; Rutkowski, P.; Jagodziński, P.; Korejwo, G.; Przygoda, J.; Lewandowska, D.; Czerwiński, J.; Kamiński, A.; Gellert, R. Current status of renal replacement therapy in Poland in 2019. Nefrol. Dial. Pol. 2020, 24, 38–50. [Google Scholar]
- Debska-Slizien, A.; Rutkowski, B.; Rutkowski, P.; Jagodziński, P.; Przygoda, J.; Lewandowska, D.; Czerwiński, J.; Kamiński, A.; Gellert, R. Current status of renal replacement therapy in Poland in 2020. Nephrol. Dial. Pol. 2020, 25, 7–20. [Google Scholar]
- Corbett, R.W.; Blakey, S.; Nitsch, D.; Loucaidou, M.; McLean, A.; Duncan, N.; Ashby, D.R.; West London, R.; Transplant, C. Epidemiology of COVID-19 in an Urban Dialysis Center. J. Am. Soc. Nephrol. 2020, 31, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Bhat, P.; Del Pilar Fernandez, M.; Bhat, J.G.; Coritsidis, G.N. COVID-19 Infection in ESKD: Findings from a Prospective Disease Surveillance Program at Dialysis Facilities in New York City and Long Island. J. Am. Soc. Nephrol. 2020, 31, 2517–2521. [Google Scholar] [CrossRef]
- Mahase, E. Coronavirus COVID-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ 2020, 368, m641. [Google Scholar] [CrossRef] [Green Version]
- Naicker, S.; Yang, C.W.; Hwang, S.J.; Liu, B.C.; Chen, J.H.; Jha, V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020, 97, 824–828. [Google Scholar] [CrossRef]
- Caplin, B.; Ashby, D.; McCafferty, K.; Hull, R.; Asgari, E.; Ford, M.L.; Cole, N.; Antonelou, M.; Blakey, S.A.; Srinivasa, V.; et al. Risk of COVID-19 Disease, Dialysis Unit Attributes, and Infection Control Strategy among London In-Center Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2021, 16, 1237–1246. [Google Scholar] [CrossRef]
- Ibernon, M.; Bueno, I.; Rodriguez-Farre, N.; Ruiz, P.; Sanchez, A.; Masso, E.; Rap, O.; Gimenez, I.; Cabrera, C. The impact of COVID-19 in hemodialysis patients: Experience in a hospital dialysis unit. Hemodial. Int. 2021, 25, 205–213. [Google Scholar] [CrossRef]
- Biedunkiewicz, B.; Tylicki, L.; Puchalska-Reglińska, E.; Dąbrowska, M.; Ślizień, W.; Kubanek, A.; Rąbalski, Ł.; Kosiński, M.; Grzybek, M.; Renke, M.; et al. SARS-CoV-2 infection in vaccinated maintenance hemodialysis patients despite anti-spike seroconversion: A report of 3 breakthrough cases. Eur. J. Transl. Clin. Med. 2021. [Google Scholar] [CrossRef]
- Christensen, P.A.; Olsen, R.J.; Long, S.W.; Subedi, S.; Davis, J.J.; Hodjat, P.; Walley, D.R.; Kinskey, J.C.; Ojeda Saavedra, M.; Pruitt, L.; et al. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am. J. Pathol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jehn, M.; McCullough, J.M.; Dale, A.P.; Gue, M.; Eller, B.; Cullen, T.; Scott, S.E. Association between K-12 School Mask Policies and School-Associated COVID-19 Outbreaks—Maricopa and Pima Counties, Arizona, July–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1372–1373. [Google Scholar] [CrossRef]
- Budzyn, S.E.; Panaggio, M.J.; Parks, S.E.; Papazian, M.; Magid, J.; Eng, M.; Barrios, L.C. Pediatric COVID-19 Cases in Counties With and Without School Mask Requirements—United States, July 1–September 4, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1377–1378. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Healthcare Personnel during the Coronavirus Disease 2019 (COVID-19) Pandemic. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html (accessed on 24 November 2021).
- Esposito, P.; Russo, R.; Conti, N.; Falqui, V.; Massarino, F.; Moriero, E.; Peloso, G.; Traverso, G.B.; Garibotto, G.; Viazzi, F. Management of COVID-19 in hemodialysis patients: The Genoa experience. Hemodial. Int. 2020, 24, 423–427. [Google Scholar] [CrossRef]
- Allinovi, M.; Laudicina, S.; Dallari, L.; Gianassi, I.; Dervishi, E.; Biagini, M.; Cirami, L. Lung ultrasound may help in the differential diagnosis of suspected oligosymptomatic COVID-19 patients on hemodialysis: A case report. Hemodial. Int. 2021, 25, E48–E52. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.; Prendecki, M.; Dhutia, A.; Ali, M.A.; Sajjad, H.; Shivakumar, O.; Lightstone, L.; Kelleher, P.; Pickering, M.C.; Thomas, D.; et al. High Prevalence of Asymptomatic COVID-19 Infection in Hemodialysis Patients Detected Using Serologic Screening. J. Am. Soc. Nephrol. 2020, 31, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
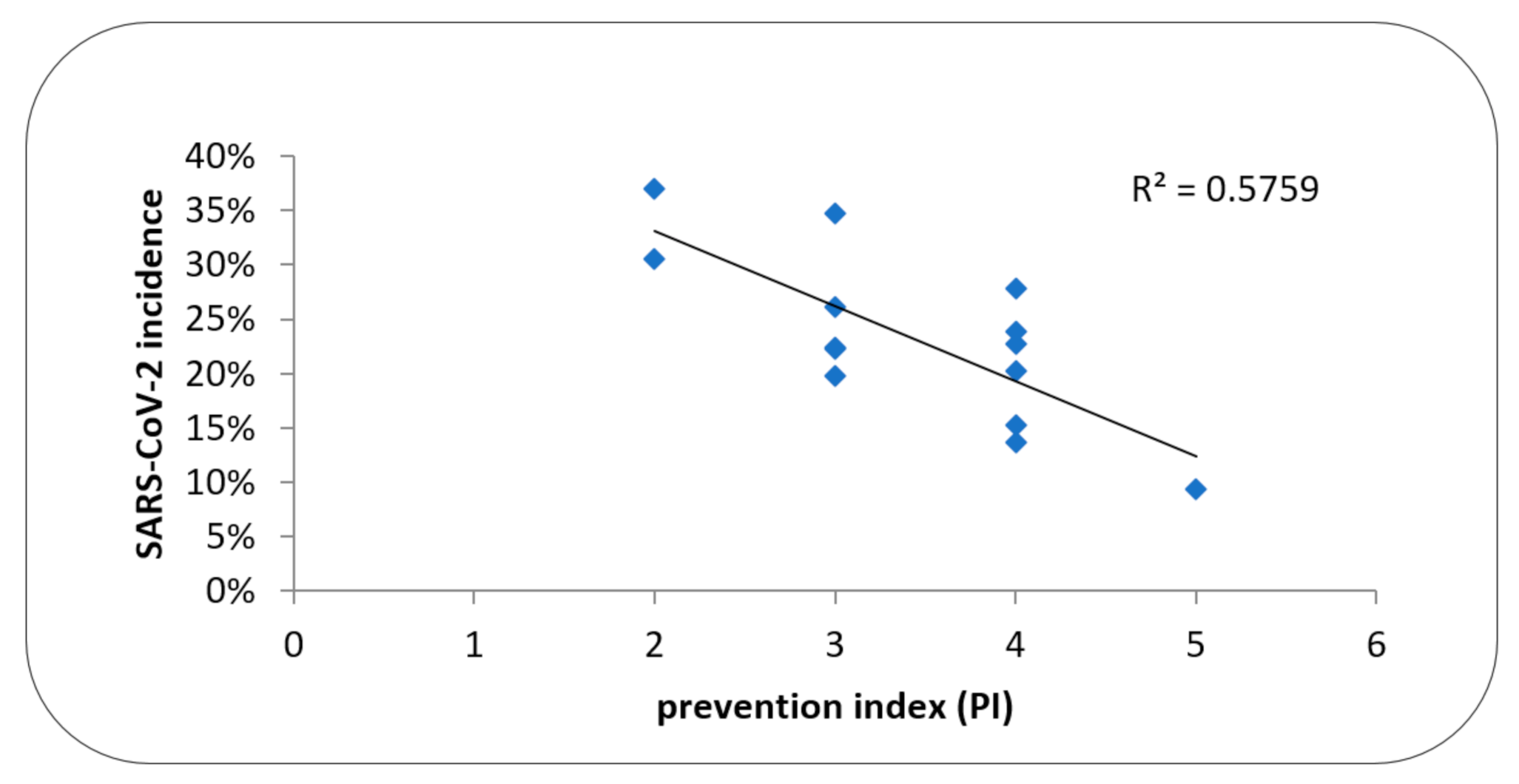
| HD Center | Size and Status of HD Center | HD Patients 31.12.2019 (n) | HD pts in the Study (n) | Incidence % | Fatality Rate % | PI |
|---|---|---|---|---|---|---|
| HDC001 | 2P | 74 | 92 | 36.4 | 32.3 | 2 |
| HDC002 | 2NP | 78 | 89 | 34.8 | 12.9 | 3 |
| HDC003 * | 2NP | 68 | 85 | 30.6 | 34.6 | 2 |
| HDC004 | 1NP | 56 | 72 | 27.8 | 35.0 | 4 |
| HDC005 | 2P | 85 | 103 | 26.2 | 40.7 | 3 |
| HDC006 * | 2NP | 83 | 109 | 23.8 | 30.8 | 4 |
| HDC007 | 1NP | 53 | 66 | 22.7 | 46.7 | 4 |
| HDC008 * | 3NP | 144 | 206 | 22.3 | 19.6 | 2 |
| HDC009 | 3NP | 109 | 139 | 22.3 | 38.7 | 3 |
| HDC010 | 3NP | 146 | 177 | 20.3 | 25.0 | 4 |
| HDC011 | 3P | 78 | 131 | 19.8 | 19.2 | 3 |
| HDC012 | 1NP | 41 | 59 | 15.2 | 11.1 | 4 |
| HDC013 | 1NP | 45 | 59 | 13.6 | 50.0 | 4 |
| HDC014 * | 3NP | 132 | 182 | 9.4 | 58.8 | 5 |
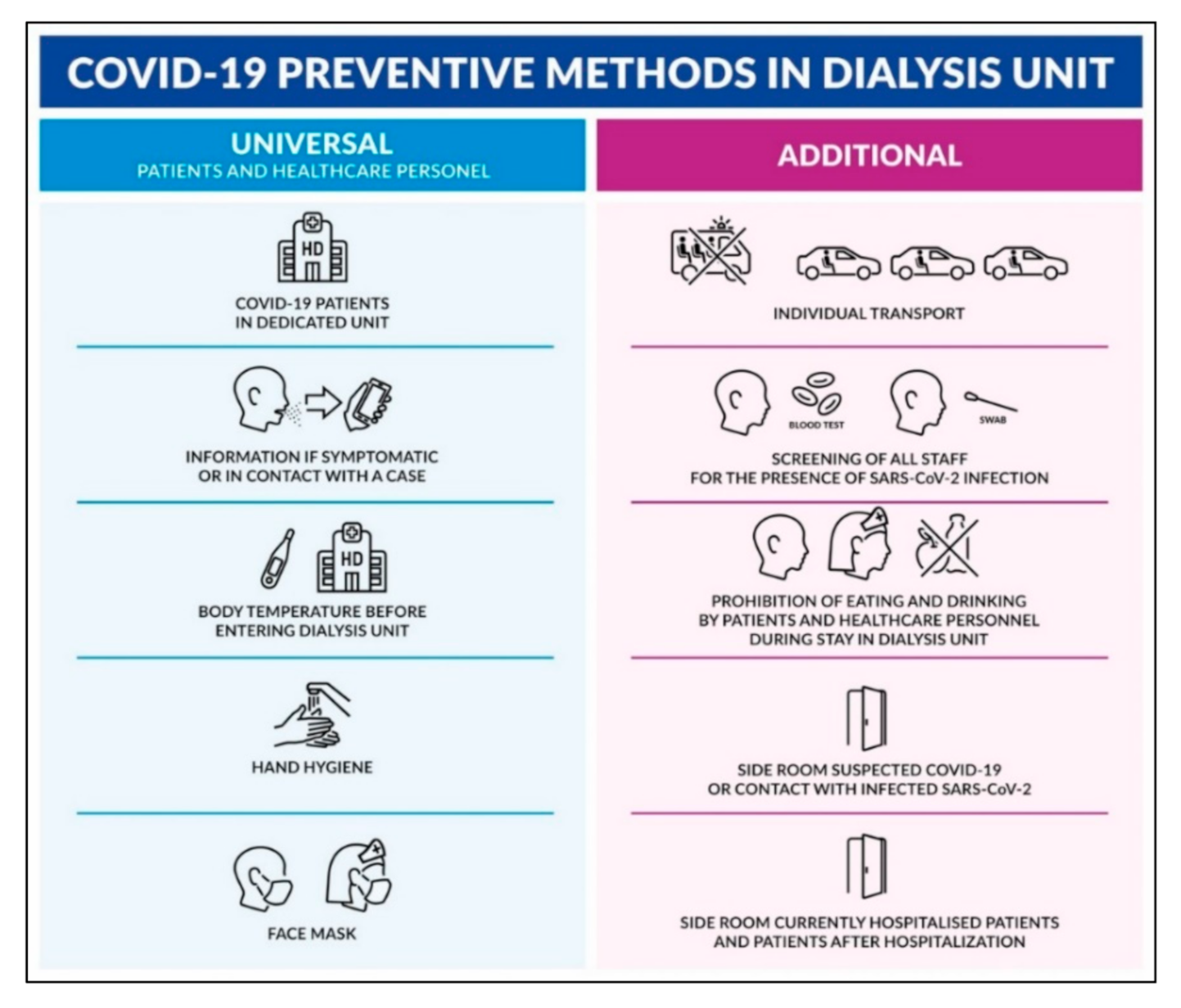
| Preventive Method | U1 a | U2 a | U3 a | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|---|---|---|
| Number of HD centers | 14 | 14 | 14 | 10 | 9 | 12 | 5 | 5 | 6 |
| % | 100% | 100% | 100% | 71% | 64% | 86% | 36% | 36% | 43% |
| Parameter | n | Incidence (%) | p |
|---|---|---|---|
| Public HD center | 4 | 26.33 ± 7.55 | ns |
| Non-public HD center | 10 | 22.06 ± 7.56 | |
| HD center 1 (small size) a | 4 | 19.83 ± 6.63 | 0.017 |
| HD center 2 (medium size) | 5 | 30.49 ± 6.4 b | |
| HD center 3 (large size) | 5 | 18.83 ± 5.42 | |
| COVID-19-positive patients in HD center | 4 | 19.33 ± 5.95 | ns |
| Only COVID-19-negative patients in HD center | 10 | 24.86 ± 8.06 | |
| Suspected patient isolation | 12 | 21.33 ± 1.41 | ns |
| Suspected patients without isolation | 2 | 29.35 ± 7.71 | |
| Isolation after hospitalization | 5 | 22.12 ± 9.13 | ns |
| No isolation after hospitalization | 9 | 23.92 ± 7.37 | |
| Isolation of hospitalized patients | 5 | 17.41 ± 6.90 | 0.028 |
| Hospitalized patients without isolation | 9 | 26.54 ± 6.34 | |
| Screening of all staff (after exposure) | 6 | 22.25 ± 8.05 | ns |
| Without screening of all staff (after exposure) | 8 | 24.90 ± 7.36 | |
| Staff not eating and drinking together | 9 | 21.90 ± 7.39 | ns |
| Staff eating and drinking together | 5 | 25.77 ± 8.58 | |
| Patients not eating and drinking in HD center | 10 | 23.32 ± 8.31 | ns |
| Patients eating and drinking in HD center | 4 | 23.17 ± 7.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biedunkiewicz, B.; Tylicki, L.; Puchalska-Reglińska, E.; Dębska-Ślizień, A. Analysis of Experiences in Preventing COVID-19 in Hemodialysis Centers of the North of Poland before the Era of Vaccination. Int. J. Environ. Res. Public Health 2022, 19, 684. https://doi.org/10.3390/ijerph19020684
Biedunkiewicz B, Tylicki L, Puchalska-Reglińska E, Dębska-Ślizień A. Analysis of Experiences in Preventing COVID-19 in Hemodialysis Centers of the North of Poland before the Era of Vaccination. International Journal of Environmental Research and Public Health. 2022; 19(2):684. https://doi.org/10.3390/ijerph19020684
Chicago/Turabian StyleBiedunkiewicz, Bogdan, Leszek Tylicki, Ewelina Puchalska-Reglińska, and Alicja Dębska-Ślizień. 2022. "Analysis of Experiences in Preventing COVID-19 in Hemodialysis Centers of the North of Poland before the Era of Vaccination" International Journal of Environmental Research and Public Health 19, no. 2: 684. https://doi.org/10.3390/ijerph19020684
APA StyleBiedunkiewicz, B., Tylicki, L., Puchalska-Reglińska, E., & Dębska-Ślizień, A. (2022). Analysis of Experiences in Preventing COVID-19 in Hemodialysis Centers of the North of Poland before the Era of Vaccination. International Journal of Environmental Research and Public Health, 19(2), 684. https://doi.org/10.3390/ijerph19020684








