Emissions of Fungal Volatile Organic Compounds in Residential Environments and Temporal Emission Patterns: Implications for Sampling Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chamber Experiments
2.2. Field Experiments
2.3. Chemical Analysis of mVOCs (Chamber Experiments)
2.4. Statistical Analyses
3. Results
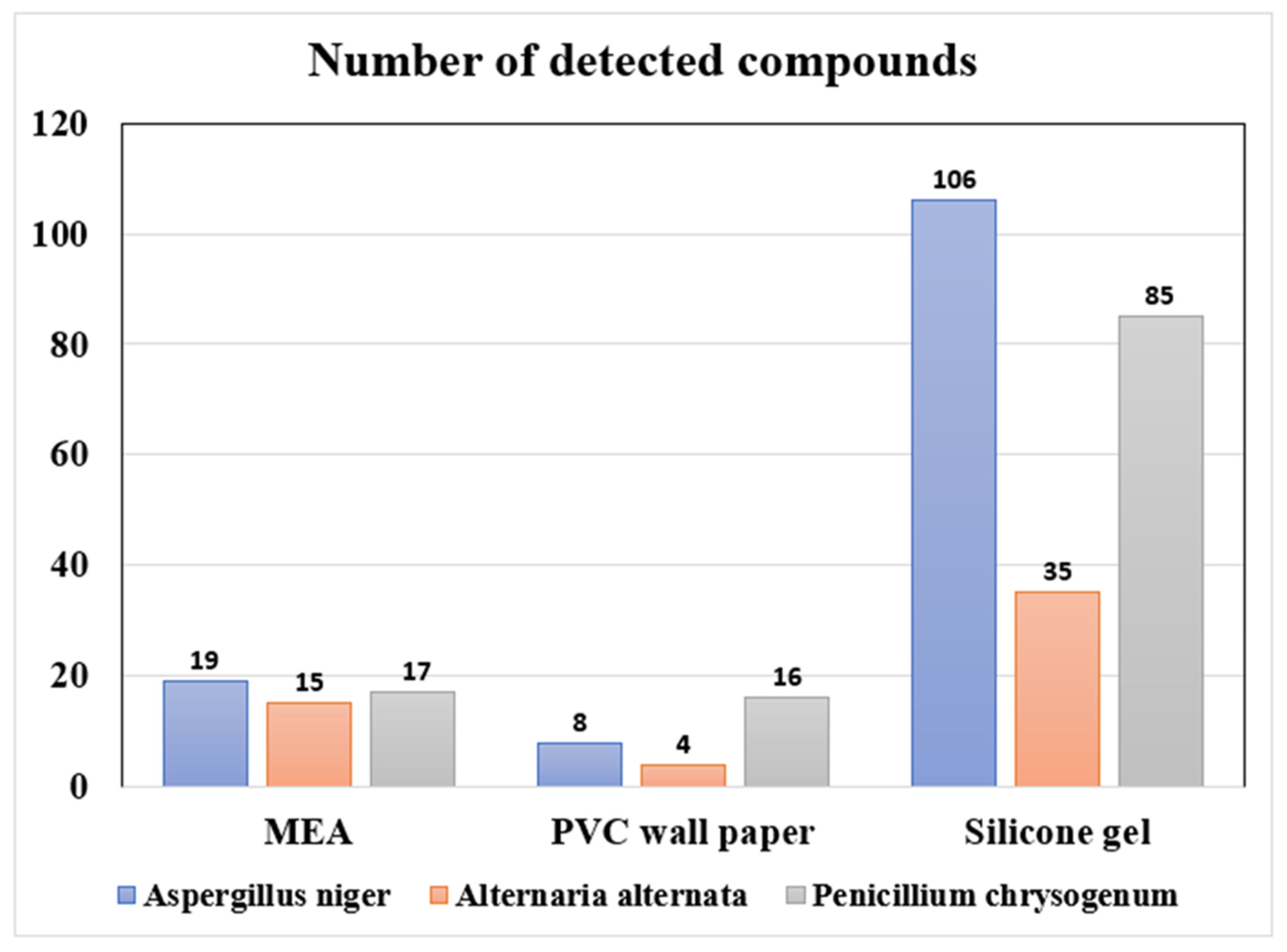
3.1. Detection of mVOCs from Fungi in the Chamber Experiments
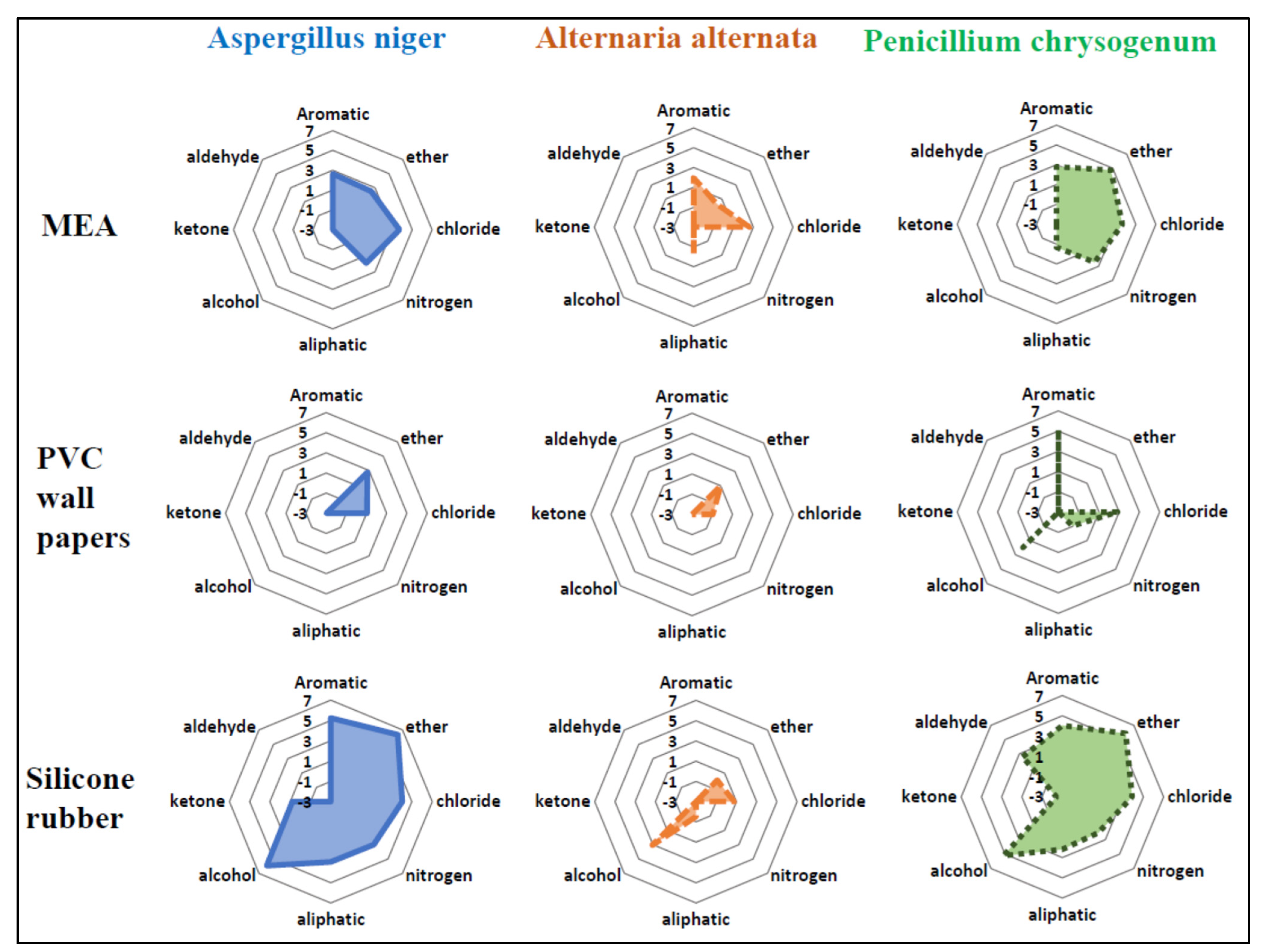
3.2. Emission Pattern of Individual Classes
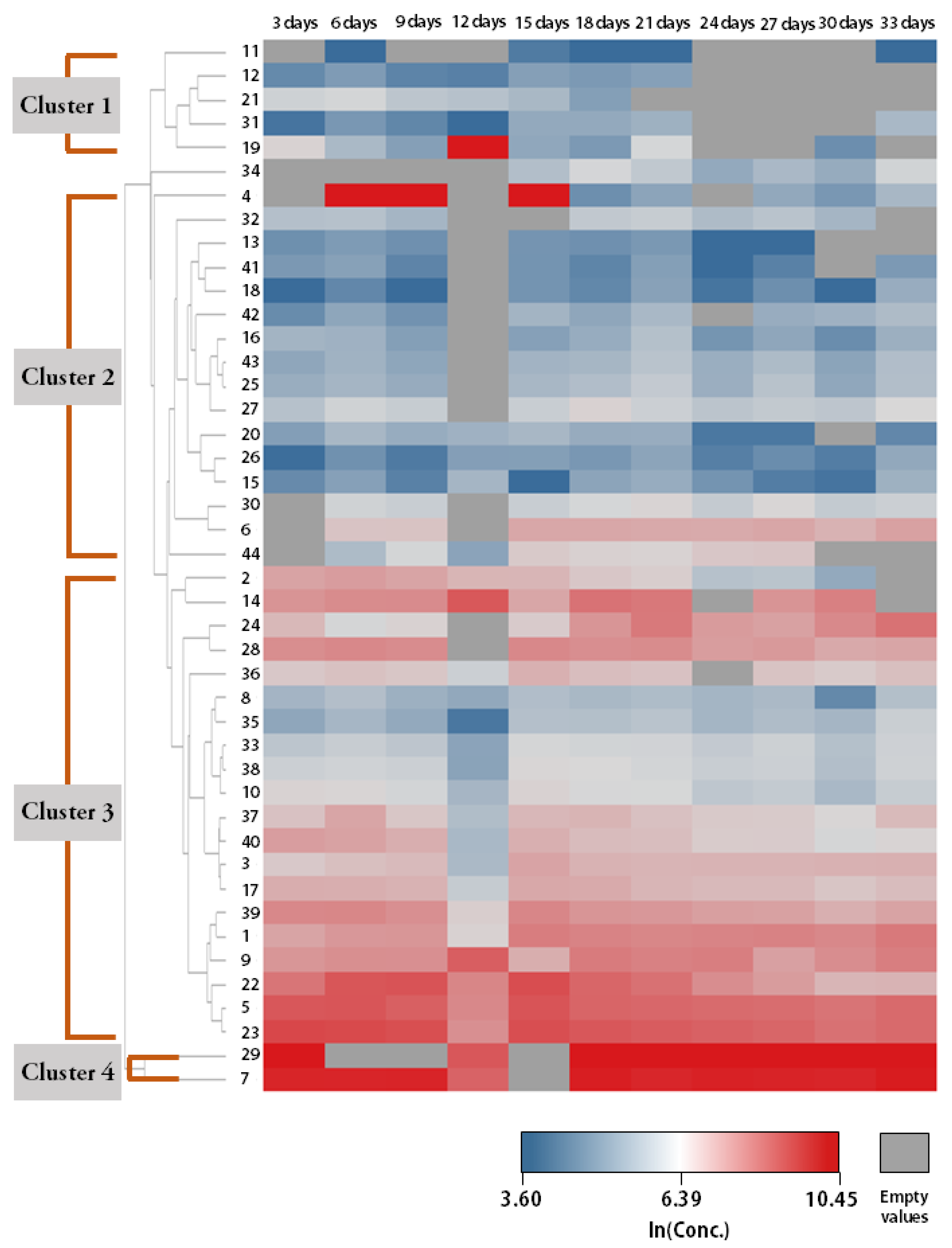
3.3. Temporal Patterns of mVOCs Emission
3.4. Occurences of mVOCs in Residential Environments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.H.; Margni, M.D.; McKone, T.E.; Jolliet, O. Intake fraction for multimedia pollutants: A tool for life cycle analysis and comparative risk assessment. Risk Anal. 2002, 22, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Nazaroff, W.W. Inhalation intake fraction of pollutants from episodic indoor emissions. Build. Environ. 2008, 43, 269–277. [Google Scholar] [CrossRef]
- Shin, H.M.; McKone, T.E.; Bennett, D.H. Intake Fraction for the Indoor Environment: A Tool for Prioritizing Indoor Chemical Sources. Environ. Sci. Technol. 2012, 46, 10063–10072. [Google Scholar] [CrossRef] [PubMed]
- Rosch, C.; Kohajda, T.; Roder, S.; von Bergen, M.; Schlink, U. Relationship between sources and patterns of VOCs in indoor air. Atmos. Pollut. Res. 2014, 5, 129–137. [Google Scholar] [CrossRef]
- Bari, M.A.; Kindzierski, W.B.; Wheeler, A.J.; Heroux, M.E.; Wallace, L.A. Source apportionment of indoor and outdoor volatile organic compounds at homes in Edmonton, Canada. Build. Environ. 2015, 90, 114–124. [Google Scholar] [CrossRef]
- Korpi, A.; Jarnberg, J.; Pasanen, A.L. Microbial Volatile Organic Compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef]
- Little, J.C.; Weschler, C.J.; Nazaroff, W.W.; Liu, Z.; Hubal, E.A.C. Rapid Methods to Estimate Potential Exposure to Semivolatile Organic Compounds in the Indoor Environment. Environ. Sci. Technol. 2012, 46, 11171–11178. [Google Scholar] [CrossRef]
- Weschler, C.J.; Nazaroff, W.W. Semivolatile organic compounds in indoor environments. Atmos. Environ. 2008, 42, 9018–9040. [Google Scholar] [CrossRef]
- Li, L.; Arnot, J.A.; Wania, F. How are Humans Exposed to Organic Chemicals Released to Indoor Air? Environ. Sci. Technol. 2019, 53, 11276–11284. [Google Scholar] [CrossRef]
- Mitro, S.D.; Dodson, R.E.; Singla, V.; Adamkiewicz, G.; Elmi, A.F.; Tilly, M.K.; Zota, A.R. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-Analysis of U.S. Studies (vol 50, pg 10661, 2016). Environ. Sci. Technol. 2016, 50, 13611. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence. Environ. Health Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Speckbacher, V.; Zeilinger, S.; Zimmermann, S.; Mayhew, C.A.; Wiesenhofer, H.; Ruzsanyi, V. Monitoring the volatile language of fungi using gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2021, 413, 3055–3067. [Google Scholar] [CrossRef]
- Walinder, R.; Ernstgard, L.; Johanson, G.; Norback, D.; Venge, P.; Wieslander, G. Acute effects of a fungal volatile compound. Environ. Health Perspect. 2005, 113, 1775–1778. [Google Scholar] [CrossRef]
- Meheust, D.; Le Cann, P.; Reboux, G.; Millon, L.; Gangneux, J.P. Indoor fungal contamination: Health risks and measurement methods in hospitals, homes and workplaces. Crit. Rev. Microbiol. 2014, 40, 248–260. [Google Scholar] [CrossRef]
- Rose, L.J.; Simmons, R.B.; Crow, S.A.; Ahearn, D.G. Volatile organic compounds associated with microbial growth in automobile air conditioning systems. Curr. Microbiol. 2000, 41, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Sahlberg, B.; Gunnbjornsdottir, M.; Soon, A.; Jogi, R.; Gislason, T.; Wieslander, G.; Janson, C.; Norback, D. Airborne molds and bacteria, microbial volatile organic compounds (MVOC), plasticizers and formaldehyde in dwellings in three North European cities in relation to sick building syndrome (SBS). Sci. Total Environ. 2013, 444, 433–440. [Google Scholar] [CrossRef]
- Khan, A.A.H.; Karuppayil, S.M. Practices contributing to biotic pollution in Air-conditioned indoor environments. Aerobiologia 2011, 27, 85–89. [Google Scholar] [CrossRef]
- Khan, A.A.H.; Karuppayil, S.M. Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef]
- Koch, A.; Heilemann, K.J.; Bischof, W.; Heinrich, J.; Wichmann, H.E. Indoor viable mold spores—A comparison between two cities, Erfurt (eastern Germany) and Hamburg (western Germany). Allergy 2000, 55, 176–180. [Google Scholar] [CrossRef]
- Noris, F.; Siegel, J.A.; Kinney, K.A. Evaluation of HVAC filters as a sampling mechanism for indoor microbial communities. Atmos. Environ. 2011, 45, 338–346. [Google Scholar] [CrossRef]
- Leow, M.E.L.; Kour, A.K.; Inglis, T.J.J.; Kumarasinghe, G.; Pho, R.W.H. Fungal colonisation in digital silicone rubber prostheses. Prosthet. Orthot. Int. 1997, 21, 195–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fiedler, K.; Schutz, E.; Geh, S. Detection of microbial volatile organic compounds (MVOCs) produced by moulds on various materials. Int. J. Hyg. Environ. 2001, 204, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Radványi, D. Detection and Identification of Microbial Volatile Organic Compounds of the Green Mold Disease: MVOC Profile on Different Media. Int. J. Agric. Environ. Inf. Syst. 2020, 11, 14–28. [Google Scholar] [CrossRef]
- Araki, A.; Eitaki, Y.; Kawai, T.; Kanazawa, A.; Takeda, M.; Kishi, R. Diffusive sampling and measurement of microbial volatile organic compounds in indoor air. Indoor Air 2009, 19, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Matysik, S.; Herbarth, O.; Mueller, A. Determination of microbial volatile organic compounds (MVOCs) by passive sampling onto charcoal sorbents. Chemosphere 2009, 76, 114–119. [Google Scholar] [CrossRef]
- Huang, C.Y.; Shan, W.P.; Xiao, H. Recent Advances in Passive Air Sampling of Volatile Organic Compounds. Aerosol Air Qual. Res. 2018, 18, 602–622. [Google Scholar] [CrossRef]
- Kuske, M.; Romain, A.C.; Nicolas, J. Microbial volatile organic compounds as indicators of fungi. Can an electronic nose detect fungi in indoor environments? Build. Environ. 2005, 40, 824–831. [Google Scholar] [CrossRef]
- Lee, S. Characteristics of Airborne Fungi-Derived VOCs in Residential Areas. Seoul National University of Science and Technology: Seoul, Korea, 2017. [Google Scholar]
- Neu, L.; Banziger, C.; Proctor, C.R.; Zhang, Y.; Liu, W.T.; Hammes, F. Ugly ducklings-the dark side of plastic materials in contact with potable water. Npj Biofilms Microbiomes 2018, 4, 7. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Miettinen, K.T.; Keinanen, M.M.; Kekki, T.K.; Laine, O.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. Microbiology, chemistry and biofilm development in a pilot drinking water distribution system with copper and plastic pipes. Water Res. 2004, 38, 3769–3779. [Google Scholar] [CrossRef]
- Yu, J.; Kim, D.; Lee, T. Microbial diversity in biofilms on water distribution pipes of different materials. Water Sci. Technol. 2010, 61, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Kristensen, K.; Lunderberg, D.M.; Liu, Y.J.; Misztal, P.K.; Tian, Y.L.; Arata, C.L.; Nazaroff, W.W.; Goldstein, A.H. Sources and dynamics of semivolatile organic compounds in a single-family residence in northern California. Indoor Air 2019, 29, 645–655. [Google Scholar] [CrossRef]
- Dodson, R.E.; Bessonneau, V.; Udesky, J.O.; Nishioka, M.; McCauley, M.; Rudel, R.A. Passive indoor air sampling for consumer product chemicals: A field evaluation study. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Tuazon, E.C.; Aschmann, S.M. Atmospheric chemistry of 2-pentanone and 2-heptanone. Environ. Sci. Technol. 2000, 34, 623–631. [Google Scholar] [CrossRef]
- Helmig, D.; Klinger, L.F.; Guenther, A.; Vierling, L.; Geron, C.; Zimmerman, P. Biogenic volatile organic compound emissions (BVOCs) I. Identifications from three continental sites in the US. Chemosphere 1999, 38, 2163–2187. [Google Scholar] [CrossRef]
- Wilkins, K.; Nielsen, E.M.; Wolkoff, P. Patterns in volatile organic compounds in dust from moldy buildings. Indoor Air 1997, 7, 128–134. [Google Scholar] [CrossRef]
- Kim, K.; Shin, H.M.; Wong, L.; Young, T.M.; Bennett, D.H. Temporal variability of indoor dust concentrations of semivolatile organic compounds. Indoor Air 2021, 31, 693–701. [Google Scholar] [CrossRef]
- Wei, W.J.; Ramalho, O.; Mandin, C. A long-term dynamic model for predicting the concentration of semivolatile organic compounds in indoor environments: Application to phthalates. Build. Environ. 2019, 148, 11–19. [Google Scholar] [CrossRef]
- Dodson, R.E.; Camann, D.E.; Morello-Frosch, R.; Brody, J.G.; Rudel, R.A. Semivolatile Organic Compounds in Homes: Strategies for Efficient and Systematic Exposure Measurement Based on Empirical and Theoretical Factors. Environ. Sci. Technol. 2015, 49, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.H.; Furtaw, E.J. Fugacity-based indoor residential pesticide fate model. Environ. Sci. Technol. 2004, 38, 4912. [Google Scholar] [CrossRef]
| Compounds | Detection Frequency (%) | ||
|---|---|---|---|
| Aspergillus niger | Alternaria alternata | Penicillium chrysogenum | |
| MEA/Wallpaper/Silicone Rubber | MEA/Wallpaper/Silicone Rubber | MEA/Wallpaper/Silicone Rubber | |
| 1,1,2,2-Tetrachloroethane | 100/70/100 | - * | 100/100/91 |
| Benzene | - * | - * | 90/41/91 |
| Ethyl methacrylate | 40/80/100 | - * | 30/100/91 |
| Ethylene dichloride | 100/80/91 | 90/70/91 | 100/100/91 |
| Isobutyronitrile | - * | - * | 90/50/91 |
| Methyl isobutyrate | 50/50/91 | - * | 100/100/91 |
| Methyl methacrylate | 100/90/73 | 70/100/91 | 100/100/91 |
| Methyl propionate | 30/10/82 | - * | - * |
| Methylacrylonitrile | - * | - * | 90/40/82 |
| Styrene | - * | - * | 100/70/91 |
| Compounds | Active Sampling | Passive Sampling | ||||
|---|---|---|---|---|---|---|
| DF a (Winter) | DF a (Summer) | r b | DF a (Winter) | DF a (Summer) | r b | |
| 2-Butanone | 27 | 9 | - | - c | - | - |
| 2-Butoxyethanol | - | 9 | - | - | - | - |
| 2-Ethyl-1-hexanol | 36 | 55 | −0.04 | 18 | 27 | 0.08 |
| Acetophenone | 45 | - | −0.81 | - | - | - |
| Benzene | 82 | 50 | −0.35 | 100 | 82 | 0.56 * |
| Chloroform | 45 | - | - | - | - | −0.04 |
| Dimethyl disulfide | 55 | - | 0.73 | 9 | - | - |
| Ethanol | - | 9 | - | 55 | 36 | 0.23 |
| Ethyl acetate | 73 | 45 | 0.37 | - | - | - |
| Ethylbenzene | 82 | 81 | −0.02 | 82 | 82 | 0.58 * |
| Isoprene | 73 | - | - | 45 | - | 0.73 |
| Isopropanol | - | - | - | 9 | - | - |
| n-Butanol | - | - | 0.90 * | - | - | - |
| n-Decane | 45 | 9 | −0.32 | 36 | - | −0.28 |
| Nitrophenyloxadiazolol | 9 | 18 | - | 54 | 36 | 0.86 * |
| n-Octane | 89 | 29 | - | 91 | - | - |
| o,m-Xylene | 100 | 81 | - | 82 | 91 | 0.46 * |
| Styrene | 82 | 44 | −0.26 | 62 | - | 0.41 |
| Toluene | 82 | 88 | −0.17 | 100 | 100 | 0.46 * |
| Trichloroethylene | - | - | −0.99 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Lee, S.; Choi, Y.; Kim, D. Emissions of Fungal Volatile Organic Compounds in Residential Environments and Temporal Emission Patterns: Implications for Sampling Methods. Int. J. Environ. Res. Public Health 2022, 19, 12601. https://doi.org/10.3390/ijerph191912601
Kim K, Lee S, Choi Y, Kim D. Emissions of Fungal Volatile Organic Compounds in Residential Environments and Temporal Emission Patterns: Implications for Sampling Methods. International Journal of Environmental Research and Public Health. 2022; 19(19):12601. https://doi.org/10.3390/ijerph191912601
Chicago/Turabian StyleKim, Kyunghoon, Suyeon Lee, Yelim Choi, and Daekeun Kim. 2022. "Emissions of Fungal Volatile Organic Compounds in Residential Environments and Temporal Emission Patterns: Implications for Sampling Methods" International Journal of Environmental Research and Public Health 19, no. 19: 12601. https://doi.org/10.3390/ijerph191912601
APA StyleKim, K., Lee, S., Choi, Y., & Kim, D. (2022). Emissions of Fungal Volatile Organic Compounds in Residential Environments and Temporal Emission Patterns: Implications for Sampling Methods. International Journal of Environmental Research and Public Health, 19(19), 12601. https://doi.org/10.3390/ijerph191912601







