Inhibition of LPS-Induced Microglial Activation by the Ethyl Acetate Extract of Pueraria mirifica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Pueraria mirifica
2.2. Cell Cultures and Treatments
2.3. Assessment of Cell Viability
2.4. Assessment of NO Production
2.5. Assessment of mRNA Expression
2.6. Western Blot
2.7. Statistical Analysis
3. Results
3.1. P. mirifica Extract Has No Effect on Cell Viability
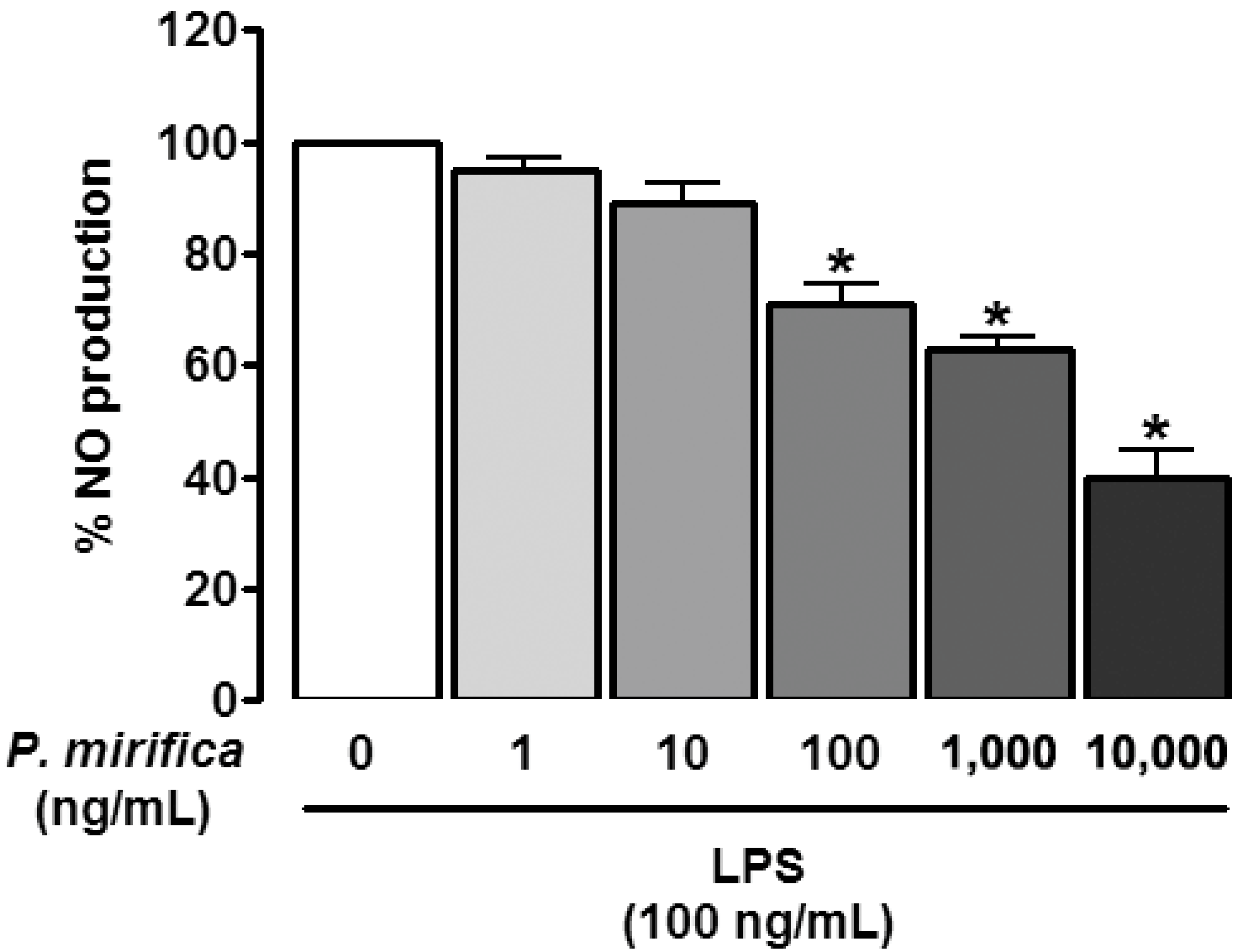
3.2. P. mirifica Extract Diminishes NO Production
3.3. P. mirifica Extract Suppresses iNOS Expression via IRF-1 Inhibition
3.4. P. mirifica Extract Inhibits Proinflammatory Cytokines Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tse, J.K.Y. Gut Microbiota, Nitric Oxide, and Microglia as Prerequisites for Neurodegenerative Disorders. ACS Chem. Neurosci. 2017, 8, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Hu, S.; Molitor, T.W.; Shaskan, E.G.; Peterson, P.K. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 1992, 149, 2736–2741. [Google Scholar] [PubMed]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurzer, M.S.; Xu, X. Dietary phytoestrogens. Annu. Rev. Nutr. 1997, 17, 353–381. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Regal, J.F.; Fraser, D.G.; Weeks, C.E.; Greenberg, N.A. Dietary phytoestrogens have anti-inflammatory activity in a guinea pig model of asthma. Proc. Soc. Exp. Biol. Med. 2000, 223, 372–378. [Google Scholar] [CrossRef]
- Dijsselbloem, N.; Berghe, W.V.; De Naeyer, A.; Haegeman, G. Soy isoflavone phyto-pharmaceuticals in interleukin-6 affections: Multi-purpose nutraceuticals at the crossroad of hormone replacement, anti-cancer and anti-inflammatory therapy. Biochem. Pharmacol. 2004, 68, 1171–1185. [Google Scholar] [CrossRef]
- Yen, G.C.; Lai, H.H. Inhibition of reactive nitrogen species effects in vitro and in vivo by isoflavones and soy-based food extracts. J. Agric. Food Chem. 2003, 51, 7892–7900. [Google Scholar] [CrossRef]
- Lam, A.N.C.; Demasi, M.; James, M.J.; Husband, A.J.; Walker, C. Effect of Red Clover Isoflavones on Cox-2 Activity in Murine and Human Monocyte/Macrophage Cells. Nutr. Cancer 2004, 49, 89–93. [Google Scholar] [CrossRef]
- Jin, D.-Q.; Lim, C.S.; Hwang, J.K.; Ha, I.; Han, J.-S. Anti-oxidant and anti-inflammatory activities of macelignan in murine hippocampal cell line and primary culture of rat microglial cells. Biochem. Biophys. Res. Commun. 2005, 331, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Hellendall, R.P.; Ting, J.P.-Y. Differential regulation of cytokine-induced major histocompatibility complex class II expression and nitric oxide release in rat microglia and astrocytes by effectors of tyrosine kinase, protein kinase C, and cAMP. J. Neuroimmunol. 1997, 74, 19–29. [Google Scholar] [CrossRef]
- Kong, L.-Y.; Lai, C.; Wilson, B.C.; Simpson, J.N.; Hong, J.-S. Protein tyrosine kinase inhibitors decrease lipopolysaccharide-induced proinflammatory cytokine production in mixed glia, microglia-enriched or astrocyte-enriched cultures. Neurochem. Int. 1997, 30, 491–497. [Google Scholar] [CrossRef]
- Cain, J.C. Mirœstrol: An Œstrogen from the Plant Pueraria Mirifica. Nature 1960, 188, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Chansakaow, S.; Ishikawa, T.; Yoshizawa), K.S.; Okada, M.; Higuchi, Y.; Kudo, M.; Chaichantipyuth, C. Isoflavonoids from Pueraria mirifica and their Estrogenic Activity. Planta Medica 2000, 66, 572–575. [Google Scholar] [CrossRef]
- Chansakaow, S.; Ishikawa, T.; Seki, H.; Sekine, K.; Okada, M.; Chaichantipyuth, C. Identification of Deoxymiroestrol as the Actual Rejuvenating Principle of “Kwao Keur”, Pueraria mirifica. The Known Miroestrol May Be an Artifact. J. Nat. Prod. 2000, 63, 173–175. [Google Scholar] [CrossRef]
- Fainanta, T.; Jaroenporn, S.; Wititsuwankul, P.; Malaivijitnond, S. Comparison of neuroprotective effects of dihydrotestosterone, 17β-estradiol, and Pueraria mirifica herb extract on cognitive impairment in androgen deficient male rats. Horm. Behav. 2022, 143, 105198. [Google Scholar] [CrossRef]
- Chulikhit, Y.; Sukhano, W.; Daodee, S.; Putalun, W.; Wongpradit, R.; Khamphukdee, C.; Umehara, K.; Noguchi, H.; Matsumoto, K.; Monthakantirat, O. Effects of Pueraria candollei var mirifica (Airy Shaw and Suvat.) Niyomdham on Ovariectomy-Induced Cognitive Impairment and Oxidative Stress in the Mouse Brain. Molecules 2021, 26, 3442. [Google Scholar] [CrossRef]
- Cheepsunthorn, P.; Radov, L.; Menzies, S.; Reid, J.; Connor, J.R. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia 2001, 35, 53–62. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Kartawy, M.; Amal, H. The role of nitric oxide in brain disorders: Autism spectrum disorder and other psychiatric, neurological, and neurodegenerative disorders. Redox Biol. 2020, 34, 101567. [Google Scholar] [CrossRef]
- Sucontphunt, A.; De-Eknamkul, W.; Nimmannit, U.; Dimitrijevich, S.D.; Gracy, R.W. Protection of HT22 neuronal cells against glutamate toxicity mediated by the antioxidant activity of Pueraria candollei var. mirifica extracts. J. Nat. Med. 2010, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Nathan, C.; Xie, Q.W. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J. Exp. Med. 1994, 180, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.T.; Ignarro, L.J. Lipopolysaccharide-induced Expression of Interferon-β Mediates the Timing of Inducible Nitric-oxide Synthase Induction in RAW 264.7 Macrophages. J. Biol. Chem. 2001, 276, 47950–47957. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, H.; Fujiwara, N.; Sakiyama, H.; Yoshihara, D.; Suzuki, K. Hydrogen peroxide enhances LPS-induced nitric oxide production via the expression of interferon beta in BV-2 microglial cells. Neurosci. Lett. 2011, 494, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ijzermans, J.N.; Marquet, R.L. Interferon-gamma: A Review. Immunobiology 1989, 179, 456–473. [Google Scholar] [CrossRef]
- Salkowski, C.A.; Detore, G.; McNally, R.; van Rooijen, N.; Vogel, S.N. Regulation of inducible nitric oxide synthase messenger RNA expression and nitric oxide production by lipopolysaccharide in vivo: The roles of macrophages, endogenous IFN-gamma, and TNF receptor-1-mediated signaling. J. Immunol. 1997, 158, 905–912. [Google Scholar]
- Gessani, S.; Belardelli, F.; Pecorelli, A.; Puddu, P.; Baglioni, C. Bacterial lipopolysaccharide and gamma interferon induce transcription of beta interferon mRNA and interferon secretion in murine macrophages. J. Virol. 1989, 63, 2785–2789. [Google Scholar] [CrossRef] [Green Version]
- Shimokawa, S.; Kumamoto, T.; Ishikawa, T.; Takashi, M.; Higuchi, Y.; Chaichantipyuth, C.; Chansakaow, S. Quantitative analysis of miroestrol and kwakhurin for standardisation of Thai miracle herb ‘Kwao Keur’ (Pueraria mirifica) and establishment of simple isolation procedure for highly estrogenic miroestrol and deoxymiroestrol. Nat. Prod. Res. 2013, 27, 371–378. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantaratnotai, N.; Thampithak, A.; Utaisincharoen, P.; Pinthong, D.; Sanvarinda, P. Inhibition of LPS-Induced Microglial Activation by the Ethyl Acetate Extract of Pueraria mirifica. Int. J. Environ. Res. Public Health 2022, 19, 12920. https://doi.org/10.3390/ijerph191912920
Jantaratnotai N, Thampithak A, Utaisincharoen P, Pinthong D, Sanvarinda P. Inhibition of LPS-Induced Microglial Activation by the Ethyl Acetate Extract of Pueraria mirifica. International Journal of Environmental Research and Public Health. 2022; 19(19):12920. https://doi.org/10.3390/ijerph191912920
Chicago/Turabian StyleJantaratnotai, Nattinee, Anusorn Thampithak, Pongsak Utaisincharoen, Darawan Pinthong, and Pimtip Sanvarinda. 2022. "Inhibition of LPS-Induced Microglial Activation by the Ethyl Acetate Extract of Pueraria mirifica" International Journal of Environmental Research and Public Health 19, no. 19: 12920. https://doi.org/10.3390/ijerph191912920
APA StyleJantaratnotai, N., Thampithak, A., Utaisincharoen, P., Pinthong, D., & Sanvarinda, P. (2022). Inhibition of LPS-Induced Microglial Activation by the Ethyl Acetate Extract of Pueraria mirifica. International Journal of Environmental Research and Public Health, 19(19), 12920. https://doi.org/10.3390/ijerph191912920





