Characteristics and Residual Health Risk of Organochlorine Pesticides in Fresh Vegetables in the Suburb of Changchun, Northeast China
Abstract
1. Introduction
2. Materials and Methods
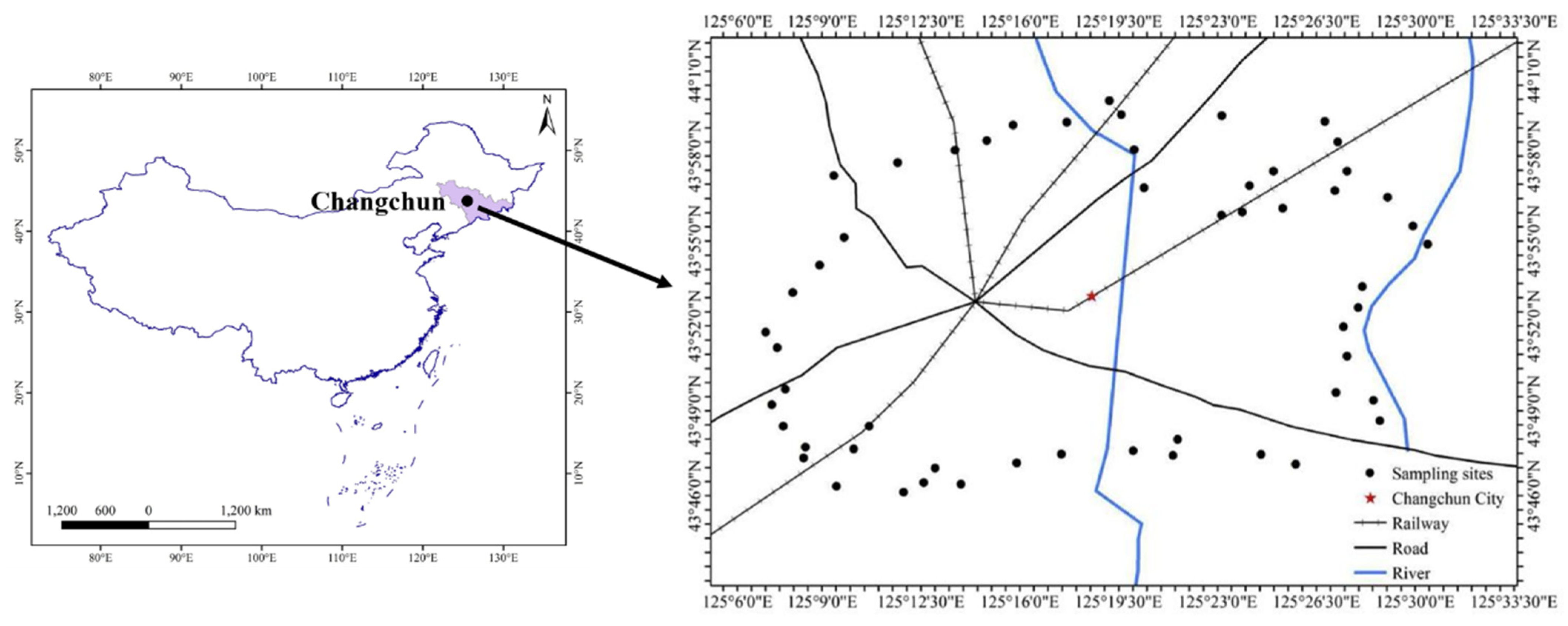
2.1. Study Area and Sampling Sites
2.2. Materials and Reagents
2.3. Analysis and Quality Control
2.4. Human Risk Assessment of OCPs through Vegetables Consumption
3. Results and Discussion
3.1. OCPs in Vegetables
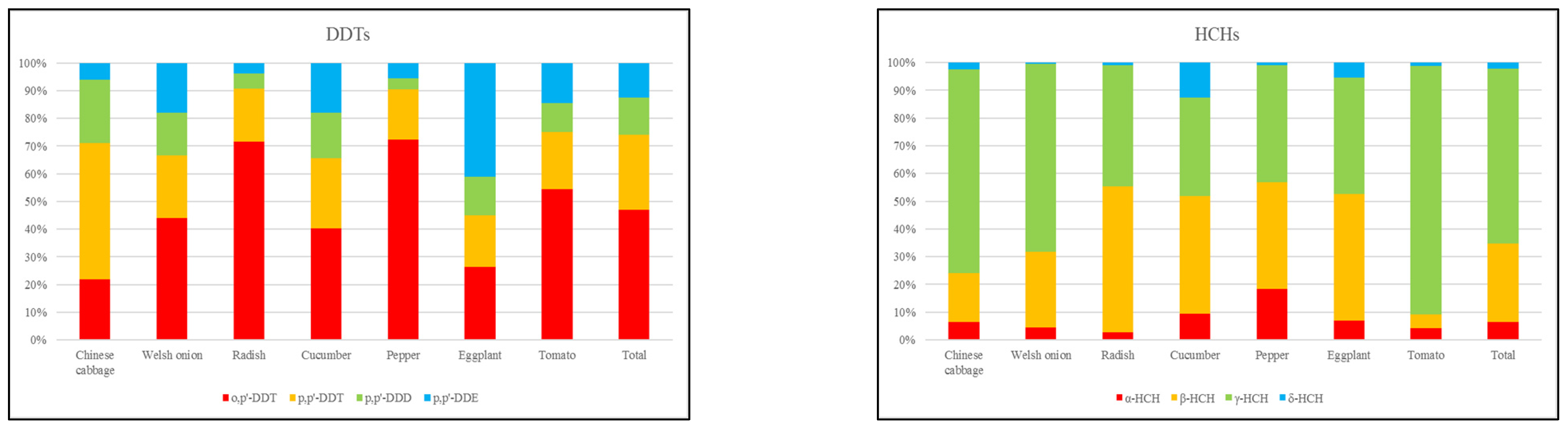
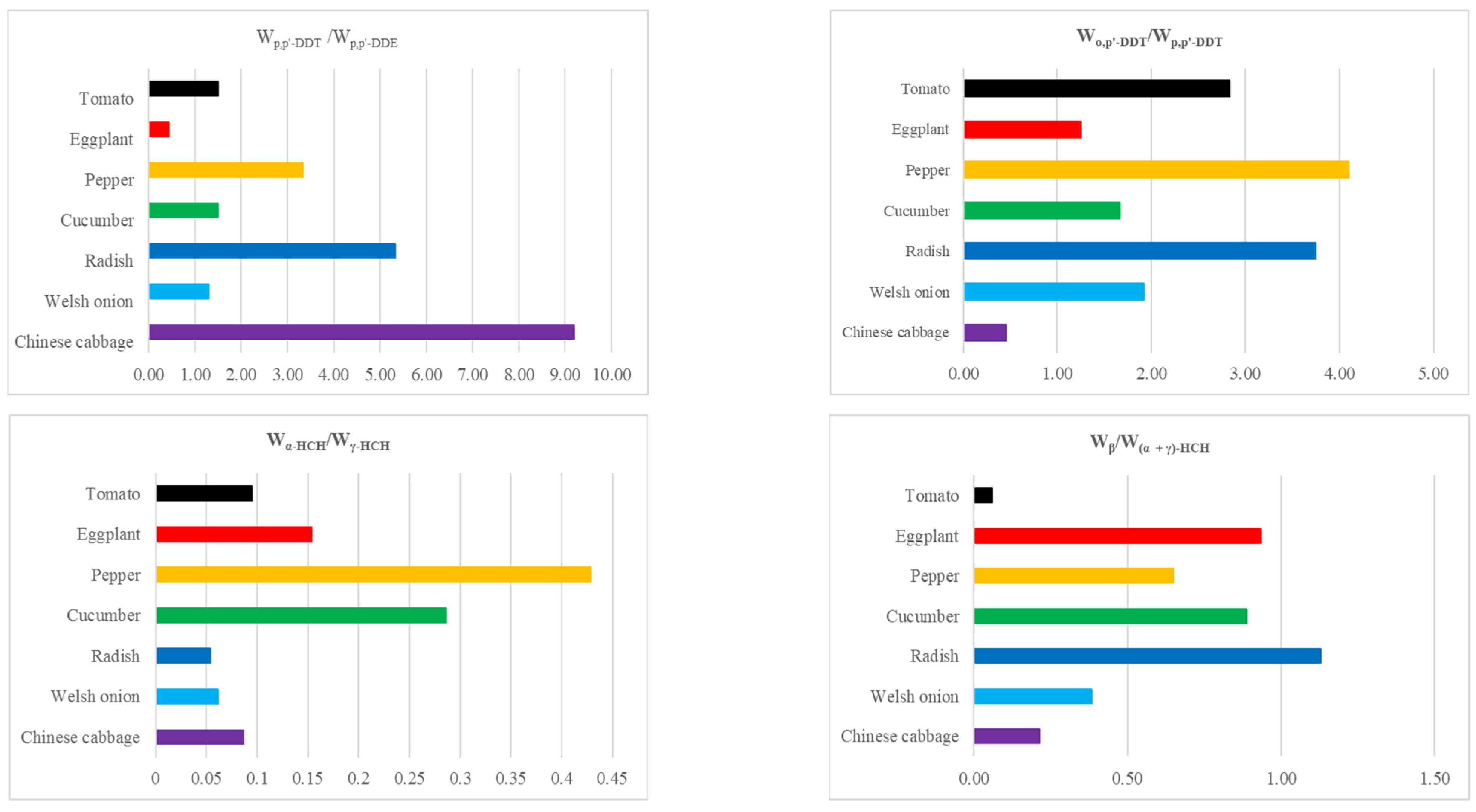
3.2. Composition and Source Analysis of OCPs
3.3. Consumption Health Risk of OCPs in Vegetable
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Xue, C.; Zhang, Y.; Li, Z.; Liu, C.; Pan, X.; Chen, F.; Liu, Y. Soil aggregate-associated distribution of DDTs and HCHs in farmland and bareland soils in the Danjiangkou Reservoir Area of China. Environ. Pollut. 2018, 243, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Pan, Z.; Bai, A.; Li, J.; Li, X. Distribution and bioaccumulation of organochlorine pesticides (OCPs) in food web of Nansi Lake, China. Environ. Monit. Assess. 2014, 186, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Nie, Z.; Die, Q.; Tian, Y.; Liu, F.; He, J.; Huang, Q. Organochlorine pesticides in soil, air, and vegetation at and around a contaminated site in southwestern China: Concentration, transmission, and risk evaluation. Chemosphere 2017, 178, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamary, N.M.; Al-Ghouti, M.A.; Al-Shaikh, I.; Al-Meer, S.H.; Ahmad, T.A. Evaluation of pesticide residues of organochlorine in vegetables and fruits in Qatar: Statistical analysis. Environ. Monit. Assess. 2016, 188, 198. [Google Scholar] [CrossRef]
- Cui, Y.; Ke, R.; Gao, W.; Tian, F.; Wang, Y.; Jiang, G. Analysis of Organochlorine Pesticide Residues in Various Vegetable Oils Collected in Chinese Markets. J. Agric. Food Chem. 2020, 68, 14594–14602. [Google Scholar] [CrossRef]
- Gonzalez, M.; Miglioranza, K.S.; de Moreno, J.E.A.; Moreno, V.J. Occurrence and distribution of organochlorine pesticides (OCPs) in tomato (Lycopersicon esculentum) crops from organic production. J. Agric. Food Chem. 2003, 51, 1353–1359. [Google Scholar] [CrossRef]
- Mansour, S.A.; Belal, M.H.; Abou-Arab, A.A.K.; Gad, M.F. Monitoring of pesticides and heavy metals in cucumber fruits produced from different farming systems. Chemosphere 2009, 75, 601–609. [Google Scholar] [CrossRef]
- Fang, Y.; Nie, Z.; Yang, Y.; Die, Q.; Liu, F.; He, J.; Huang, Q. Human health risk assessment of pesticide residues in market-sold vegetables and fish in a northern metropolis of China. Environ. Sci. Pollut. Res. Int. 2015, 22, 6135–6143. [Google Scholar] [CrossRef]
- Liang, X.; Xie, Q.; Zheng, Q.; Yang, B.; Ye, J.; Tang, C. Soil-crop Distribution and Health Risk Assessment of Organochlorine Pesticides on Typical Agricultural Land in Southern Leizhou Peninsula. Environ. Sci. 2022, 43, 500–509. (In Chinese) [Google Scholar]
- Midmore, D.J.; Jansen, H.G.P. Supplying vegetables to Asian cities: Is there a case for peri-urban production? Food Policy 2003, 28, 13–27. [Google Scholar] [CrossRef]
- Kumar Sharma, R.; Agrawal, M.; Marshall, F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007, 66, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Bi, C.; Zhang, J.; Jin, X.; Chen, Z. Characterization of polycyclic aromatic hydrocarbons (PAHs) in vegetables near industrial areas of Shanghai, China: Sources, exposure, and cancer risk. Environ. Pollut. 2018, 241, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Liu, Q.; Liu, J.; Wang, Q.; Wang, Y. Concentrations of organophosphorus pesticides in fresh vegetables and related human health risk assessment in Changchun, Northeast China. Food Control 2016, 60, 353–360. [Google Scholar] [CrossRef]
- Zhang, A.; Luo, W.; Sun, J.; Xiao, H.; Liu, W. Distribution and uptake pathways of organochlorine pesticides in greenhouse and conventional vegetables. Sci. Total Environ. 2015, 505, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Chanda, A.; Gogoi, P.; Bhattacharyya, S. Organochlorine pesticides and heavy metals in the zooplankton, fishes, and shrimps of tropical shallow tidal creeks and the associated human health risk. Mar. Pollut. Bull. 2021, 165, 112170. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, Q.; Zhang, X.; Zheng, D.; Zhang, Z.; Zhang, S. Population health risk due to dietary intake of heavy metals in the industrial area of Huludao City, China. Sci. Total Environ. 2007, 387, 96–104. [Google Scholar] [CrossRef]
- GB 2763-2021; National Food Safety Standard-Maximum Residue Limits for Pesticides in Food. China Agricultural Press: Beijing, China, 2021. (In Chinese)
- Chourasiya, S.; Khillare, P.S.; Jyethi, D.S. Health risk assessment of organochlorine pesticide exposure through dietary intake of vegetables grown in the periurban sites of Delhi, India. Environ. Sci. Pollut. Res. Int. 2015, 22, 5793–5806. [Google Scholar] [CrossRef]
- Li, W.; Tai, L.; Liu, J.; Gai, Z.; Ding, G. Monitoring of pesticide residues levels in fresh vegetable form Heibei Province, North China. Environ. Monit. Assess. 2014, 186, 6341–6349. [Google Scholar] [CrossRef]
- Olatunji, O.S. Evaluation of selected polychlorinated biphenyls (PCBs) congeners and dichlorodiphenyltrichloroethane (DDT) in fresh root and leafy vegetables using GC-MS. Sci. Rep. 2019, 9, 538. [Google Scholar] [CrossRef]
- Sapbamrer, R.; Hongsibsong, S. Organophosphorus pesticide residues in vegetables from farms, markets, and a supermarket around Kwan Phayao Lake of Northern Thailand. Arch. Environ. Contam. Toxicol. 2014, 67, 60–67. [Google Scholar] [CrossRef]
- Wong, M.H.; Leung, A.O.; Chan, J.K.; Choi, M.P. A review on the usage of POP pesticides in China, with emphasis on DDT loadings in human milk. Chemosphere 2005, 60, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Impacts of Urbaniztion Degree on the Accumulatin of Polyaromatic Hydrocarbons and Organochlorine Pesticides in Regional Environmental Media and Related Health Risk Assessment. Ph.D. Thesis, The Beijing Jiaotong University, Beijing, China, 2018. (In Chinese). [Google Scholar]
- Yang, Y.; Xie, X.; Weng, J. Contents and Assessment of Soil Heavy Metal Pollution in Different Types of Green Space in Changchun. Chin. J. Soil Sci. 2020, 51, 1454–1460. (In Chinese) [Google Scholar]
- Islam, M.M.; Kadiyala, V.; Dharmarajan, R.; Annamalai, P.; Mallavarapu, M. Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 2020, 711, 134612. [Google Scholar]
- Martinez, A.; Erdman, N.R.; Rodenburg, Z.L.; Eastling, P.M.; Hornbuckle, K.C. Spatial distribution of chlordanes and pcb congeners in soil in cedar rapids, Iowa, USA. Environ. Pollut. 2012, 161, 222–228. [Google Scholar] [CrossRef]
- Man, Y.B.; Chan, J.K.; Wu, S.C.; Wong, C.K.; Wong, M.H. Dietary exposure to DDTs in two coastal cities and an inland city in China. Sci. Total Environ. 2013, 463–464, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Polder, A.; Savinova, T.N.; Tkachev, A.; Løken, K.B.; Odland, J.O.; Skaare, J.U. Levels and patterns of Persistent Organic Pollutants (POPS) in selected food items from Northwest Russia (1998–2002) and implications for dietary exposure. Sci. Total Environ. 2010, 408, 5352–5361. [Google Scholar] [CrossRef] [PubMed]
- Doong, R.A.; Peng, C.K.; Sun, Y.C.; Liao, P.L. Composition and distribution of organochlorine pesticide residues in surface sediments from the Wu-Shi River Estuary, Taiwan. Mar. Pollut. Bull. 2002, 45, 246–253. [Google Scholar] [CrossRef]
- Wang, H.S.; Sthiannopkao, S.; Du, J.; Chen, Z.J.; Kim, K.W.; Mohamed Yasin, M.S.; Hashim, J.H.; Wong, C.K.; Wong, M.H. Daily intake and human risk assessment of organochlorine pesticides (OCPs) based on Cambodian market basket data. J. Hazard. Mater. 2011, 192, 1441–1449. [Google Scholar] [CrossRef]
- World Health Organization (WHO). DDT and Its Derivatives; World Health Organization: New York, NY, USA, 1979.
- Gong, X.; Li, Q.; Zhang, L.; Zhao, Z.; Xue, B.; Yao, S.; Wang, X.; Cai, Y. The occurrence of organochlorine pesticides (OCPs) in riverine sediments of hilly region of southern China: Implications for sources and transport processes. J. Geochem. Explor. 2020, 216, 106580. [Google Scholar] [CrossRef]
- Qiu, X.; Zhu, T.; Yao, B.; Hu, J.; Hu, S. Contribution of dicofol to the current DDT pollution in China. Environ. Sci. Technol. 2005, 39, 4385–4390. [Google Scholar] [CrossRef]
- Yi, Z.; Yang, W.; Wu, Y.; Du, Z.; Bi, J. Residues and Sources of HCHs and DDTs in Tea Leaves in Fujian Tea Gardens. J. Agro-Environ. Sci. 2012, 31, 24–29. (In Chinese) [Google Scholar]
- Wang, X.; Ren, N.; Qi, H.; Ma, W.; Li, Y. Levels, distributions, and source identification of organochlorine pesticides in the topsoils in Northeastern China. J. Environ. Sci. 2009, 21, 1386–1392. [Google Scholar] [CrossRef]
- Jia, H.; Chang, Y.; Sun, Y.; Wang, D.; Liu, X.; Yang, M.; Xu, D.; Meng, B.; Li, Y. Distribution and potential human risk of organochlorine pesticides in market mollusks from Dalian, China. Bull. Environ. Contam. Toxicol. 2010, 84, 278–284. [Google Scholar] [CrossRef]
- Liu, F.; Liao, C.; Fu, J.; Lv, J.; Xue, Q.; Jiang, G. Polycyclic aromatic hydrocarbons and organochlorine pesticides in rice hull from a typical e-waste recycling area in southeast China: Temporal trend, source, and exposure assessment. Environ. Geochem. Health 2014, 36, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M.; Qadir, A.; Mahmood, A.; Mehmood, A.; Malik, R.N.; Li, J.; Yousaf, Z.; Jamil, N.; Shaikh, I.A.; Ali, H.; et al. Human health risk assessment, congener specific analysis and spatial distribution pattern of organochlorine pesticides (OCPs) through rice crop from selected districts of Punjab Province, Pakistan. Sci. Total Environ. 2015, 511, 354–361. [Google Scholar] [CrossRef]
- Shoiful, A.; Fujita, H.; Watanabe, I.; Honda, K. Concentrations of organochlorine pesticides (OCPs) residues in foodstuffs collected from traditional markets in Indonesia. Chemosphere 2013, 90, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Fromberg, A.; Granby, K.; Højgård, A.; Fagt, S.; Larsen, J.C. Estimation of dietary intake of PCB and organochlorine pesticides for children and adults. Food Chem. 2011, 125, 1179–1187. [Google Scholar] [CrossRef]
- Mahmood, A.; Malik, R.N.; Li, J.; Zhang, G. Human health risk assessment and dietary intake of organochlorine pesticides through air, soil and food crops (wheat and rice) along two tributaries of river Chenab, Pakistan. Food Chem. Toxicol. 2014, 71, 17–25. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, J.; Zhang, S.; An, Y.; Hao, L.; Yang, X.; Wang, C.; Wang, Z.; Wu, Q. Novel N-riched covalent organic framework for solid-phase microextraction of organochlorine pesticides in vegetable and fruit samples. Food Chem. 2022, 388, 133007. [Google Scholar] [CrossRef]
- Wang, X.; Gao, M.; Wang, B.; Tan, Y.; Guo, Y.; Li, Q.; Ge, S.; Lan, C.; Chen, J.; Jiangtulu, B.; et al. Risk of dietary intake of organochlorine pesticides among the childbearing-age women: A multiple follow-up study in North China. Ecotoxicol. Environ. Saf. 2021, 224, 112607. [Google Scholar] [CrossRef]
- Hao, Q.; Sun, Y.; Xu, X.; Luo, X.; Wang, S.; Zhang, Z.; Mai, B. Residual levels of PCBs and DDTs in wholesale fish in Guangzhou, China and potential health risks. J. Trop. Oceanogr. 2014, 33, 84–91. (In Chinese) [Google Scholar]
- Wang, X. Assessment of Exposure to Environmental Pollutants among the Community-Dwelling Elderly in Wuhan. Ph.D. Thesis, The Huazhong University of Science and Technology, Wuhan, China, 2021. (In Chinese). [Google Scholar]
- Fujii, Y.; Haraguchi, K.; Harada, K.H.; Hitomi, T.; Inoue, K.; Itoh, Y.; Watanabe, T.; Takenaka, K.; Uehara, S.; Yang, H.R.; et al. Detection of dicofol and related pesticides in human breast milk from China, Korea and Japan. Chemosphere 2011, 82, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wu, Y.; Yin, S.; Li, J.; Zhao, Y.; Zhang, L.; Chen, H.; Liu, Y.; Yang, X.; Li, X. National survey of the levels of persistent organochlorine pesticides in the breast milk of mothers in China. Environ. Pollut. 2011, 159, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Espinosa, M.J.; Murcia, M.; Iniguez, C.; Vizcaino, E.; Llop, S.; Vioque, J.; Grimalt, J.O.; Rebagliato, M.; Ballester, F. Prenatal exposure to organochlorine compounds and birth size. Pediatrics 2011, 128, e127–e134. [Google Scholar] [CrossRef]
- Gyalpo, T.; Fritsche, L.; Bouwman, H.; Bornman, R.; Scheringer, M.; Hungerbühler, K. Estimation of human body concentrations of DDT from indoor residual spraying for malaria control. Environ. Pollut. 2012, 169, 235–241. [Google Scholar] [CrossRef]
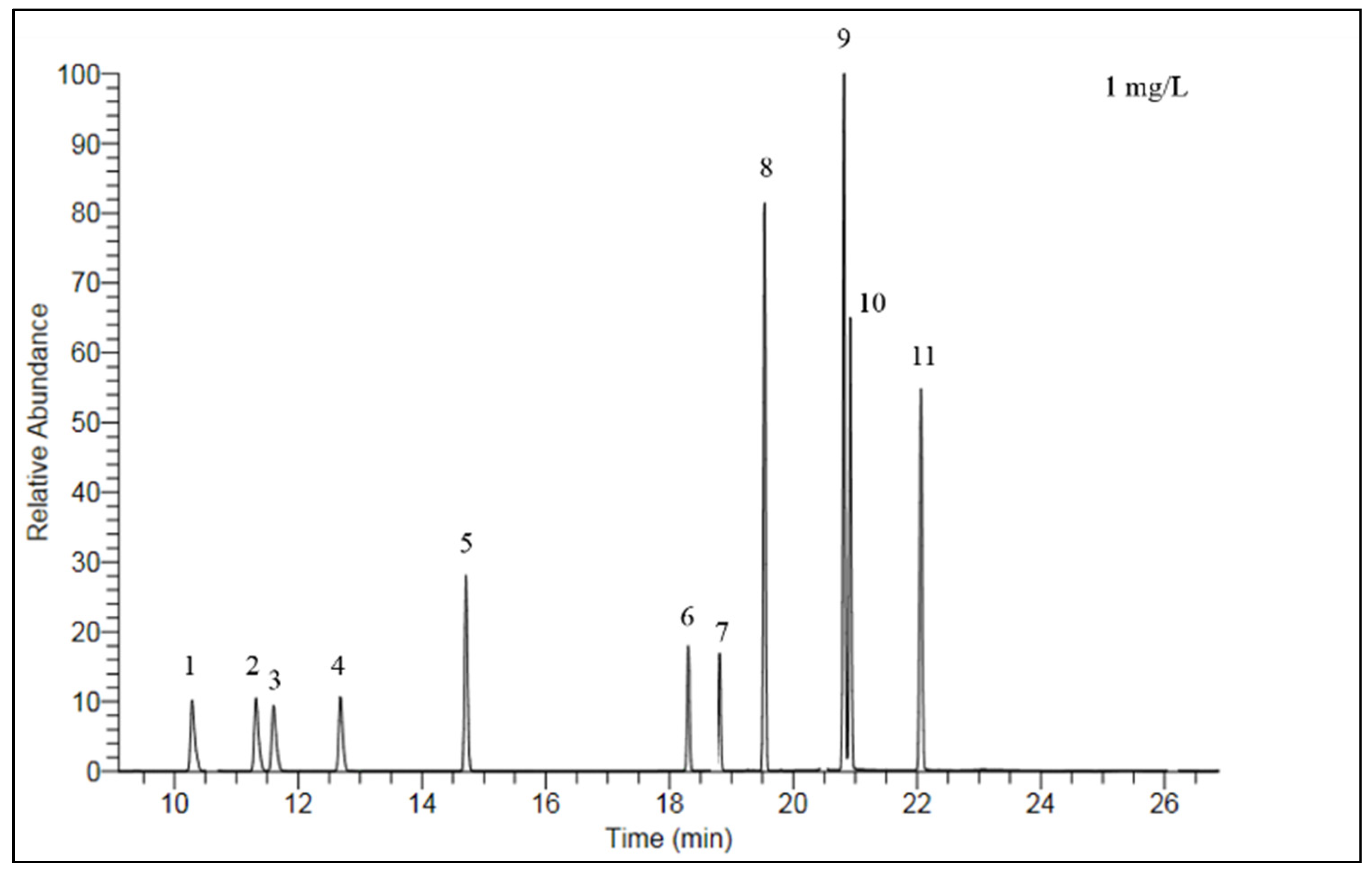
| Pesticides | LOD (µg·kg−1) | Spiked Concentration (µg·kg−1) | Recovery (%) (n = 3) | RSD% |
|---|---|---|---|---|
| o,p′-DDT | 0.13 | 50.00 | 87.61 | 5.65 |
| p,p′-DDT | 0.17 | 50.00 | 83.53 | 7.32 |
| p,p′-DDD | 0.11 | 50.00 | 92.95 | 4.76 |
| p,p′-DDE | 0.10 | 50.00 | 89.66 | 9.82 |
| α-HCH | 0.080 | 50.00 | 91.23 | 6.45 |
| β-HCH | 0.18 | 50.00 | 84.93 | 8.71 |
| γ-HCH | 0.090 | 50.00 | 93.51 | 10.20 |
| δ-HCH | 0.10 | 50.00 | 81.39 | 9.80 |
| heptachlor | 0.11 | 50.00 | 76.21 | 11.15 |
| cis-chlordane | 0.070 | 50.00 | 80.57 | 5.81 |
| tran-chlordane | 0.080 | 50.00 | 81.60 | 9.14 |
| OCPs | Leafy Vegetables (n = 68) | Root Vegetables (n = 16) | Fruit Vegetables (n = 130) | Total (n = 214) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chinese Cabbage (n = 28) | Welsh Onion (n = 40) | Radish (n = 16) | Cucumber (n = 31) | Pepper (n = 29) | Eggplant (n = 34) | Tomato (n = 36) | ||||||||||
| Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | |
| ∑HCHs | nd-69.13 | 9.43 | nd-53.76 | 11.94 | nd-53.71 | 8.42 | nd-9.08 | 1.91 | nd-68.57 | 6.64 | nd-26.56 | 3.14 | nd-54.41 | 5.40 | nd-69.13 | 6.60 |
| α-HCH | nd-5.55 | 0.62 | nd-3.40 | 0.52 | nd-1.68 | 0.22 | nd-2.28 | 0.18 | nd-14.64 | 1.22 | nd-3.04 | 0.22 | nd-1.98 | 0.23 | nd-14.64 | 0.42 |
| β-HCH | nd-10.02 | 1.64 | nd-14.12 | 3.28 | nd-33.92 | 4.44 | nd-8.61 | 0.81 | nd-20.02 | 2.56 | nd-21.68 | 1.43 | nd-3.90 | 0.26 | nd-33.92 | 1.88 |
| γ-HCH | nd-67.59 | 6.93 | nd-50.01 | 8.08 | nd-39.94 | 3.68 | nd-5.59 | 0.68 | nd-34.03 | 2.80 | nd-19.96 | 1.32 | nd-47.76 | 4.84 | nd-67.59 | 4.16 |
| δ-HCH | nd-3.03 | 0.24 | nd-1.58 | 0.06 | nd-0.79 | 0.08 | nd-1.73 | 0.24 | nd-1.08 | 0.06 | nd-2.08 | 0.17 | nd-0.68 | 0.070 | nd-3.03 | 0.14 |
| ∑DDTs | nd-59.65 | 9.34 | nd-56.4 | 11.43 | nd-56.28 | 8.36 | nd-9.29 | 1.34 | nd-86.65 | 5.64 | nd-14.84 | 2.04 | nd-55.99 | 3.05 | nd-86.65 | 5.82 |
| o,p′-DDT | nd-28.27 | 2.05 | nd-32.77 | 5.04 | nd-37.96 | 5.98 | nd-3.77 | 0.54 | nd-73.19 | 4.08 | nd-5.53 | 0.54 | nd-40.33 | 1.66 | nd-73.19 | 2.74 |
| p,p′-DDT | nd-31.72 | 4.60 | nd-14.98 | 2.58 | nd-14.46 | 1.60 | nd-4.46 | 0.34 | nd-11.93 | 1.02 | nd-4.21 | 0.38 | nd-6.88 | 0.63 | nd-31.72 | 1.57 |
| p,p′-DDD | nd-21.54 | 2.12 | nd-9.93 | 1.76 | nd-2.98 | 0.46 | nd-2.88 | 0.22 | nd-2.57 | 0.23 | nd-2.62 | 0.28 | nd-4.59 | 0.32 | nd-21.54 | 0.78 |
| p,p′-DDE | nd-3.77 | 0.57 | nd-15.42 | 2.05 | nd-2.46 | 0.32 | nd-2.69 | 0.24 | nd-4.62 | 0.31 | nd-7.38 | 0.84 | nd-4.14 | 0.44 | nd-15.42 | 0.73 |
| ∑Chlordans | nd-37.16 | 2.93 | nd-29.55 | 3.31 | nd-21.81 | 2.74 | nd-34.47 | 1.85 | nd-33.74 | 2.93 | nd-29.96 | 2.02 | nd-20.92 | 1.02 | nd-37.16 | 2.37 |
| cis-chlordane | nd-18.05 | 1.51 | nd-13.66 | 1.64 | nd-16.47 | 2.16 | nd-26.02 | 1.27 | nd-15.41 | 1.32 | nd-22.33 | 1.58 | nd-12.65 | 0.72 | nd-26.02 | 1.43 |
| tran-chlordane | nd-19.14 | 1.42 | nd-16.42 | 1.67 | nd-5.33 | 0.58 | nd-8.59 | 0.58 | nd-18.22 | 1.61 | nd-7.70 | 0.44 | nd-8.23 | 0.30 | nd-19.14 | 0.94 |
| heptachlor | nd-7.34 | 0.66 | nd-8.75 | 0.48 | nd-1.51 | 0.14 | nd-4.34 | 0.27 | nd-4.48 | 0.32 | nd-2.32 | 0.08 | nd-6.49 | 0.33 | nd-8.75 | 0.29 |
| OCPs | ADI µg·kg−1·d−1 | Ave EDI µg·kg−1·d−1 | Ave THQ | Ave HI | Max EDI µg·kg−1·d−1 | Max THQ | Max HI | |
|---|---|---|---|---|---|---|---|---|
| Children | HCHs | 5 | 0.022 | 0.0040 | 0.036 | 0.23 | 0.046 | 0.62 |
| DDTs | 10 | 0.019 | 0.0020 | 0.29 | 0.029 | |||
| Chlordan | 0.5 | 0.0080 | 0.016 | 0.12 | 0.25 | |||
| heptachlor | 0.1 | 0.0010 | 0.010 | 0.029 | 0.29 | |||
| Adults | HCHs | 5 | 0.029 | 0.0060 | 0.043 | 0.30 | 0.060 | 0.80 |
| DDTs | 10 | 0.025 | 0.0030 | 0.38 | 0.038 | |||
| Chlordan | 0.5 | 0.010 | 0.021 | 0.16 | 0.32 | |||
| heptachlor | 0.1 | 0.0012 | 0.013 | 0.038 | 0.38 |
| Sites | HCHs | DDTs | Reference |
|---|---|---|---|
| Dalian, China | 0.001 | 0.003 | [36] |
| Taizhou, China | 0.137 | 0.076 | [37] |
| Punjab Province, Pakistan | 0.0039 | 0.019 | [38] |
| Jakarta, Bogor, and Yogyakarta, Indonesia | 0.002 | 0.040 | [39] |
| Denmark | 0.0022 | 0.0037 | [40] |
| This study | 0.022–0.029 | 0.019–0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Cui, Z.; Wang, Y.; Zhang, J. Characteristics and Residual Health Risk of Organochlorine Pesticides in Fresh Vegetables in the Suburb of Changchun, Northeast China. Int. J. Environ. Res. Public Health 2022, 19, 12547. https://doi.org/10.3390/ijerph191912547
Wang N, Cui Z, Wang Y, Zhang J. Characteristics and Residual Health Risk of Organochlorine Pesticides in Fresh Vegetables in the Suburb of Changchun, Northeast China. International Journal of Environmental Research and Public Health. 2022; 19(19):12547. https://doi.org/10.3390/ijerph191912547
Chicago/Turabian StyleWang, Nan, Zhengwu Cui, Yang Wang, and Jingjing Zhang. 2022. "Characteristics and Residual Health Risk of Organochlorine Pesticides in Fresh Vegetables in the Suburb of Changchun, Northeast China" International Journal of Environmental Research and Public Health 19, no. 19: 12547. https://doi.org/10.3390/ijerph191912547
APA StyleWang, N., Cui, Z., Wang, Y., & Zhang, J. (2022). Characteristics and Residual Health Risk of Organochlorine Pesticides in Fresh Vegetables in the Suburb of Changchun, Northeast China. International Journal of Environmental Research and Public Health, 19(19), 12547. https://doi.org/10.3390/ijerph191912547





