Magnetic Beads of Zero Valent Iron Doped Polyethersolfun Developed for Removal of Arsenic from Apatite-Soil Treated Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Preparation of Apatite-Soil
2.3. Treatment of Apatite-Soil Experiment
2.4. Beads Charactrization
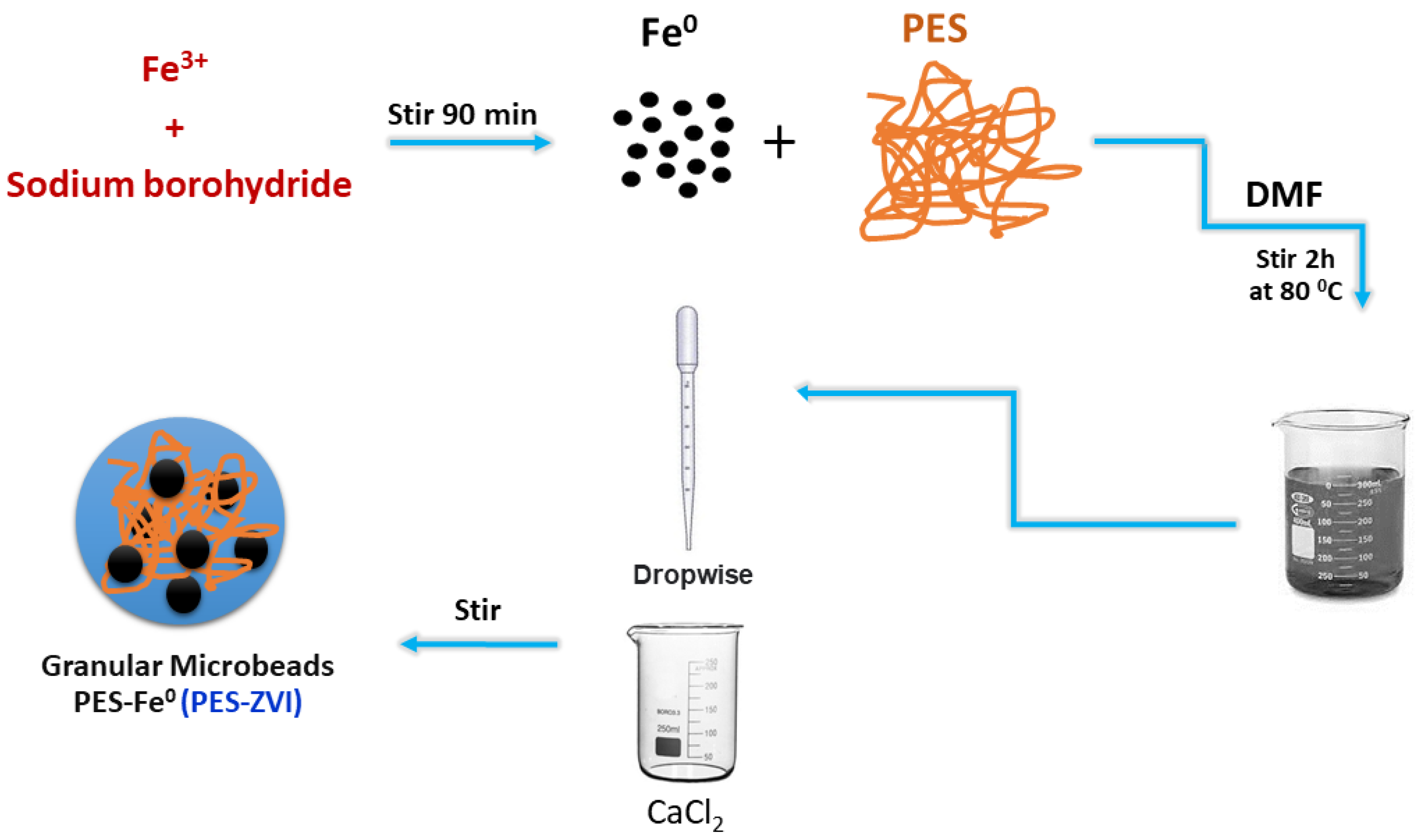
2.5. Synthesis of PES/ZVI Microbeaads
2.6. Adsorption/Removal Procedure
3. Results and Discussions
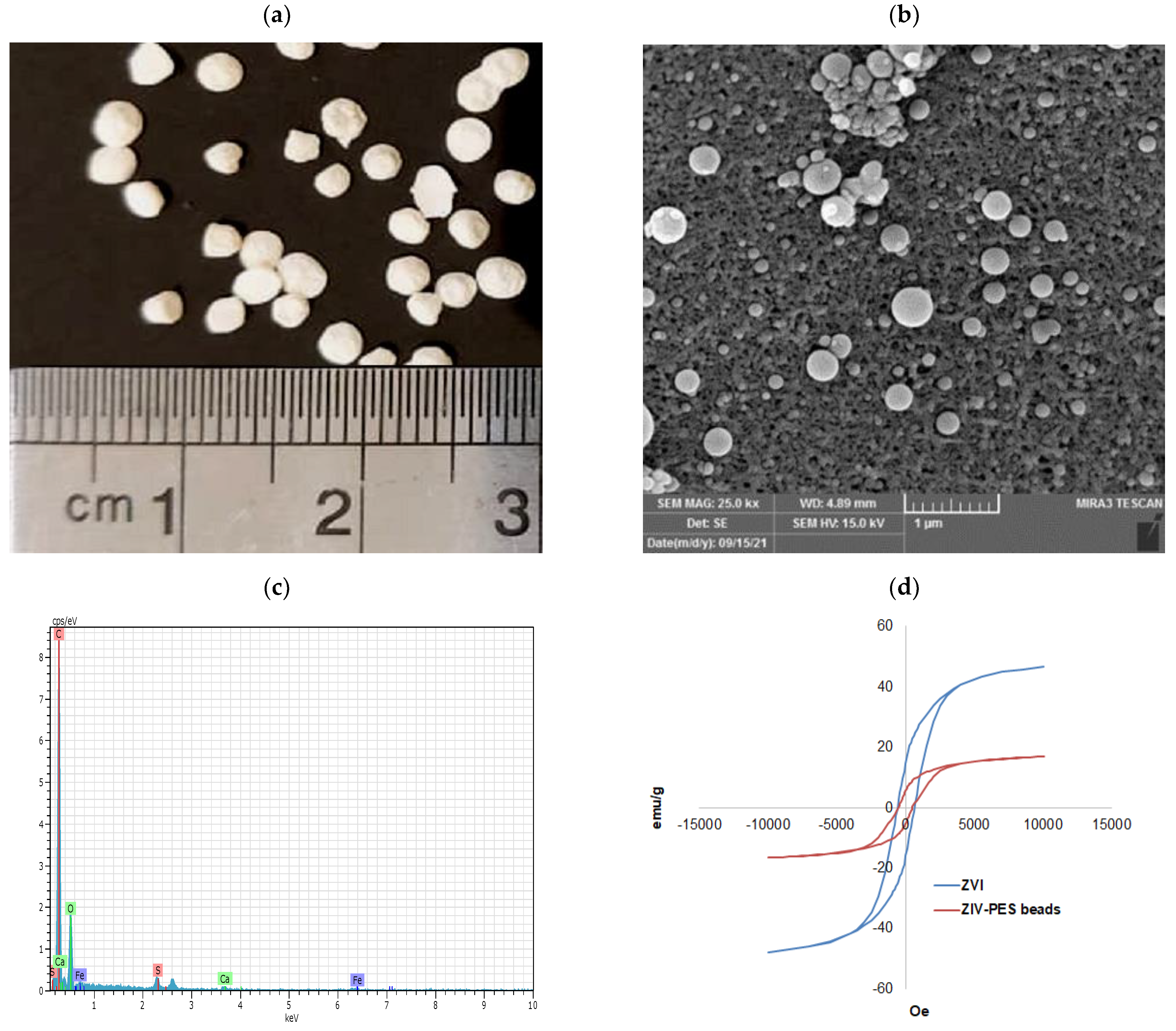
3.1. Characterization
3.1.1. FTIR
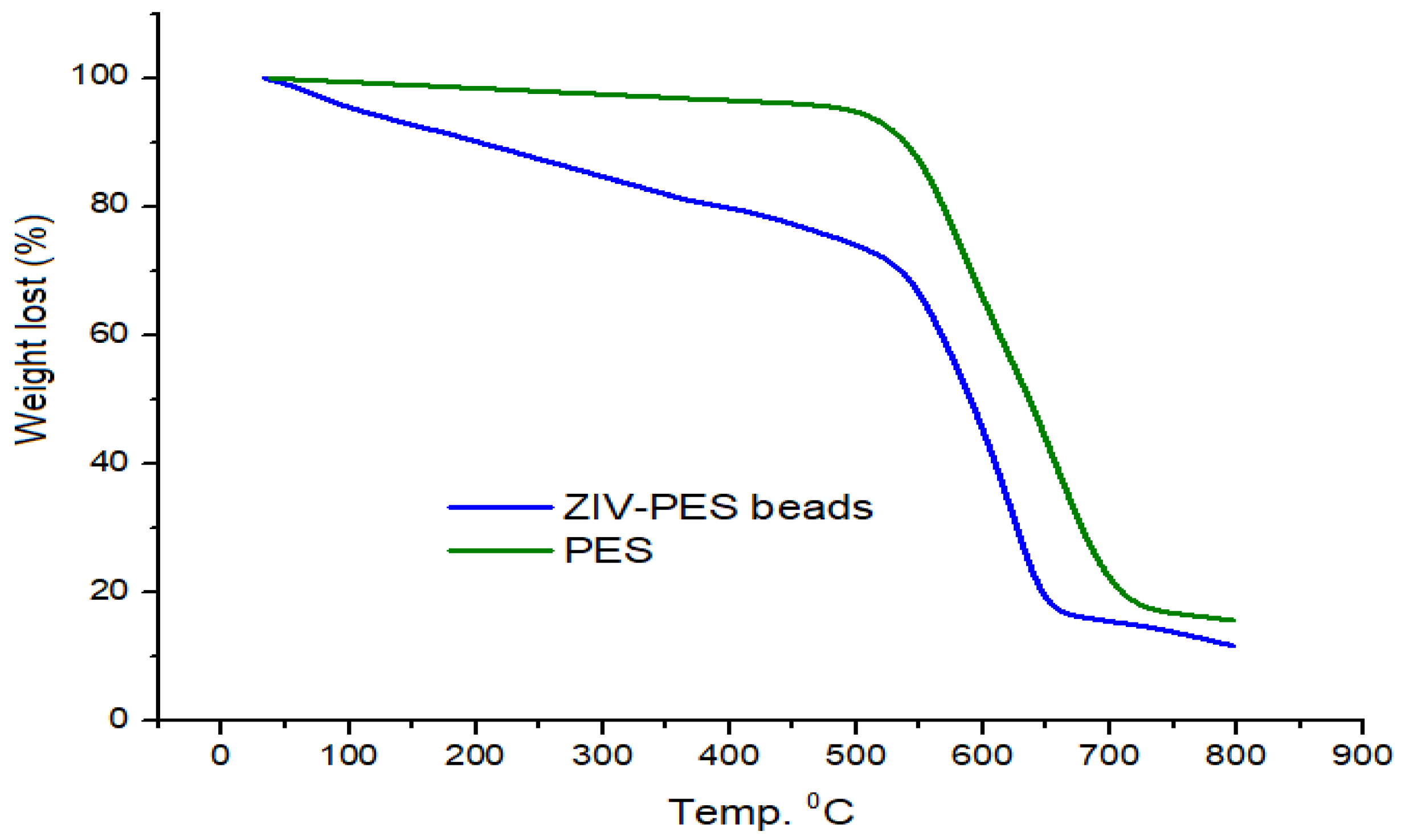
3.1.2. TGA
3.1.3. SEM Microscopy
3.1.4. VSM
3.2. Effect of Parameters on Removal Efficiency
3.2.1. Type of Adsorbent
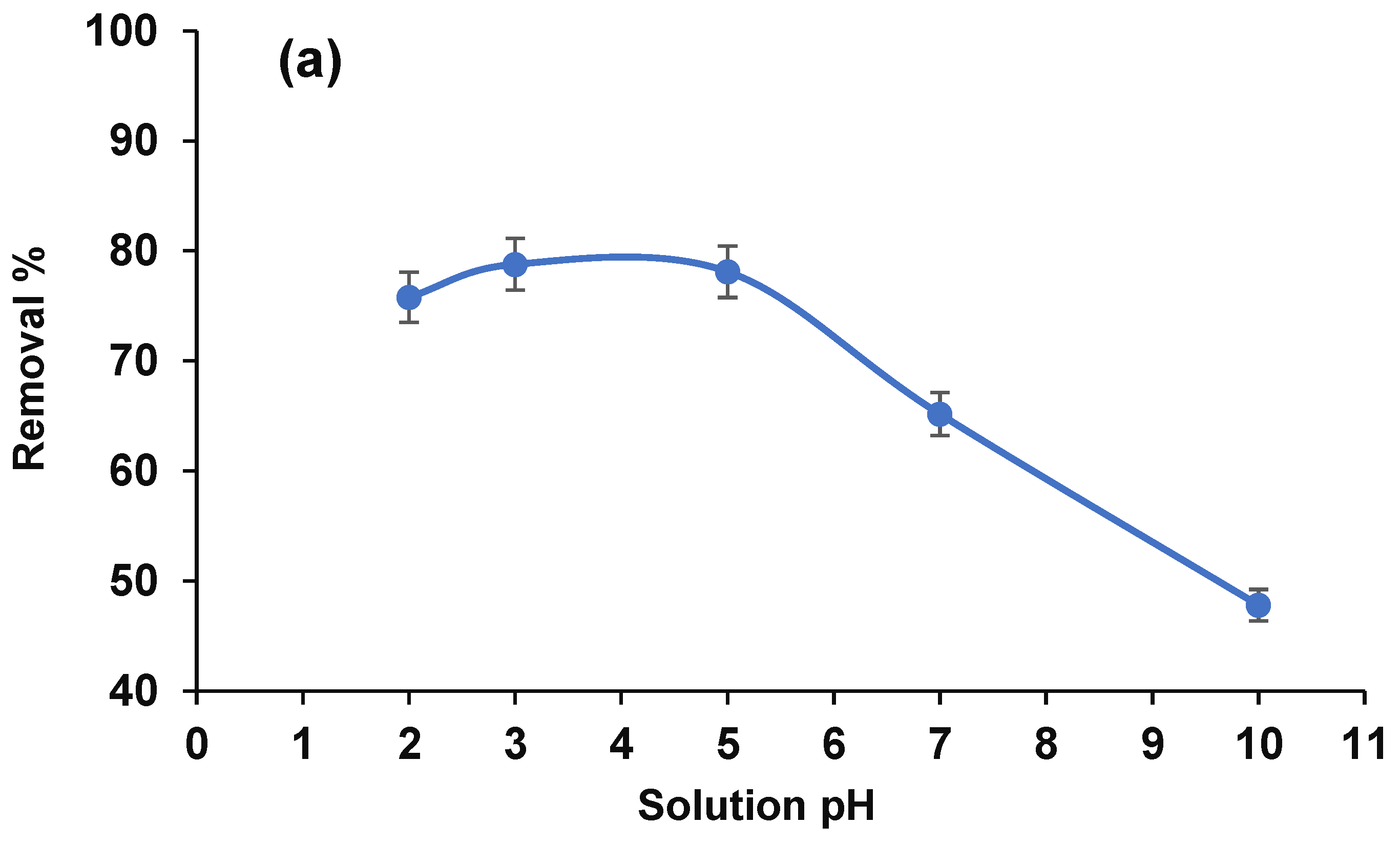
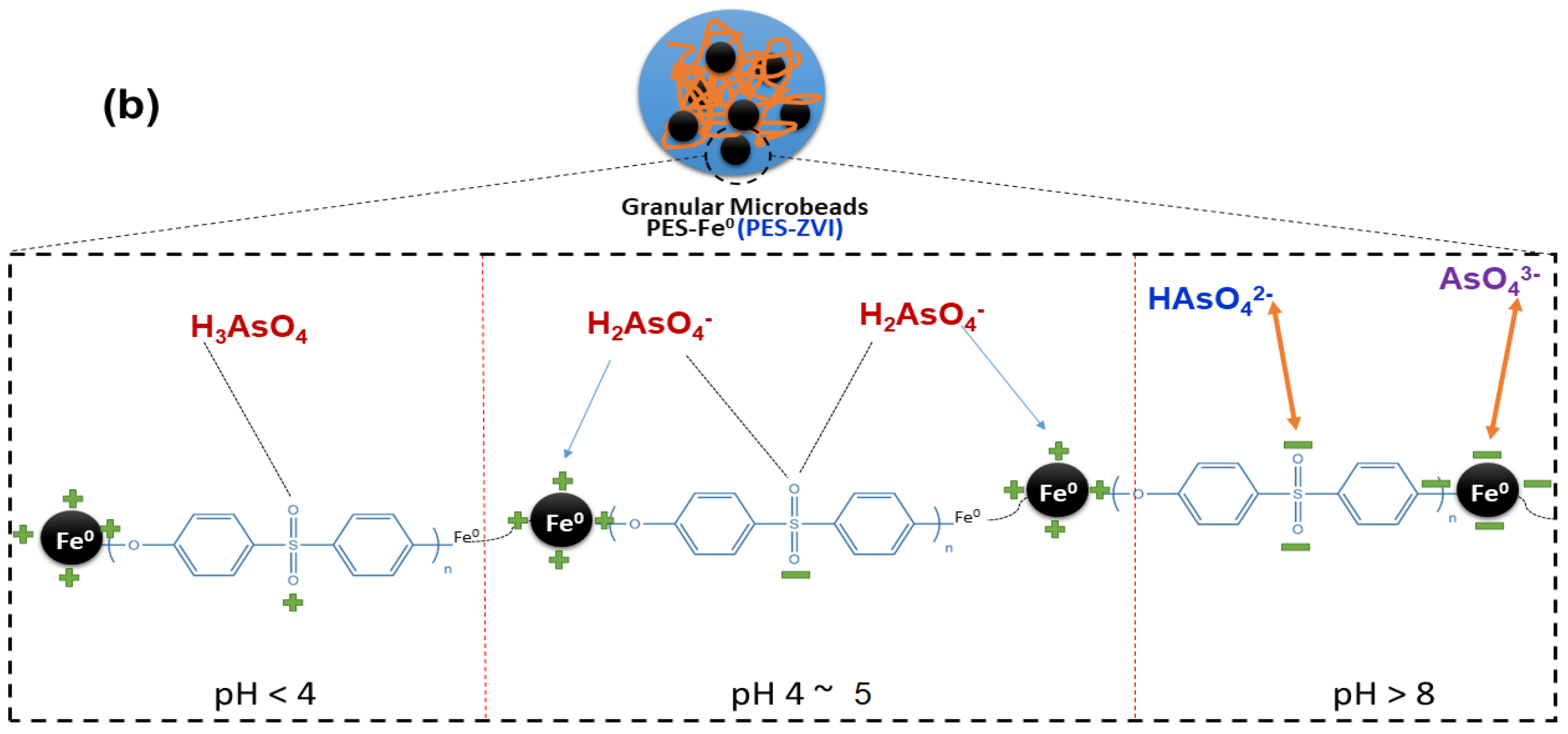
3.2.2. Solution pH and Proposed Mechanism
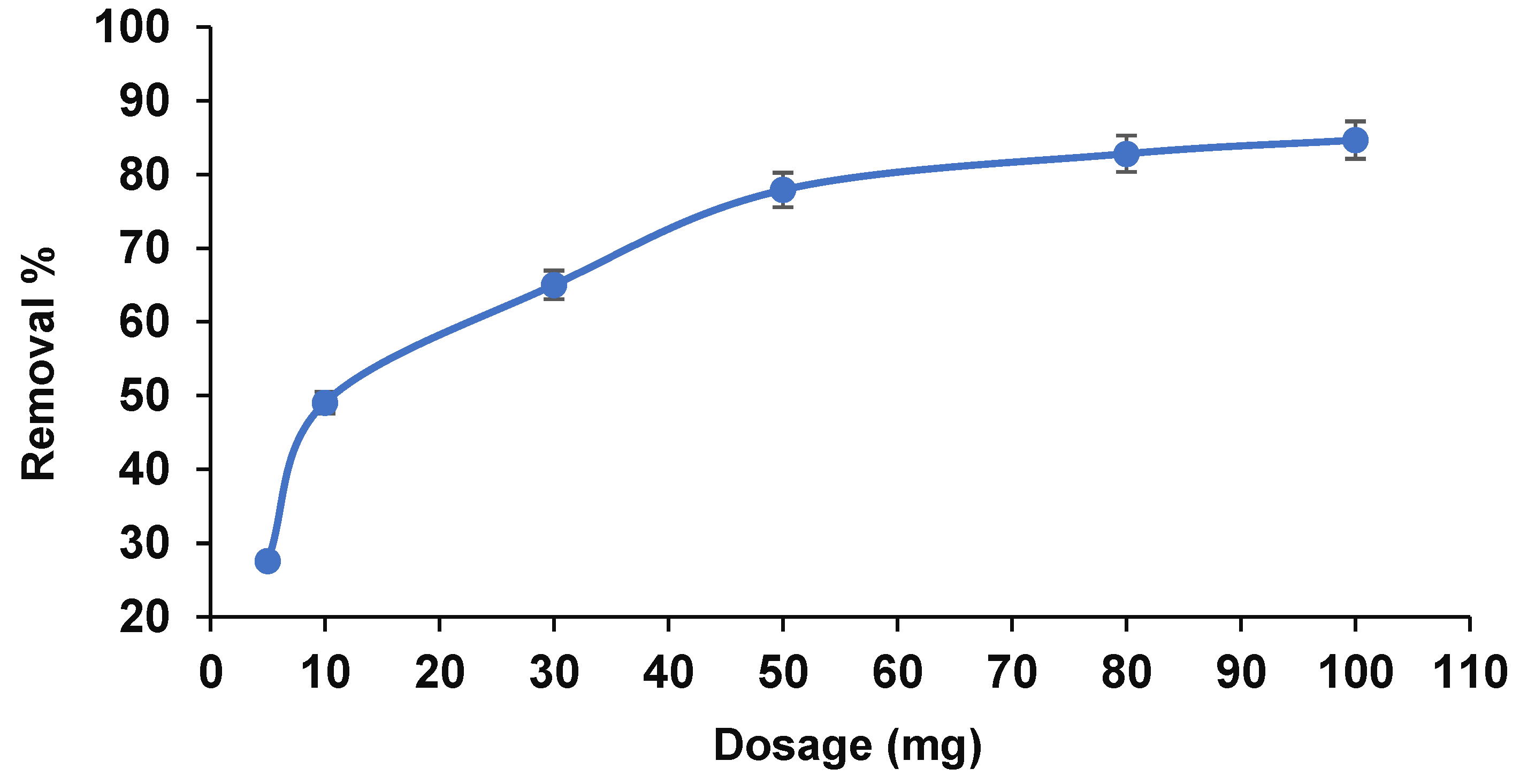
3.2.3. Adsorbent Dosage
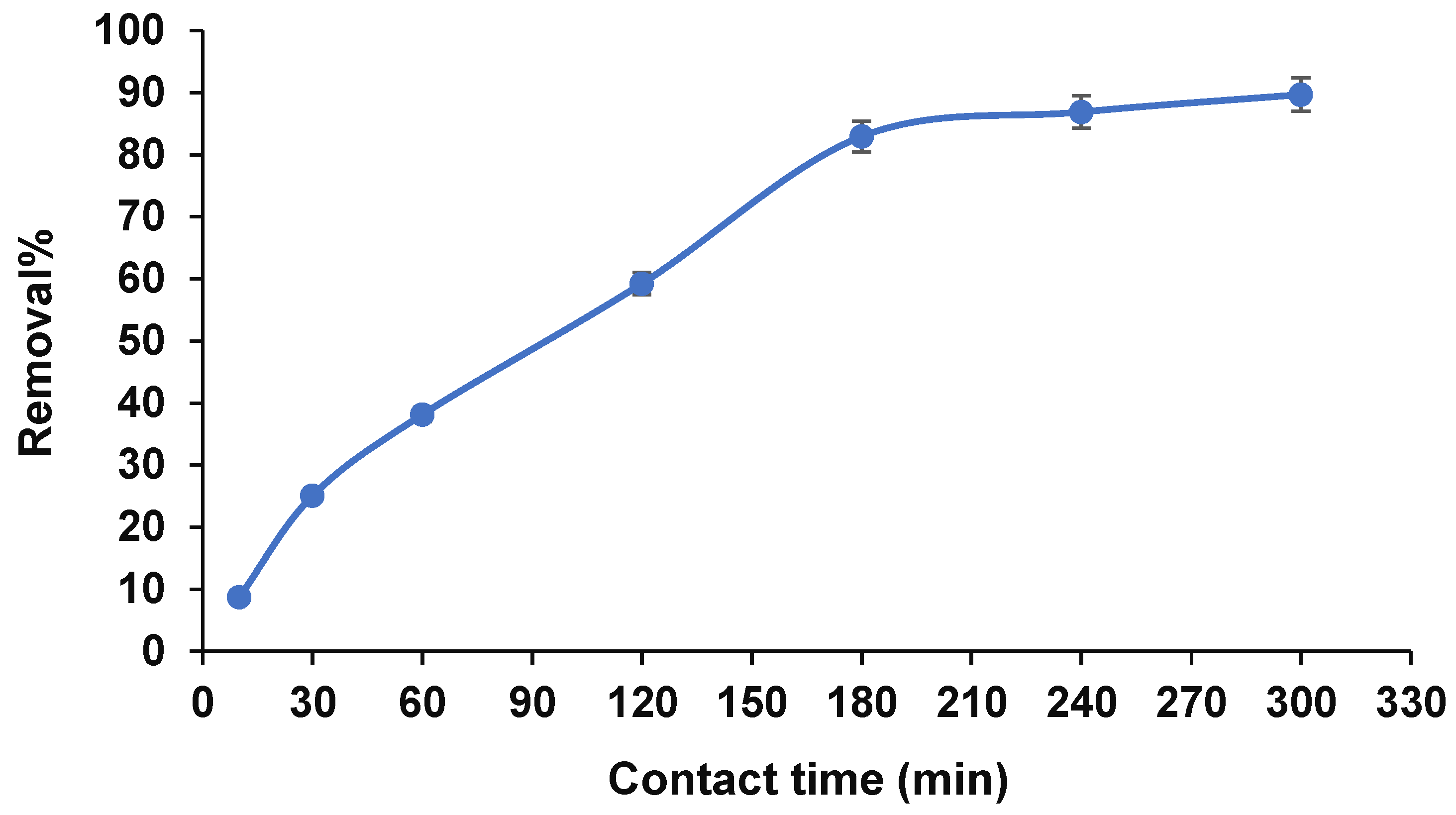
3.2.4. Contact Time
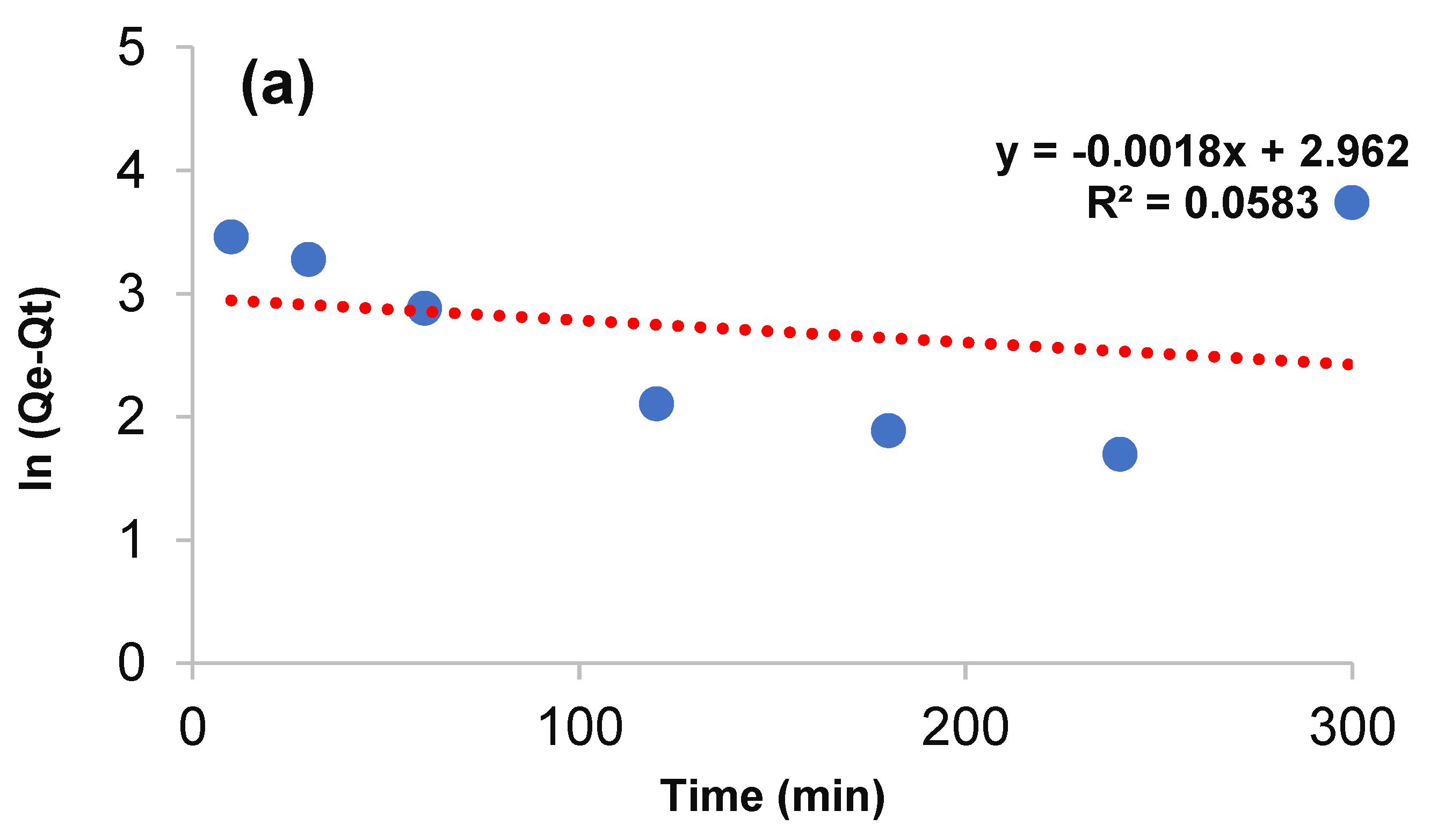
3.3. Kinetic Study
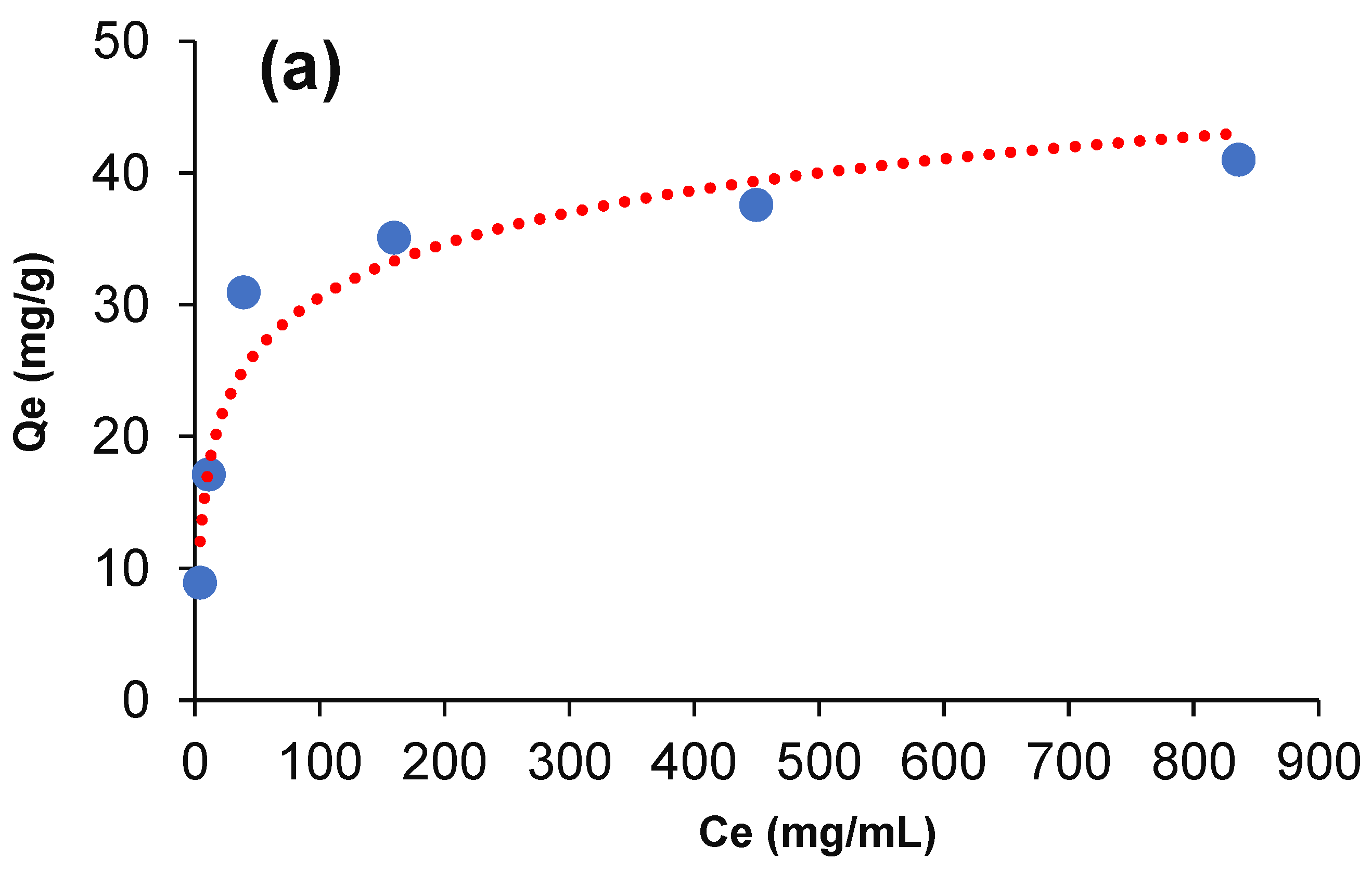
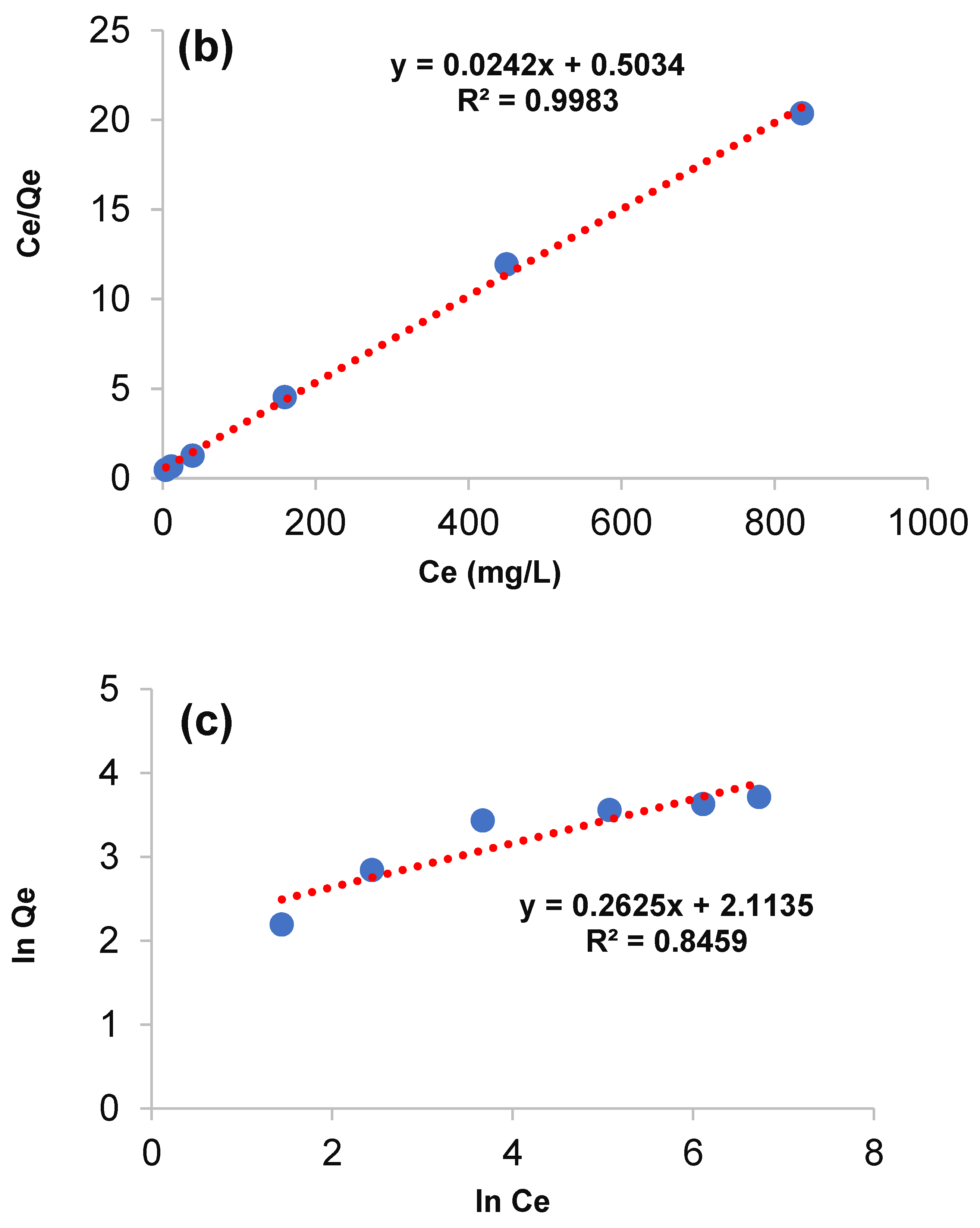
3.4. Equilibrium Adsorption and Isotherm Models
3.5. Temperature and Thermodynamic Studies
3.6. Real Samples
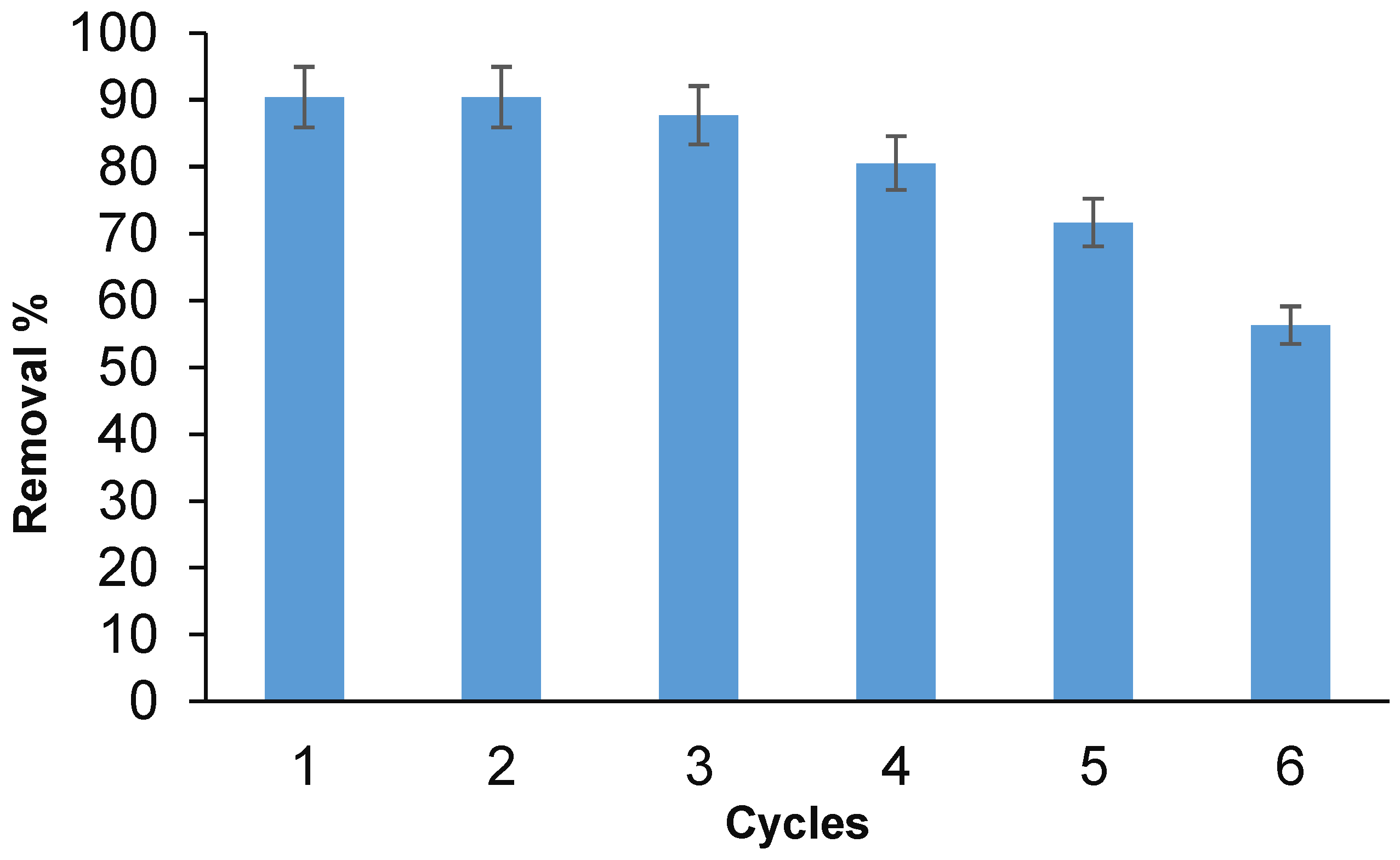
3.7. Regeneration Anc Recycle
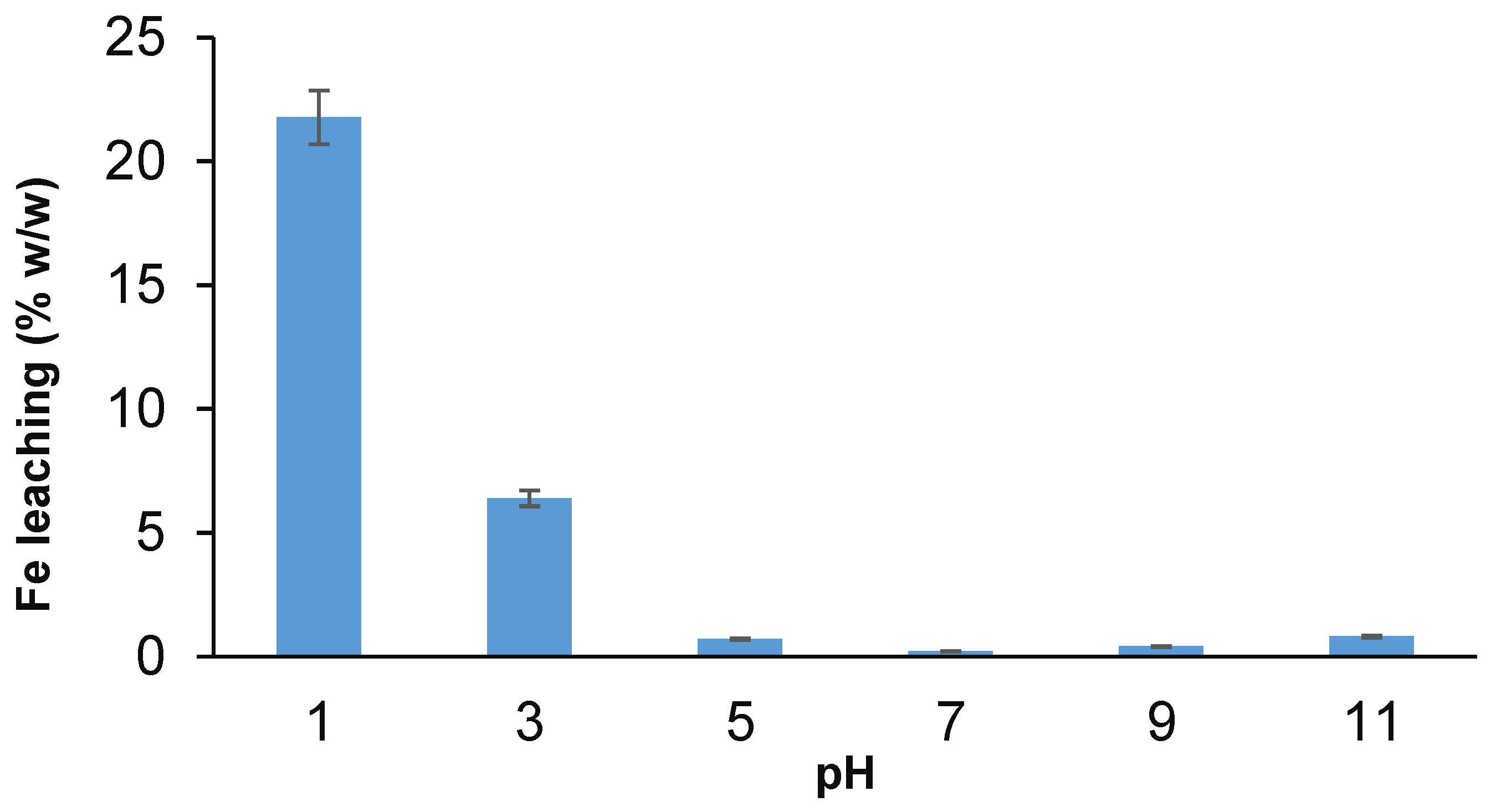
3.8. Iron Leaching Test
3.9. Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gabris, M.A.; Rezania, S.; Rafieizonooz, M.; Khankhaje, E.; Devanesan, S.; AlSalhi, M.S.; Aljaafreh, M.J.; Shadravan, A. Chitosan magnetic graphene grafted polyaniline doped with cobalt oxide for removal of Arsenic(V) from water. Environ. Res. 2022, 207, 112209. [Google Scholar] [CrossRef] [PubMed]
- Nodeh, M.K.M.; Gabris, M.A.; Nodeh, H.R.; Bidhendi, M.E. Efficient removal of arsenic(III) from aqueous media using magnetic polyaniline-doped strontium–titanium nanocomposite. Environ. Sci. Pollut. Res. 2018, 25, 16864–16874. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, O.M.; Tawabini, B.S.; Soupios, P.; Ntarlagiannis, D. Removal of arsenic from contaminated groundwater using biochar: A technical review. Int. J. Environ. Sci. Technol. 2021, 19, 651–664. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Roy, A.; Mukherjee, S.; Kumar, M. Performance evaluation of crop residue and kitchen waste-derived biochar for eco-efficient removal of arsenic from soils of the Indo-Gangetic plain: A step towards sustainable pollution management. Environ. Res. 2021, 200, 111758. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhattacharya, T. Removal of Arsenic by Wheat Straw Biochar from Soil. Bull. Environ. Contam. Toxicol. 2022, 108, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Zheng, B.; Zhang, Y.; Hu, Y. Rapid and effective removal of As(III) and As(V) using spore@Ti4+ microspheres. Chemosphere 2018, 206, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Rezania, S.; Talaiekhozani, A.; Oryani, B.; Cho, J.; Barghi, M.; Rupani, P.F.; Kamali, M. Occurrence of persistent organic pollutants (POPs) in the atmosphere of South Korea: A review. Environ. Pollut. 2022, 307, 119586. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Lilhare, S.; Mathew, S.B.; Singh, A.K.; Carabineiro, S.A.C. Calcium Alginate Beads with Entrapped Iron Oxide Magnetic Na-noparticles Functionalized with Methionine—A Versatile Adsorbent for Arsenic Removal. Nanomaterials 2021, 11, 1345. [Google Scholar] [CrossRef]
- Sayed, M.; Khan, A.; Rauf, S.; Shah, N.S.; Rehman, F.; Al-Kahtani, A.A.; Khan, J.A.; Iqbal, J.; Boczkaj, G.; Gul, I.; et al. Bismuth-Doped Nano Zerovalent Iron: A Novel Catalyst for Chloramphenicol Degradation and Hydrogen Production. ACS Omega 2020, 5, 30610–30624. [Google Scholar] [CrossRef]
- Nejad, Z.D.; Rezania, S.; Jung, M.C.; Al-Ghamdi, A.A.; Mustafa, A.E.-Z.M.; Elshikh, M.S. Effects of fine fractions of soil organic, semi-organic, and inorganic amendments on the mitigation of heavy metal(loid)s leaching and bioavailability in a post-mining area. Chemosphere 2021, 271, 129538. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, Q.; Li, Y.; Qin, L.; Zhang, K.; Liu, G.; Yuan, C.-G. Stabilization of nano zero-valent iron by electrospun composite mat with good catalysis and recyclability. J. Clean. Prod. 2022, 363, 132459. [Google Scholar] [CrossRef]
- Liu, W.; Mei, Y.; Etschmann, B.; Brugger, J.; Pearce, M.; Ryan, C.G.; Borg, S.; Wykes, J.; Kappen, P.; Paterson, D.; et al. Arsenic in hydrothermal apatite: Oxidation state, mechanism of uptake, and comparison between experiments and nature. Geochim. Cosmochim. Acta 2017, 196, 144–159. [Google Scholar] [CrossRef]
- Alvand, Z.M.; Rajabi, H.R.; Mirzaei, A.; Masoumiasl, A.; Sadatfaraji, H. Rapid and green synthesis of cadmium telluride quantum dots with low toxicity based on a plant-mediated approach after microwave and ultrasonic assisted extraction: Synthesis, characterization, biological potentials and comparison study. Mater. Sci. Eng. C 2019, 98, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Goyal, A.K.; Rath, G. Development and Characterization of Gastroretentive High-Density Pellets Lodged with Zero Valent Iron Nanoparticles. J. Pharm. Sci. 2018, 107, 2663–2673. [Google Scholar] [CrossRef]
- Tshangana, C.S.; Muleja, A.A.; Nxumalo, E.N.; Mhlanga, S.D. Poly (ether) sulfone electrospun nanofibrous membranes em-bedded with graphene oxide quantum dots with antimicrobial activity. Environ. Sci. Pollut. Res. 2020, 27, 26845–26855. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Baltazar, S.; Garcia, A.; Romero, A.; Rubio, M.A.; Altbir, D. Lead removal by nano-scale zero valent iron: Surface analysis and pH effect. Mater. Res. Bull. 2014, 59, 341–348. [Google Scholar] [CrossRef]
- Prabha, G.; Raj, V. Preparation and characterization of chitosan-Polyethylene glycol-polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles as carrier system: Drug loading and in vitro drug release study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 808–816. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Xie, H.; Pan, Y.; Liu, J.; Huang, Z.; Long, X.; Xiao, H. Cellulose-based adsorbents loaded with zero-valent iron for removal of metal ions from contaminated water. Environ. Sci. Pollut. Res. 2020, 27, 33234–33247. [Google Scholar] [CrossRef] [PubMed]
- Mosleh, N.; Najmi, M.; Parandi, E.; Nodeh, H.R.; Vasseghian, Y.; Rezania, S. Magnetic sporopollenin supported polyaniline developed for removal of lead ions from wastewater: Kinetic, isotherm and thermodynamic studies. Chemosphere 2022, 300, 134461. [Google Scholar] [CrossRef] [PubMed]
- Inam, M.A.; Khan, R.; Park, D.R.; Ali, B.A.; Uddin, A.; Yeom, I.T. Influence of pH and Contaminant Redox Form on the Competitive Removal of Arsenic and Antimony from Aqueous Media by Coagulation. Minerals 2018, 8, 574. [Google Scholar] [CrossRef]
- Rezania, S.; Mojiri, A.; Park, J.; Nawrot, N.; Wojciechowska, E.; Marraiki, N.; Zaghloul, N.S. Removal of lead ions from wastewater using lanthanum sulfide nanoparticle decorated over magnetic graphene oxide. Environ. Res. 2022, 204, 111959. [Google Scholar] [CrossRef]
- Mbareck, C.; Nguyen, Q.T.; Alaoui, O.T.; Barillier, D. Elaboration, characterization and application of polysulfone and polyacrylic acid blends as ultrafiltration membranes for removal of some heavy metals from water. J. Hazard. Mater. 2009, 171, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chen, J.; Wang, X. Removal of arsenic and cadmium with sequential soil washing techniques using Na 2 EDTA, oxalic and phosphoric acid: Optimization conditions, removal effectiveness and ecological risks. Chemosphere 2016, 156, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, B. Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem. Eng. J. 2013, 226, 286–292. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, L.; Huang, Y.; Song, Z.; Qiu, W. Effects of a manganese oxide-modified biochar composite on adsorption of arsenic in red soil. J. Environ. Manag. 2015, 163, 155–162. [Google Scholar] [CrossRef]
- Jeong, Y.; Fan, M.; Singh, S.; Chuang, C.-L.; Saha, B.; van Leeuwen, J.H. Evaluation of iron oxide and aluminum oxide as potential arsenic(V) adsorbents. Chem. Eng. Process. Process Intensif. 2007, 46, 1030–1039. [Google Scholar] [CrossRef]
- Goswami, A.; Raul, P.; Purkait, M. Arsenic adsorption using copper (II) oxide nanoparticles. Chem. Eng. Res. Des. 2012, 90, 1387–1396. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Sepúlveda, P.; Manquián-Cerda, K.; Ramírez-Tagle, R.; Rubio, M.A.; Bolan, N.; Sarkar, B.; Arancibia-Miranda, N. Synthesis and characterization of zeolite-based composites functionalized with nanoscale zero-valent iron for removing arsenic in the presence of selenium from water. J. Hazard. Mater. 2019, 373, 810–819. [Google Scholar] [CrossRef] [PubMed]



| Sample | Original Concentration of Arsenic (mg·L−1) | Concentration of Arsenic in Apatite Treated Water at Different pHs (after Treatment) | ||
|---|---|---|---|---|
| pH = 3 | pH = 6 | pH = 10 | ||
| G2 | 103.7 | 101.2 | 76.2 | 47.2 |
| T1 | 147.3 | 142 | 118 | 63 |
| GTSP | 170.59 | 163 | 129 | 77 |
| T15-20 | 156.67 | 147.11 | 124.8 | 82 |
| Q3 | 87.7 | 86.9 | 62.4 | 32.7 |
| Q8 | 72.32 | 72.14 | 53.7 | 29.11 |
| Q12 | 146.2 | 139 | 123 | 67 |
| Q16 | 134.5 | 130.4 | 99.4 | 60.5 |
| Models | Parameters | Arsenic |
|---|---|---|
| Pseudo first order | Qe (mg·g−1) | 18.916 |
| K1 (min−1) | 0.0018 | |
| R2 | 0.0583 | |
| Pseudo second order | Qe (mg·g−1) | 55.556 |
| K2 (g·mg−1·min−1) | 0.000324 | |
| R2 | 0.981 |
| Metal | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qm (mg·g−1) | KL (L·mg−1) | R2 | 1/n | KF (mg·L−1)−1/n | R2 | |
| Arsenic | 41.32 | 2.07 | 0.998 | 3.81 | 7.14 | 0.845 |
| Adsorbate | T(K) | Qe (mg·g−1) | ΔG (kJ/mol) | ΔH (kJ/mol·K) | ΔS (kJ/mol·K) |
|---|---|---|---|---|---|
| arsenic | 20 | 34.45 | −0.76 | 28.31 | 0.097 |
| 25 | 35.77 | −1.45 | |||
| 45 | 38.03 | −3.31 |
| Samples | Native Arsenic (ppb) | C0 (after Acidic Treatment) | Ce (after Removal) | R% |
|---|---|---|---|---|
| G2 | 103.7 | 101.2 | 19 | 81.22 |
| GTSP | 147.3 | 142 | 28 | 80.28 |
| T1 | 170.59 | 163 | 45 | 72.39 |
| T15-20 | 156.67 | 147.11 | 39 | 73.48 |
| Q3 | 87.7 | 86.9 | 11.8 | 86.42 |
| Q8 | 72.32 | 72.4 | 8.1 | 88.81 |
| Q12 | 146.2 | 139 | 33 | 76.25 |
| Q16 | 134.5 | 130.4 | 29.4 | 77.45 |
| Adsorbent | Adsorbate | Qm (mg·g−1) | Ref. |
|---|---|---|---|
| Biochar/AlOOH | Arsenic | 17.41 | [27] |
| Magnetic oxide biochar | Arsenic | 14.36 | [28] |
| Fe2O3 Al2O3 | Arsenic | 0.66 0.17 | [29] |
| Copper (II) oxide | Arsenic | 1.086 | [30] |
| Zeolite-based/ZVI | Arsenic | 38.26 | [31] |
| PES/ZVI | Arsenic | 41.32 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noorbakhsh, R.; Koohi, M.K.; Hassan, J.; Rahmani, A.; Rashidi Nodeh, H.; Rezania, S. Magnetic Beads of Zero Valent Iron Doped Polyethersolfun Developed for Removal of Arsenic from Apatite-Soil Treated Water. Int. J. Environ. Res. Public Health 2022, 19, 12697. https://doi.org/10.3390/ijerph191912697
Noorbakhsh R, Koohi MK, Hassan J, Rahmani A, Rashidi Nodeh H, Rezania S. Magnetic Beads of Zero Valent Iron Doped Polyethersolfun Developed for Removal of Arsenic from Apatite-Soil Treated Water. International Journal of Environmental Research and Public Health. 2022; 19(19):12697. https://doi.org/10.3390/ijerph191912697
Chicago/Turabian StyleNoorbakhsh, Roya, Mohammad Kazem Koohi, Jalal Hassan, Anosheh Rahmani, Hamid Rashidi Nodeh, and Shahabaldin Rezania. 2022. "Magnetic Beads of Zero Valent Iron Doped Polyethersolfun Developed for Removal of Arsenic from Apatite-Soil Treated Water" International Journal of Environmental Research and Public Health 19, no. 19: 12697. https://doi.org/10.3390/ijerph191912697
APA StyleNoorbakhsh, R., Koohi, M. K., Hassan, J., Rahmani, A., Rashidi Nodeh, H., & Rezania, S. (2022). Magnetic Beads of Zero Valent Iron Doped Polyethersolfun Developed for Removal of Arsenic from Apatite-Soil Treated Water. International Journal of Environmental Research and Public Health, 19(19), 12697. https://doi.org/10.3390/ijerph191912697








