Grafting of R4N+-Bearing Organosilane on Kaolinite, Montmorillonite, and Zeolite for Simultaneous Adsorption of Ammonium and Nitrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
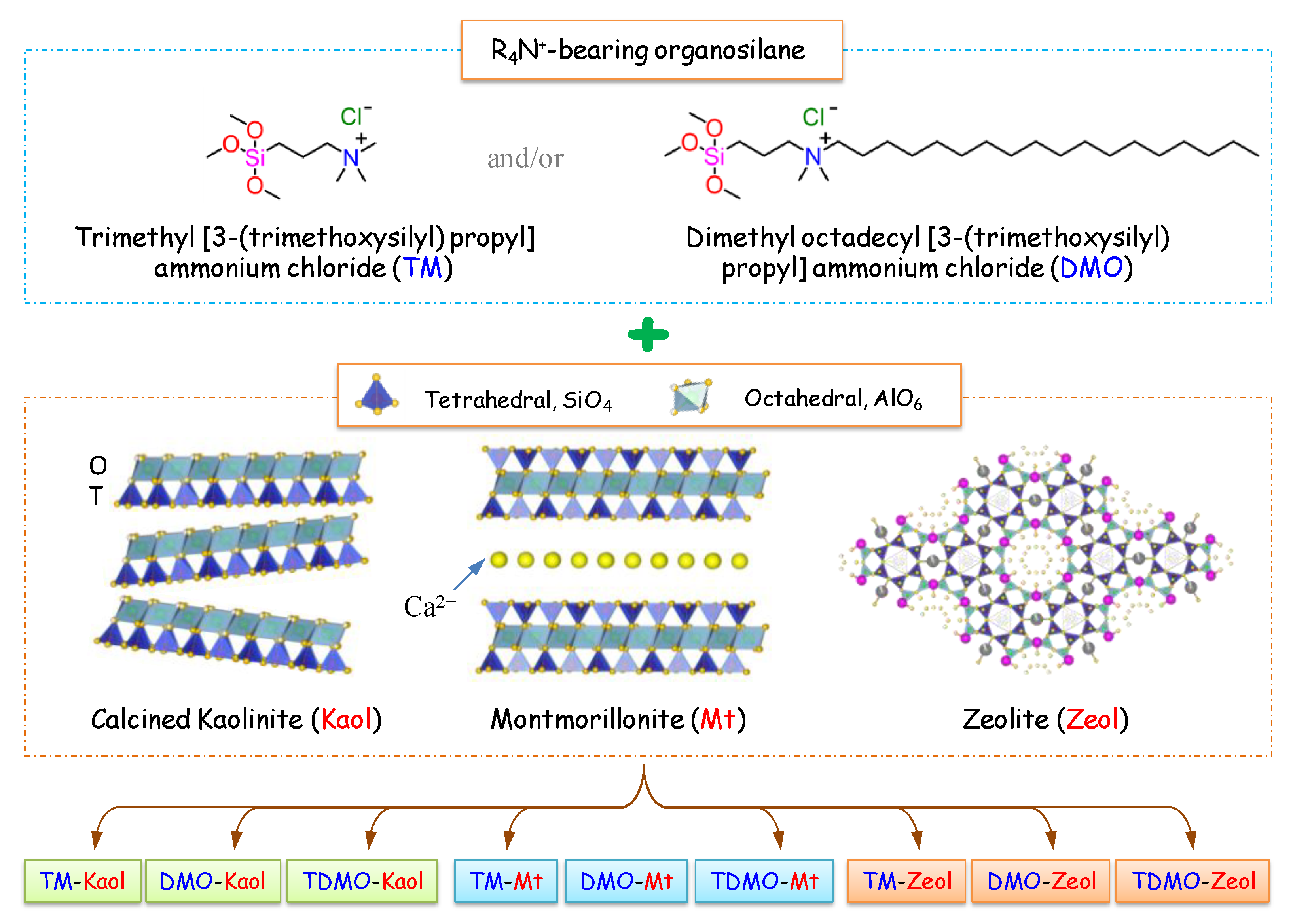
2.2. Preparation of the Grafted Kaol, Mt, and Zeol
2.3. Solid Characterization
2.4. Batch Adsorption Experiment
3. Results and Discussion
3.1. Solid Characterization
3.1.1. X-ray Diffraction
3.1.2. Thermal Gravimetric Analysis
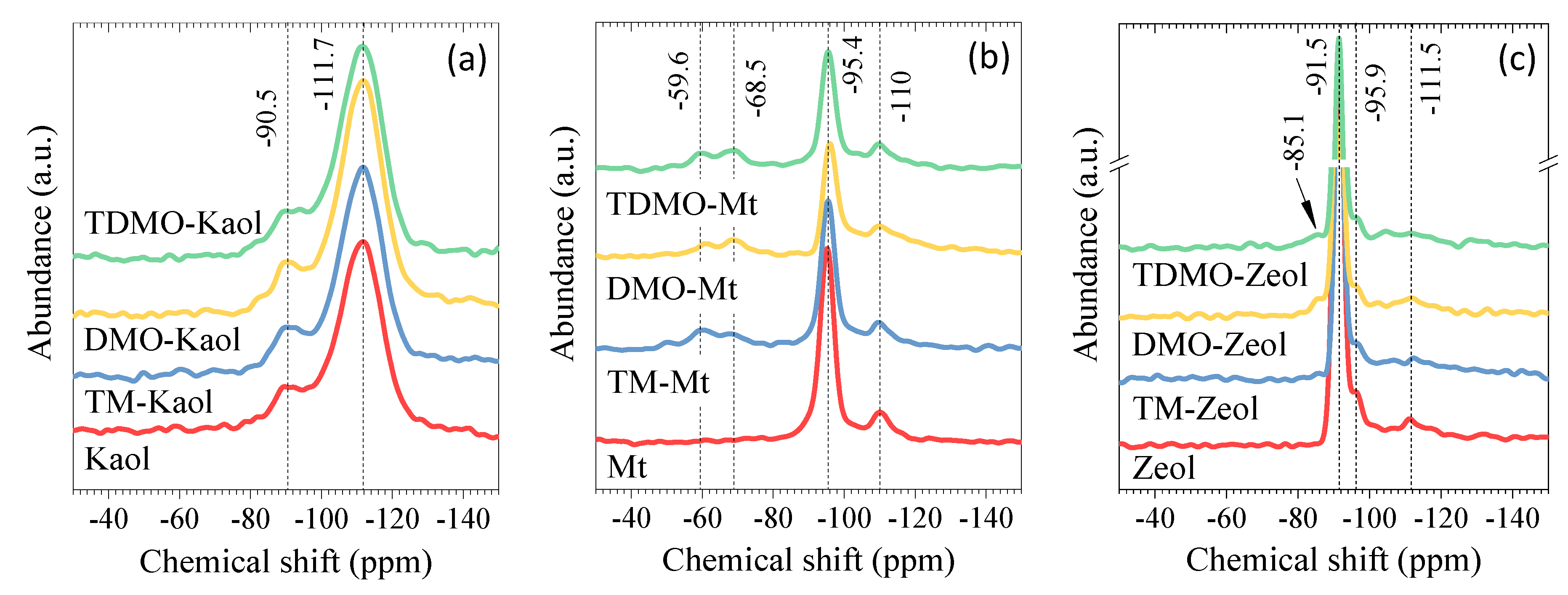
3.1.3. Solid-State Nuclear Magnetic Resonance
3.2. Adsorption Characteristics
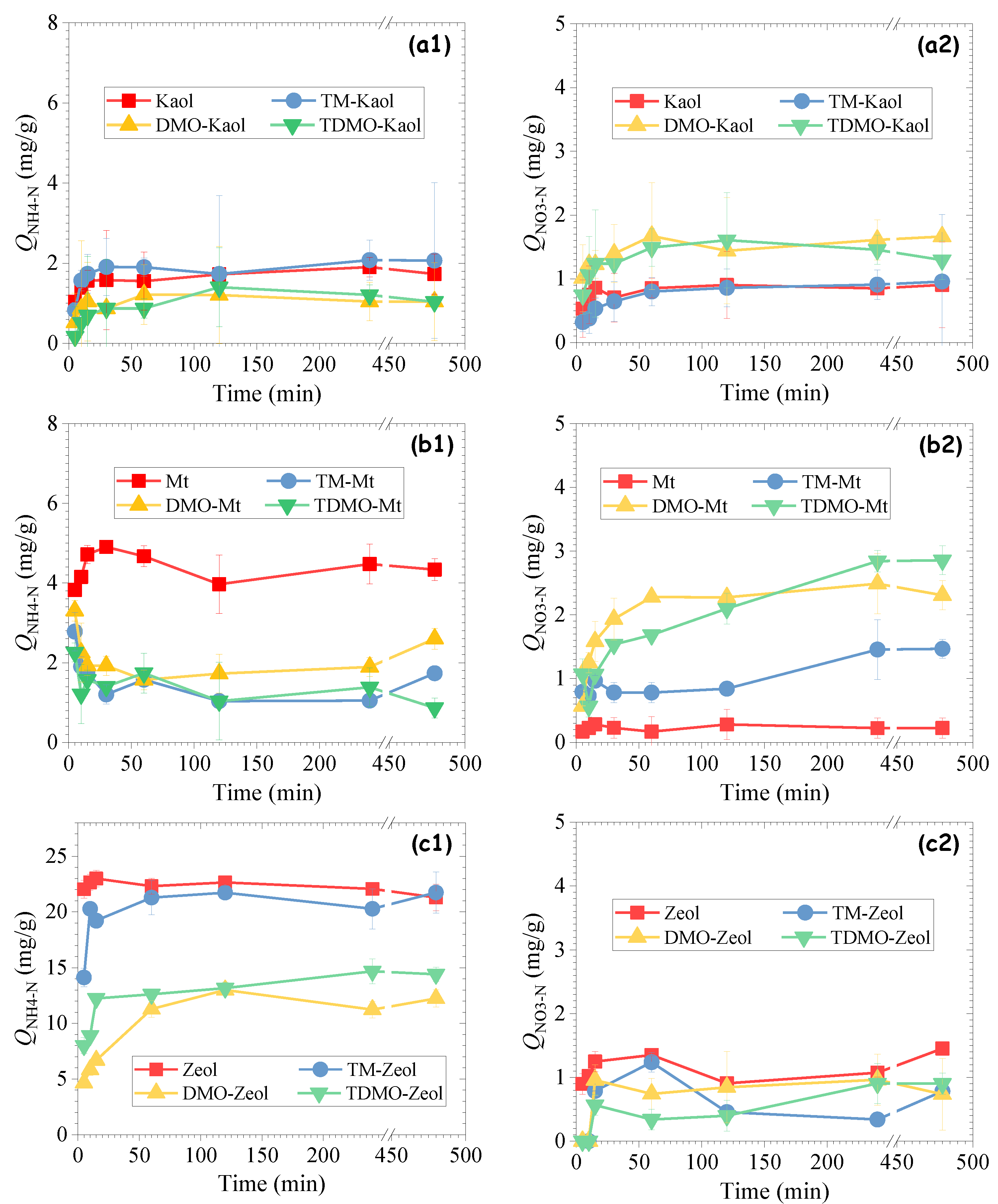
3.2.1. Influence of Contact Time
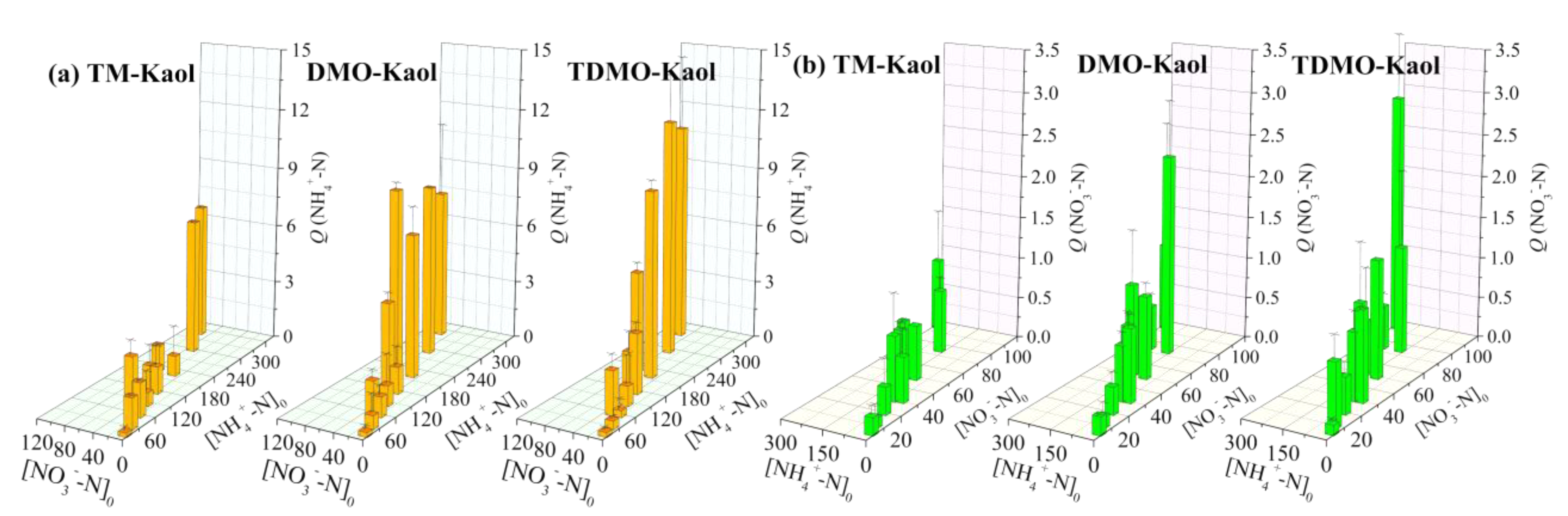
3.2.2. Influence of the Initial Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shabtai, I.A.; Lynch, L.M.; Mishael, Y.G. Designing clay-polymer nanocomposite sorbents for water treatment: A review and meta-analysis of the past decade. Water Res. 2021, 188, 116571. [Google Scholar] [CrossRef] [PubMed]
- Padil, V.V.T.; Kumar, K.P.A.; Murugesan, S.; Torres-Mendieta, R.; Wacławek, S.; Cheong, J.Y.; Černík, M.; Varma, R.S. Sustainable and safer nanoclay composites for multifaceted applications. Green Chem. 2022, 24, 3081–3114. [Google Scholar] [CrossRef]
- Phuekphong, A.F.; Imwiset, K.J.; Ogawa, M. Designing nanoarchitecture for environmental remediation based on the clay minerals as building block. J. Hazard. Mater. 2020, 399, 122888. [Google Scholar] [CrossRef] [PubMed]
- Atkin, R.; Craig, V.S.J.; Wanless, E.J.; Biggs, S. Mechanism of cationic surfactant adsorption at the solid–aqueous interface. Adv. Colloid Interface Sci. 2003, 103, 219–304. [Google Scholar] [CrossRef]
- He, H.; Tao, Q.; Zhu, J.; Yuan, P.; Shen, W.; Yang, S. Silylation of clay mineral surfaces. Appl. Clay Sci. 2013, 71, 15–20. [Google Scholar] [CrossRef]
- Luo, W.; Hirajima, T.; Sasaki, K. Selective adsorption of inorganic anions on unwashed and washed hexadecyl pyridinium-modified montmorillonite. Sep. Purif. Technol. 2017, 176, 120–125. [Google Scholar] [CrossRef]
- Luo, W.; Ouyang, J.; Antwi, P.; Wu, M.; Huang, Z.; Qin, W. Microwave/ultrasound-assisted modification of montmorillonite by conventional and gemini alkyl quaternary ammonium salts for adsorption of chromate and phenol: Structure-function relationship. Sci. Total Environ. 2019, 655, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kancharla, S.; Sasaki, K. Synthesis and characterization of imidazole-bearing polymer-modified montmorillonite for adsorption of perchlorate. Appl. Clay Sci. 2020, 199, 105859. [Google Scholar] [CrossRef]
- Luo, W.; Inoue, A.; Hirajima, T.; Sasaki, K. Synergistic effect of Sr2+ and ReO4− adsorption on hexadecyl pyridinium-modified montmorillonite. Appl. Surf. Sci. 2017, 394, 431–439. [Google Scholar] [CrossRef]
- Thiebault, T.; Brendlé, J.; Auge, G.; Limousy, L. Cleaner synthesis of silylated clay minerals for the durable recovery of ions (Co2+ and Sr2+) from aqueous solutions. Ind. Eng. Chem. Res. 2020, 59, 2104–2112. [Google Scholar] [CrossRef]
- Guimarães, A.d.M.F.; Ciminelli, V.S.T.; Vasconcelos, W.L. Smectite organofunctionalized with thiol groups for adsorption of heavy metal ions. Appl. Clay Sci. 2009, 42, 410–414. [Google Scholar]
- Li, Z.; Pan, Z.; Wang, Y. Enhanced adsorption of cationic Pb (II) and anionic Cr (VI) ions in aqueous solution by amino-modified nano-sized illite-smectite clay. Environ. Sci. Pollut. Res. 2019, 26, 11126–11139. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, X.; Mamadiev, M.; Zhao, C.; Wang, Z.; Xu, H. Synthesis of interstratified graphene/montmorillonite composite material through organics-pillared, delamination and co-stacking and its application in hexavalent chromium removal from aqueous solution. Adv. Powder Technol. 2017, 28, 521–533. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Hirotsu, T.; Sonoda, A. Montmorillonite modified with hexadecylpyridinium chloride as highly efficient anion exchanger for perchlorate ion. Chem. Eng. J. 2012, 191, 141–146. [Google Scholar] [CrossRef]
- Yang, J.; Shi, K.; Gao, X.; Hou, X.; Wu, W.; Shi, W. Hexadecylpyridinium (HDPy) modified bentonite for efficient and selective removal of 99Tc from wastewater. Chem. Eng. J. 2020, 382, 122894. [Google Scholar] [CrossRef]
- Bagherifam, S.; Komarneni, S.; Lakzian, A.; Fotovat, A.; Khorasani, R.; Huang, W.; Ma, J.; Hong, S.; Cannon, F.S.; Wang, Y. Highly selective removal of nitrate and perchlorate by organoclay. Appl. Clay Sci. 2014, 95, 126–132. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.; Chen, T.; Chen, D.; Frost, R.L. An insight into the removal of Pb (II), Cu (II), Co (II), Cd (II), Zn (II), Ag (I), Hg (I), Cr (VI) by Na (I)-montmorillonite and Ca (II)-montmorillonite. Appl. Clay Sci. 2015, 118, 239–247. [Google Scholar] [CrossRef]
- Funes, I.G.A.; Peralta, M.E.; Pettinari, G.R.; Carlos, L.; Parolo, M.E. Facile modification of montmorillonite by intercalation and grafting: The study of the binding mechanisms of a quaternary alkylammonium surfactant. Appl. Clay Sci. 2020, 195, 105738. [Google Scholar] [CrossRef]
- Dedzo, G.K.; Rigolet, S.; Josien, L.; Ngameni, E.; Dzene, L. Functionalization of synthetic saponite: Identification of grafting sites and application for anions sequestration. Appl. Surf. Sci. 2021, 567, 150911. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, H. Controlling and tuning the dispersion properties of calcined kaolinite particles in various organic solvents via stepwise modification method using 3-glycidoxypropyltrimethoxysilane and dodecylamine. Appl. Surf. Sci. 2013, 277, 281–287. [Google Scholar] [CrossRef]
- Umezawa, Y.; Hosono, T.; Onodera, S.-I.; Siringan, F.; Buapeng, S.; Delinom, R.; Yoshimizu, C.; Tayasu, I.; Nagata, T.; Taniguchi, M. Sources of nitrate and ammonium contamination in groundwater under developing Asian megacities. Sci. Total Environ. 2008, 404, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Follett, R.F.; Hatfield, J.L. Nitrogen in the environment: Sources, problems, and management. Sci. World J. 2001, 1, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Constable, M.; Charlton, M.; Jensen, F.; McDonald, K.; Craig, G.; Taylor, K.W. An ecological risk assessment of ammonia in the aquatic environment. Hum. Ecol. Risk Assess. 2003, 9, 527–548. [Google Scholar] [CrossRef]
- Piscitelli, F.; Posocco, P.; Toth, R.; Fermeglia, M.; Pricl, S.; Mensitieri, G.; Lavorgna, M. Sodium montmorillonite silylation: Unexpected effect of the aminosilane chain length. J. Colloid Interface Sci. 2010, 351, 108–115. [Google Scholar] [CrossRef]
- Peixoto, A.F.; Fernandes, A.C.; Pereira, C.; Pires, J.; Freire, C. Physicochemical characterization of organosilylated halloysite clay nanotubes. Microporous Mesoporous Mater. 2016, 219, 145–154. [Google Scholar] [CrossRef]
- Ren, H.; Tian, S.; Zhu, M.; Zhao, Y.; Li, K.; Ma, Q.; Ding, S.; Gao, J.; Miao, Z. Modification of montmorillonite by Gemini surfactants with different chain lengths and its adsorption behavior for methyl orange. Appl. Clay Sci. 2018, 151, 29–36. [Google Scholar] [CrossRef]
- Saeed, M.; Munir, M.; Nafees, M.; Shah, S.S.A.; Ullah, H.; Waseem, A. Synthesis, characterization and applications of silylation based grafted bentonites for the removal of Sudan dyes: Isothermal, kinetic and thermodynamic studies. Microporous Mesoporous Mater. 2020, 291, 109697. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Q.; Cheng, H. Recent advances in kaolinite-based material for photocatalysts. Chin. Chem. Lett. 2021, 32, 2617–2628. [Google Scholar] [CrossRef]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- He, H.; Frost, R.L.; Bostrom, T.; Yuan, P.; Duong, L.; Yang, D.; Xi, Y.; Kloprogge, J.T. Changes in the morphology of organoclays with HDTMA+ surfactant loading. Appl. Clay Sci. 2006, 31, 262–271. [Google Scholar] [CrossRef]
- Luo, W.; Huang, Q.; Zeng, P.; Cheng, C.; Yuan, X.; Xiao, T.; Zhang, M.; Antwi, P.; Xing, J.; Ren, S. Gemini surfactant-modified montmorillonite with tetrachloroferrate (FeCl4−) as a counterion simultaneously sequesters nitrate and phosphate from aqueous solution. J. Hazard. Mater. 2021, 409, 124829. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, X.; Yang, B.; Sun, R. Rapid modification of montmorillonite with novel cationic Gemini surfactants and its adsorption for methyl orange. Mater. Chem. Phys. 2011, 130, 1220–1226. [Google Scholar] [CrossRef]
- Luo, W.; Inoue, A.; Hirajima, T.; Sasaki, K. Sequential modification of montmorillonite with dimethyl dioctadecyl ammonium chloride and benzyl octadecyl dimethyl ammonium chloride for removal of perchlorate. Microporous Mesoporous Mater. 2016, 233, 117–124. [Google Scholar] [CrossRef]
- Luo, W.; Sasaki, K.; Hirajima, T. Influence of the pre-dispersion of montmorillonite on organic modification and the adsorption of perchlorate and methyl red anions. Appl. Clay Sci. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Xi, Y.; Frost, R.L.; He, H.; Kloprogge, T.; Bostrom, T. Modification of Wyoming montmorillonite surfaces using a cationic surfactant. Langmuir 2005, 21, 8675–8680. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.R.; Jia, D.C.; He, P.G.; Zhou, Y. Influence of calcination temperature of kaolin on the structure and properties of final geopolymer. Mater. Lett. 2010, 64, 2551–2554. [Google Scholar] [CrossRef]
- Kornas, A.; Olszówka, J.E.; Urbanova, M.; Mlekodaj, K.; Brabec, L.; Rathousky, J.; Dedecek, J.; Pashkova, V. Milling Activation for the Solvent-Free Synthesis of the Zeolite Mordenite. Eur. J. Inorg. Chem. 2020, 2020, 2791–2797. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Qi, X.; Liu, R.; Chen, M.; Li, Z.; Qin, T.; Qian, Y.; Zhao, S.; Liu, M.; Zeng, Q.; Shen, J. Removal of copper ions from water using polysaccharide-constructed hydrogels. Carbohydr. Polym. 2019, 209, 101–110. [Google Scholar] [CrossRef]
- Luukkonen, T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J.; Lassi, U. Metakaolin geopolymer characterization and application for ammonium removal from model solutions and landfill leachate. Appl. Clay Sci. 2016, 119, 266–276. [Google Scholar] [CrossRef]
- El-Shafey, O.I.; Fathy, N.A.; El-Nabarawy, T.A. Sorption of ammonium ions onto natural and modified Egyptian kaolinites: Kinetic and equilibrium studies. Adv. Phys. Chem. 2014, 2014, 935854. [Google Scholar] [CrossRef]
- Matusik, J. Arsenate, orthophosphate, sulfate, and nitrate sorption equilibria and kinetics for halloysite and kaolinites with an induced positive charge. Chem. Eng. J. 2014, 246, 244–253. [Google Scholar] [CrossRef]
- Meleshyn, A.; Bunnenberg, C. Interlayer expansion and mechanisms of anion sorption of Na-montmorillonite modified by cetylpyridinium chloride: A Monte Carlo study. J. Phys. Chem. B 2006, 110, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.-J.; Tay, J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef]
- Kithome, M.; Paul, J.W.; Lavkulich, L.M.; Bomke, A.A. Kinetics of ammonium adsorption and desorption by the natural zeolite clinoptilolite. Soil Sci. Soc. Am. J. 1998, 62, 622–629. [Google Scholar] [CrossRef]




| Sample | SiO2 | Al2O3 | MgO | CaO | Fe2O3 | Na2O | K2O | TiO2 |
|---|---|---|---|---|---|---|---|---|
| Kaol | 44.88 | 44.03 | 0.11 | 0.25 | 0.41 | 0.21 | 0.13 | 1.39 |
| Mt | 67.06 | 16.26 | 3.80 | 2.47 | 1.94 | 0.23 | 0.55 | / |
| Zeol | 34.36 | 28.50 | 0.28 | 0.15 | 0.01 | 17.67 | 0.07 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Cui, Z.; Fu, H.; Cao, H.; Chen, M.; Zhang, D.; Luo, W.; Ren, S. Grafting of R4N+-Bearing Organosilane on Kaolinite, Montmorillonite, and Zeolite for Simultaneous Adsorption of Ammonium and Nitrate. Int. J. Environ. Res. Public Health 2022, 19, 12562. https://doi.org/10.3390/ijerph191912562
Peng W, Cui Z, Fu H, Cao H, Chen M, Zhang D, Luo W, Ren S. Grafting of R4N+-Bearing Organosilane on Kaolinite, Montmorillonite, and Zeolite for Simultaneous Adsorption of Ammonium and Nitrate. International Journal of Environmental Research and Public Health. 2022; 19(19):12562. https://doi.org/10.3390/ijerph191912562
Chicago/Turabian StylePeng, Wang, Zhanpeng Cui, Hongyan Fu, Hongkai Cao, Ming Chen, Dachao Zhang, Wuhui Luo, and Sili Ren. 2022. "Grafting of R4N+-Bearing Organosilane on Kaolinite, Montmorillonite, and Zeolite for Simultaneous Adsorption of Ammonium and Nitrate" International Journal of Environmental Research and Public Health 19, no. 19: 12562. https://doi.org/10.3390/ijerph191912562
APA StylePeng, W., Cui, Z., Fu, H., Cao, H., Chen, M., Zhang, D., Luo, W., & Ren, S. (2022). Grafting of R4N+-Bearing Organosilane on Kaolinite, Montmorillonite, and Zeolite for Simultaneous Adsorption of Ammonium and Nitrate. International Journal of Environmental Research and Public Health, 19(19), 12562. https://doi.org/10.3390/ijerph191912562







