Enhancing Immediate Memory, Potential Learning, and Working Memory with Transcranial Direct Current Stimulation in Healthy Older Adults
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. Instruments
2.3. Procedure
2.4. Analysis
3. Results
3.1. Characteristics of Participants
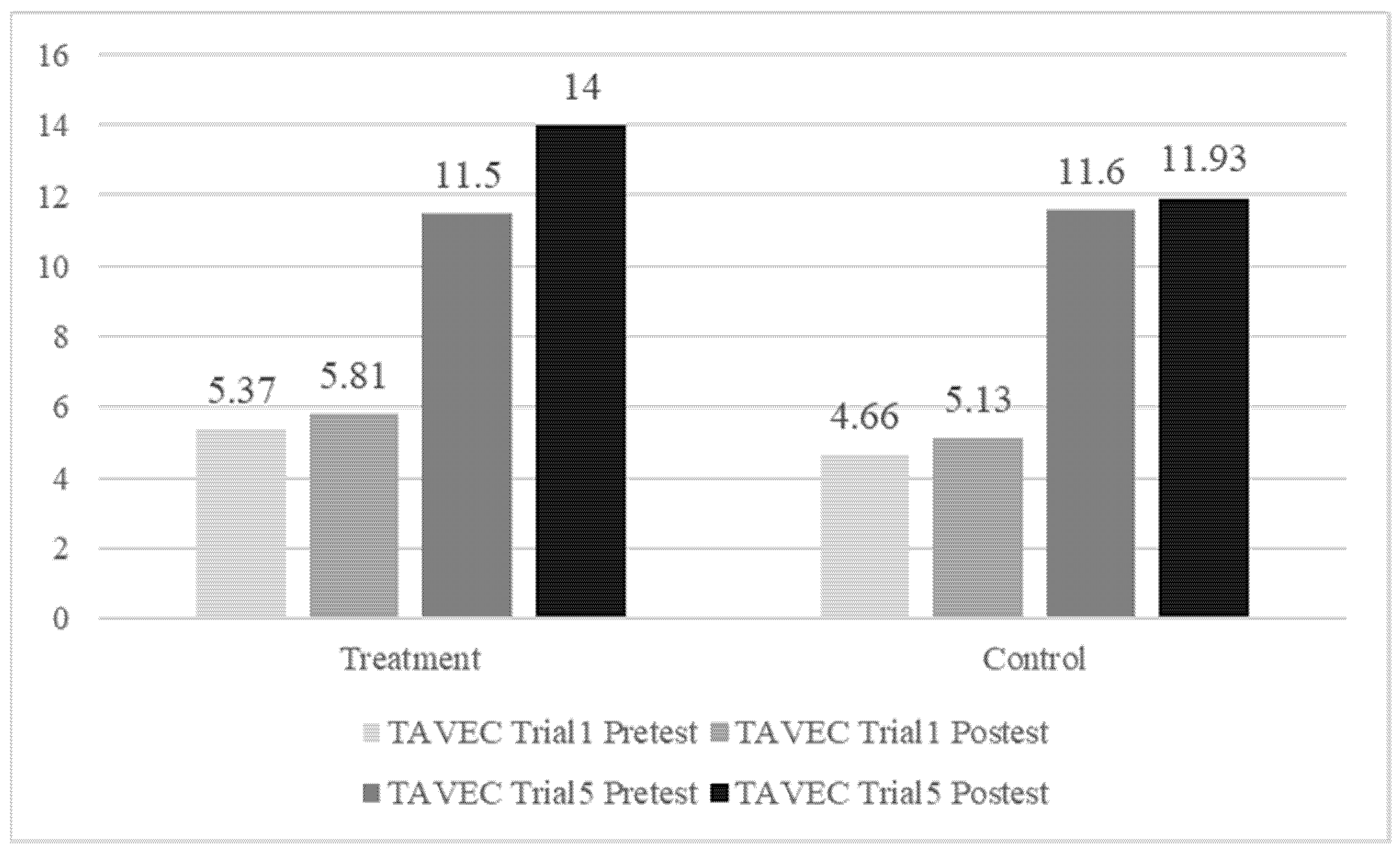
3.2. Memory
3.3. Learning Potential
3.4. Working Memory
4. Discussion
Limitations and Future Lines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Summers, J.J.; Kang, N.; Cauraugh, J.H. Does transcranial direct current stimulation enhance cognitive and motor functions in the ageing brain? A systematic review and meta-analysis. Ageing Res. Rev. 2016, 25, 42–54. [Google Scholar] [CrossRef]
- Stephens, J.A.; Berryhill, M.E. Older adults improve on everyday tasks after working memory training and neurostimulation. Brain Stimul. 2016, 9, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Jaušovec, N.; Jaušovec, K. Working memory training: Improving intelligence–changing brain activity. Brain Cogn. 2012, 79, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Fontenau, C.; Mondino, M.; Arns, M.; Baeken, C.; Bikson, M.; Brunoni, A.R.; Burke, M.; Neuvonen, T.; Padberg, F.; Pascual-Leone, A.; et al. Sham tDCS: A hidden soucre of variability? Reflections for further blinded, controlled trails. Brain Stimul. 2019, 12, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Westwood, S.J.; Olson, A.; Miall, R.C.; Nappo, R.; Romani, C. Limits to tDCS effects in language: Failures to modulate word production in healthy participants with frontal or temporal tDCS. Cortex 2017, 86, 64–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.F.; Nitsche, M. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Javadi, A.H.; Walsh, V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimul. 2012, 5, 231–241. [Google Scholar] [CrossRef]
- Dedoncker, J.; Brunoni, A.R.; Baeken, C.; Vanderhasselt, M.A. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: Influence of stimulation parameters. Brain Stimul. 2016, 9, 501–517. [Google Scholar] [CrossRef] [Green Version]
- Medvedeva, A.; Materassi, M.; Neacsu, V.; Beresford-Webb, J.; Hussin, A.; Khan, N.; Newton, F.; Galli, G. Effects of anodal transcranial direct current stimulation over the ventrolateral prefrontal cortex on episodic memory formation and retrieval. Cereb. Cortex 2019, 29, 657–665. [Google Scholar] [CrossRef]
- Shin, Y.I.; Foerster, Á.; Nitsche, M.A. Reprint of: Transcranial direct current stimulation (tDCS)– Application in neuropsychology. Neuropsychologia 2015, 74, 74–95. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Ku, Y.; Zanto, T.P.; Gazzaley, A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: A systematic review and meta-analysis. Neurobiol. Aging 2015, 36, 2348–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manenti, R.; Brambilla, M.; Petesi, M.; Ferrari, C.; Cotelli, M. Enhancing verbal episodic memory in older and young subjects after non-invasive brain stimulation. Front. Aging Neurosci. 2013, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Flöel, A.; Suttorp, W.; Kohl, O.; Kürten, J.; Lohmann, H.; Breitenstein, C.; Knecht, S. Non-invasive brain stimulation improves object-location learning in the elderly. Neurobiol. Aging 2012, 33, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, M.; Manenti, R.; Brambilla, M.; Cobelli, C.; Cohen, L.G.; Cotelli, M. Older adults get episodic memory boosting from noninvasive stimulation of prefrontal cortex during learning. Neurobiol. Aging 2016, 39, 210–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandrini, M.; Brambilla, M.; Manenti, R.; Rosini, S.; Cohen, L.G.; Cotelli, M. Noninvasive stimulation of prefrontal cortex strengthens existing episodic memories and reduces forgetting in the elderly. Front. Aging Neurosci. 2014, 6, 289. [Google Scholar] [CrossRef]
- Javadi, A.H.; Cheng, P.; Walsh, V. Short duration transcranial direct current stimulation (tDCS) modulates verbal memory. Brain Stimul. 2012, 5, 468–474. [Google Scholar] [CrossRef]
- Sandrini, M.; Manenti, R.; Gobbi, E.; Rusich, D.; Bartl, G.; Cotelli, M. Transcranial direct current stimulation applied after encoding facilitates episodic memory consolidation in older adults. Neurobiol. Learn Mem. 2019, 163, 107037. [Google Scholar] [CrossRef]
- Huo, L.; Zheng, Z.; Huang, J.; Li, R.; Li, J.; Li, J. Transcranial direct current stimulation enhances episodic memory in healthy older adults by modulating retrieval-specific activation. Neural. Plast. 2020, 2020, 8883046. [Google Scholar] [CrossRef]
- Coffman, B.A.; Clark, V.P.; Parasuraman, R. Battery powered thought: Enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage 2014, 85, 895–908. [Google Scholar] [CrossRef]
- Kincses, T.Z.; Antal, A.; Nitsche, M.A.; Bártfai, O.; Paulus, W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia 2004, 42, 113–117. [Google Scholar] [CrossRef]
- Nikolin, S.; Loo, C.K.; Bai, S.; Dokos, S.; Martin, D.M. Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. Neuroimage 2015, 117, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Richmond, L.L.; Wolk, D.; Chein, J.; Olson, I.R. Transcranial direct current stimulation enhances verbal working memory training performance over time and near transfer outcomes. J. Cogn. Neurosci. 2014, 26, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Nitsche, M.; Bermpohl, F.; Antal, A.; Feredoes, E.; Marcolin, M.A.; Rigonatti, S.; Silva, M.T.A.; Paulu, S.W.; et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 2005, 166, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nikolin, S.; Martin, D.; Loo, C.K.; Boonstra, T.W. Effects of tDCS dosage on working memory in healthy participants. Brain Stimul. 2018, 11, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.T.; Fitzgerald, P.B.; Hoy, K.E. Effects of anodal transcranial direct current stimulation on working memory: A systematic review and meta-analysis of findings from healthy and neuropsychiatric populations. Brain Stimul. 2016, 9, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Imburgio, M.J.; Orr, J.M. Effects of prefrontal tDCS on executive function: Methodological considerations revealed by meta-analysis. Neuropsychologia 2018, 117, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Vadillo, M.A.; Sirota, M.; Feurra, M.; Medvedeva, A. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) on episodic memory. Brain Stimul. 2019, 12, 231–241. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini Mental State. A practical method for grading the cognitive state of patients for the clinical. J. Psychiatry Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Benedet, M.J.; Alejandre, M.Á. TAVEC: Test de Aprendizaje Verbal España-Complutense; TEA Ediciones: Madrid, Spain, 1998. [Google Scholar]
- Kliegl, R.; Smith, J.; Baltes, P.B. Testing-the-limits and the study of adult age differences in cognitive plasticity of a mnemonic skill. Dev. Psychol. 1989, 25, 247–256. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Adults-III (WAIS-III); TEA Ediciones: Madrid, Spain, 2001. [Google Scholar]
- Nejati, V.; Salehinejad, M.A.; Nitsche, M.A. Interaction of the left dorsolateral prefrontal cortex (l-DLPFC) and right orbitofrontal cortex (OFC) in hot and cold executive functions: Evidence from transcranial direct current stimulation (tDCS). Neuroscience 2018, 369, 109–123. [Google Scholar] [CrossRef]
- Otero, T.M.; Barker, L.A. The frontal lobes and executive functioning. In Handbook of Executive Functioning; Goldstein, S., Naglieri, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 29–44. [Google Scholar]
- Boggio, P.S.; Khoury, L.P.; Martins, D.C.; Martins, O.E.; De Macedo, E.C.; Fregni, F. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. J. Neurol. Neurosurg. Psychiatr. 2009, 80, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Fileccia, E.; Di Stasi, V.; Poda, R.; Rizzo, G.; Stanzani-Maserati, M.; Oppi, F.; Avoni, P.; Capellari, S.; Liguori, R. Effects on cognition of 20-day anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex in patients affected by mild cognitive impairment: A case-control study. Neurol. Sci. 2019, 40, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.S.D.S.D.; Zortea, M.; Alves, R.L.; Naziazeno, C.C.D.S.; Saldanha, J.S.; Carvalho, S.D.C.R.D.; Leite, A.J.D.C.; Torres, I.L.D.S.; Souza, A.; Calvetti, P.U.; et al. Cognitive effects of transcranial direct current stimulation combined with working memory training in fibromyalgia: A randomized clinical trial. Sci. Rep. 2018, 8, 12477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wen, J.B.; Li, X.L. No effect of transcranial direct current stimulation of the dorsolateral prefrontal cortex on short-term memory. CNS Neurosci. Ther. 2018, 24, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirulli, C.; Fertonani, A.; Miniussi, C. The role of timing in the induction of neuromodulation in perceptual learning by transcranial electric stimulation. Brain Stimul. 2013, 6, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandrini, M.; Manenti, R.; Sahin, H.; Cotelli, M. Effects of transcranial electrical stimulation on episodic memory in physiological and pathological ageing. Ageing Res. Rev. 2020, 61, 101065. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, J.C.; Satorres, E.; Pitarque, A.; Delhom, I.; Real, E.; Escudero, J. Effectiveness of tDCS at improving recognition and reducing false memories in older adults. Int. J. Environ. Res. Public Health 2021, 18, 1317. [Google Scholar] [CrossRef]
- Borst, J.P.; Anderson, J.R. Using model-based functional MRI to locate working memory updates and declarative memory retrievals in the fronto-parietal network. Proc. Natl. Acad. Sci. USA 2013, 110, 1628–1633. [Google Scholar] [CrossRef] [Green Version]
- Lara, G.A.D.; Knechtges, P.N.; Paulus, W.; Antal, A. Anodal tDCS over the left DLPFC did not affect the encoding and retrieval of verbal declarative information. Front. Neurosci. 2017, 11, 452. [Google Scholar] [CrossRef] [Green Version]
- Pisoni, A.; Turi, Z.; Raithel, A.; Ambrus, G.G.; Alekseichuk, I.; Schacht, A.; Paulus, W.; Antal, A. Separating recognition processes of declarative memory via anodal tDCS: Boosting old item recognition by temporal and new item detection by parietal stimulation. PLoS ONE 2015, 10, e0123085. [Google Scholar] [CrossRef]
- Tonegawa, S.; Pignatelli, M.; Roy, D.S.; Ryan, T.J. Memory engram storage and retrieval. Curr. Res. Neurobiol. 2015, 35, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perceval, G.; Martin, A.K.; Copland, D.A.; Laine, M.; Meinzer, M. Multisession transcranial direct current stimulation facilitates verbal learning and memory consolidation in young and older adults. Brain Lang. 2020, 205, 104788. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian-Shirvan, E.; Farnad, L.; Mosayebi-Samani, M.; Verstraelen, S.; Meesen, R.L.; Kuo, M.F.; Nitsche, M.A. Age-related differences of motor cortex plasticity in adults: A transcranial direct current stimulation study. Brain Stimul. 2020, 13, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Fertonani, A.; Brambilla, M.; Cotelli, M.; Miniussi, C. The timing of cognitive plasticity in physiological aging: A tDCS study of naming. Front. Aging Neurosci. 2014, 6, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polanía, R.; Nitsche, M.A.; Paulus, W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum. Brain Mapp. 2011, 32, 1236–1249. [Google Scholar] [CrossRef]
- Peña-Gómez, C.; Sala-Lonch, R.; Junqué, C.; Clemente, I.C.; Vidal, D.; Bargalló, N.; Falcón, C.; Valls-Solè, J.; Pascual-Leone, A.; Bartrés-Faz, D. Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimul. 2012, 5, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Deldar, Z.; Rustamov, N.; Blanchette, I.; Piché, M. Improving working memory and pain inhibition in older persons using transcranial direct current stimulation. Neurosci. Res. 2019, 148, 19–27. [Google Scholar] [CrossRef]
- Jones, K.T.; Stephens, J.A.; Alam, M.; Bikson, M.; Berryhill, M.E. Longitudinal neurostimulation in older adults improves working memory. PLoS ONE 2015, 10, e0121904. [Google Scholar] [CrossRef]
- Nissim, N.R.; O’Shea, A.; Indahlastari, A.; Kraft, J.N.; Von Mering, O.; Aksu, S.; Progres, E.; Cohen, R.; Woods, A.J. Effects of transcranial direct current stimulation paired with cognitive training on functional connectivity of the working memory network in older adults. Front. Aging Neurosci. 2019, 11, 340. [Google Scholar] [CrossRef]
- Stoynova, N.; Laske, C.; Plewnia, C. Combining electrical stimulation and cognitive control training to reduce concerns about subjective cognitive decline. Brain Stimul. 2019, 12, 1083–1085. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Vanderhasselt, M.A. Working memory improvement with noninvasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain Cogn. 2014, 86, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, L.E.; Ilieva, I.P.; Hamilton, R.H.; Farah, M.J. Does transcranial direct current stimulation improve healthy working memory? A meta-analytic review. J. Cogn. Neurosci. 2016, 28, 1063–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goethals, I.; Audenaert, K.; Van de Wiele, C.; Dierckx, R. The prefrontal cortex: Insights from functional neuroimaging using cognitive activation tasks. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Haque, Z.Z.; Samandra, R.; Mansouri, F.A. Neural substrate and underlying mechanisms of working memory: Insights from brain stimulation studies. J. Neurophysiol. 2021, 125, 2038–2053. [Google Scholar] [CrossRef]
| Active Group (n = 16) | Sham Group (n = 15) | p-Value | |
|---|---|---|---|
| Age | 69.8 ± 3.4 | 70.13 ± 4.7 | 0.217 a |
| Gender (female/male) | 8/8 | 8/7 | 0.987 b |
| Years of Education | 12.25 ± 3.29 | 11.67 ± 3.3 | 0.627 a |
| MMSE | 29.75 ± 0.57 | 29.86 ± 0.51 | 0.559 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satorres, E.; Meléndez, J.C.; Pitarque, A.; Real, E.; Abella, M.; Escudero, J. Enhancing Immediate Memory, Potential Learning, and Working Memory with Transcranial Direct Current Stimulation in Healthy Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 12716. https://doi.org/10.3390/ijerph191912716
Satorres E, Meléndez JC, Pitarque A, Real E, Abella M, Escudero J. Enhancing Immediate Memory, Potential Learning, and Working Memory with Transcranial Direct Current Stimulation in Healthy Older Adults. International Journal of Environmental Research and Public Health. 2022; 19(19):12716. https://doi.org/10.3390/ijerph191912716
Chicago/Turabian StyleSatorres, Encarnación, Juan C. Meléndez, Alfonso Pitarque, Elena Real, Mireia Abella, and Joaquin Escudero. 2022. "Enhancing Immediate Memory, Potential Learning, and Working Memory with Transcranial Direct Current Stimulation in Healthy Older Adults" International Journal of Environmental Research and Public Health 19, no. 19: 12716. https://doi.org/10.3390/ijerph191912716
APA StyleSatorres, E., Meléndez, J. C., Pitarque, A., Real, E., Abella, M., & Escudero, J. (2022). Enhancing Immediate Memory, Potential Learning, and Working Memory with Transcranial Direct Current Stimulation in Healthy Older Adults. International Journal of Environmental Research and Public Health, 19(19), 12716. https://doi.org/10.3390/ijerph191912716








