“Ghost”, a Well-Known but Not Fully Explained Echocardiographic Finding during Transvenous Lead Extraction: Clinical Significance
Abstract
:1. Introduction
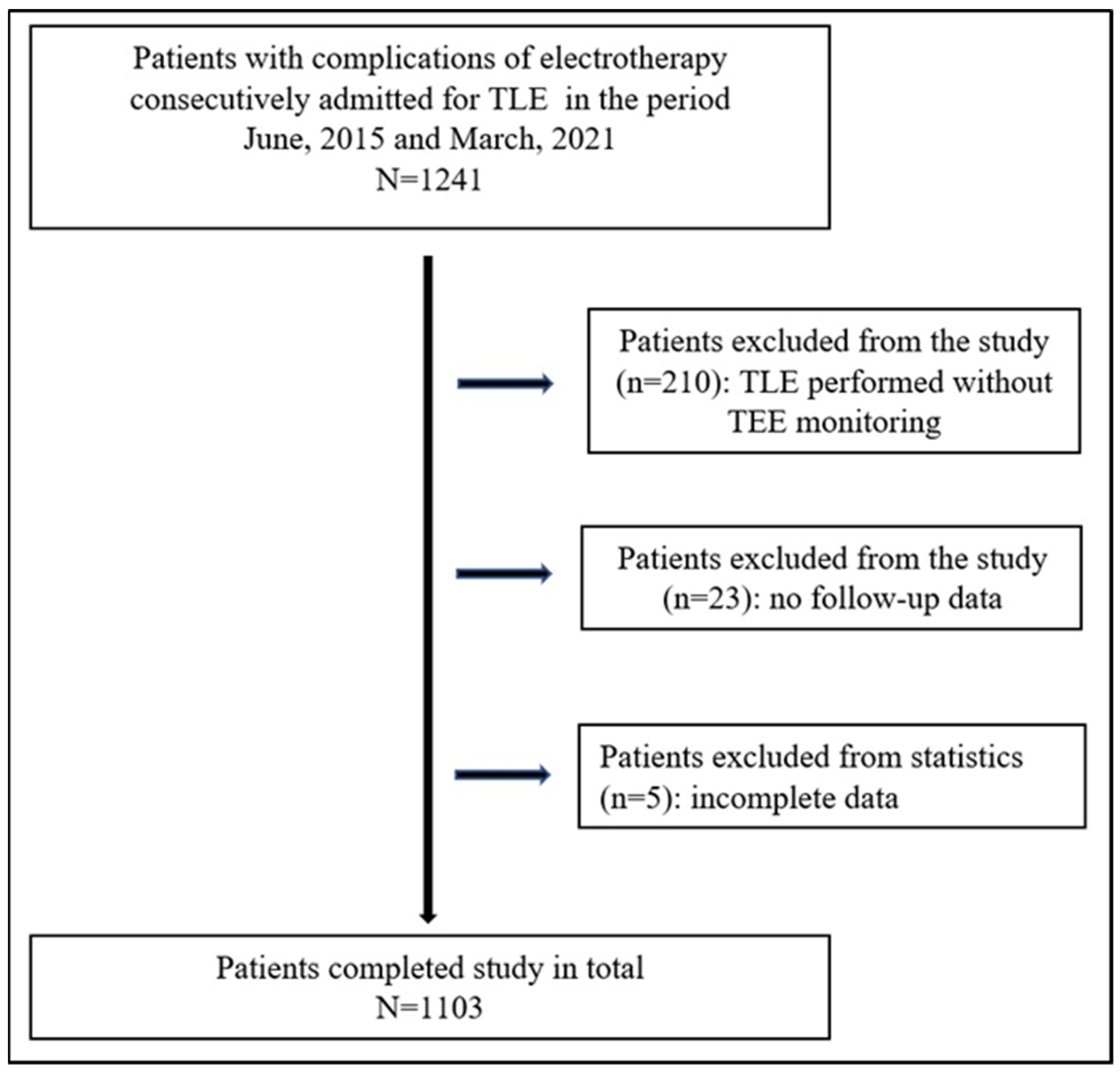
2. Materials and Methods
2.1. Study Population
2.2. TLE Indications
2.3. TEE Monitoring of the Lead Extraction Procedure
2.4. Definitions of Echocardiographic Phenomena
2.5. Lead Extraction Procedure
2.6. Approval of the Bioethics Committee
2.7. Statistical Analysis
3. Results
3.1. Uni- and Multivariable Linear Regression Analysis
3.2. Survival Analysis
4. Discussion
5. Conclusions
- In approximately 30% of TLE procedures ghosts remain attached to the CVS wall (SG) but in around 15% of the extraction procedures freed ghosts (FG) travel spontaneously to the pulmonary vascular bed and disappear.
- Younger patient age and the number of leads but not infectious indications are the factors predisposing to ghost formation during and after TLE.
- The degree of growth and maturation of the connective tissue surrounding the lead before TLE is the strongest predictor of both types of ghosts.
- The occurrence of ghosts is associated with complicated procedures, but it seems to be related to implant duration and scar growth.
- The presence of both types of ghosts does not reduce survival after TLE.
Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keiler, J.; Schulze, M.; Dreger, R.; Springer, A.; Öner, A.; Wree, A. Quantitative and Qualitative Assessment of Adhesive Thrombo-Fibrotic Lead Encapsulations (TFLE) of Pacemaker and ICD Leads in Arrhythmia Patients—A Post Mortem Study. Front. Cardiovasc. Med. 2020, 7, 602179. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, P.; Novak, M.; Kamaryt, P.; Slana, B.; Lipoldova, J.; Dvorak, P. Histological findings around electrodes in pacemaker and implantable cardioverter-defibrillator patients: Comparison of steroid-eluting and non-steroid-eluting electrodes. Europace 2012, 14, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, D.; Dubaniewicz, A.; Koźluk, E.; Grzybiak, M.; Krupa, W.; Kołodziej, P.; Pazdyga, A.; Adamowicz-Kornacka, M.; Walczak, E.; Walczak, F. The morphological conditions of the permanent pacemaker lead extraction. Folia Morphol. 2000, 59, 25–29. [Google Scholar]
- Kołodzińska, A.; Kutarski, A.; Koperski, Ł.; Grabowski, M.; Małecka, B.; Opolski, G. Differences in encapsulating lead tissue in patients who underwent transvenous lead removal. Europace 2012, 14, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- Caiati, C.; Luzzi, G.; Pollice, P.; Favale, S.; Lepera, M.E. Novel Clinical Perspective on New Masses after Lead Extraction (Ghosts) by Means of Intracardiac Echocardiography. J. Clin. Med. 2020, 9, 2571. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Polewczyk, A.; Jacheć, W.; Tułecki, Ł.; Tomków, K.; Stefańczyk, P.; Kleinrok, A.; Kutarski, A. A new approach to the continuous monitoring of transvenous lead extraction using transesophageal echocardiography—Analysis of 936 procedures. Echocardiography 2020, 37, 601–611. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Polewczyk, A.; Jacheć, W.; Tułecki, Ł.; Kleinrok, A.; Kutarski, A. Echocardiographic findings in patients with cardiac implantable electronic devices-analysis of factors predisposing to lead-associated changes. Clin. Physiol. Funct. Imaging 2021, 41, 25–41. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Tułecki, Ł.; Kleinrok, A.; Kutarski, A. Prognostic Value of Preoperative Echocardiographic Findings in Patients Undergoing Transvenous Lead Extraction. Int. J. Environ. Res. Public Health 2021, 18, 1862. [Google Scholar] [CrossRef]
- Segreti, L.; Di Cori, A.; Soldati, E.; Zucchelli, G.; Viani, S.; Paperini, L.; De Lucia, R.; Coluccia, G.; Valsecchi, S.; Bongiorni, M.G. Major predictors of fibrous adherences in transvenous implantable cardioverter-defibrillator lead extraction. Heart Rhythm 2014, 11, 2196–2201. [Google Scholar] [CrossRef]
- Ho, G.; Bhatia, P.; Mehta, I.; Maus, T.; Khoche, S.; Pollema, T.; Pretorius, V.G. Prevalence and Short-Term Clinical Outcome of Mobile Thrombi Detected on Transvenous Leads in Patients Undergoing Lead Extraction. JACC Clin. Electrophysiol. 2019, 5, 657–664. [Google Scholar] [CrossRef]
- Beaser, A.D.; Aziz, Z.; Besser, S.A.; Jones, C.I.; Jameria, Z.; Kannan, A.; Upadhyay, G.A.; Broman, M.T.; Ozcan, C.; Tung, R.; et al. Characterization of Lead Adherence Using Intravascular Ultrasound to Assess Difficulty of Transvenous Lead Extraction. Circ. Arrhythm. Electrophysiol. 2020, 13, e007726. [Google Scholar] [CrossRef] [PubMed]
- Golzio, P.G.; Errigo, D.; Peyracchia, M.; Gallo, E.; Frea, S.; Castagno, D.; Budano, C.; Giustetto, C.; Rinaldi, M. Prevalence and prognosis of lead masses in patients with cardiac implantable electronic devices without infection. J. Cardiovasc. Med. 2019, 20, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley, G.H.; Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.; Mitkowski, P.; et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: This document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009, 6, 1085–1104. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1217. [Google Scholar] [CrossRef] [PubMed]
- Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Kleinrok, A.; Tułecki, Ł.; Kutarski, A. The prognostic value of transesophageal echocardiography after transvenous lead extraction: Landscape after battle. Cardiovasc. Diagn. Ther. 2021, 11, 394–410. [Google Scholar] [CrossRef]
- Poterała, M.; Kutarski, A.; Brzozowski, W.; Tomaszewski, M.; Gromadziński, L.; Tomaszewski, A. Echocardiographic assessment of residuals after transvenous intracardiac lead extraction. Int. J. Cardiovasc. Imaging 2020, 36, 423–430. [Google Scholar] [CrossRef]
- Narducci, M.L.; Di Monaco, A.; Pelargonio, G.; Leoncini, E.; Boccia, S.; Mollo, R.; Perna, F.; Bencardino, G.; Pennestrì, F.; Scoppettuolo, G.; et al. Presence of ‘ghosts’ and mortality after transvenous lead extraction. Europace 2017, 19, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Le Dolley, Y.; Thuny, F.; Mancini, J.; Casalta, J.P.; Riberi, A.; Gouriet, F.; Bastard, E.; Ansaldi, S.; Franceschi, F.; Renard, S.; et al. Diagnosis of cardiac device-related infective endocarditis after device removal. JACC Cardiovasc. Imaging 2010, 3, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Diemberger, I.; Biffi, M.; Lorenzetti, S.; Martignani, C.; Raffaelli, E.; Ziacchi, M.; Rapezzi, C.; Pacini, D.; Boriani, G. Predictors of long-term survival free from relapses after extraction of infected CIED. Europace 2018, 20, 1018–1027. [Google Scholar] [CrossRef]
- Alizadehasl, A.; Sarrafi Rad, N.; Pourafkari, L.; Haghjoo, M. Persistence of a pacemaker lead-like “ghost” 6 months after lead extraction. Echocardiography 2019, 36, 201–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreas, M.; Wiedemann, D.; Kocher, A.; Khazen, C. Materialization of ghosts: Severe intracardiac masses after pacemaker lead extraction requiring immediate surgical intervention. Heart Rhythm 2013, 10, 1826. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.M.; Ailiani, R.G. Pseudoleads on Transesophageal Echocardiography. CASE Cardiovasc. Imaging Case Rep. 2018, 3, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Pettemerides, V.; Macnab, A. Right atrial ghost following device extraction for infective endocarditis. Echo Res. Pract. 2019, 6, I3–I4. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, V.; Dello Russo, A.; Casella, M.; Biddau, R. Residual fibrous tissue floating in the right atrium after percutaneous pacemaker lead extraction: An unusual complication early detected by intracardiac echocardiography. Int. J. Cardiol. 2008, 127, e67–e68. [Google Scholar] [CrossRef]
- Nazir, S.A.; Hudsmith, L.; Newton, J.D.; Betts, T.R. Chronic fibrous sheath mistaken for retained pacemaker product. Eur. J. Echocardiogr. 2009, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Kiuchi, K.; Fukuzawa, K.; Mori, S.; Nishii, T.; Matsumoto, K.; Ichibori, H.; Yamada, T. The details of an unusual “ghost” after transvenous lead extraction: Three-dimensional computed tomography analysis. J. Arrhythm. 2017, 33, 640–642. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Tułecki, Ł.; Tomkow, K.; Stefańczyk, P.; Tomaszewski, A.; Brzozowski, W.; Szcześniak-Stańczyk, D.; Kleinrok, A.; et al. Transesophageal Echocardiography as a Monitoring Tool During Transvenous Lead Extraction-Does It Improve Procedure Effectiveness? J. Clin. Med. 2020, 9, 1382. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Polewczyk, A.; Jacheć, W.; Kleinrok, A.; Tułecki, Ł.; Kutarski, A. Transesophageal echocardiography for the monitoring of transvenous lead extraction. Kardiol. Pol. 2020, 78, 1206–1214. [Google Scholar] [CrossRef]
- Endo, Y.; O’Mara, J.E.; Weiner, S.; Han, J.; Goldberger, M.H.; Gordon, G.M.; Nanna, M.; Ferrick, K.J.; Gross, J.N. Clinical utility of intraprocedural transesophageal echocardiography during transvenous lead extraction. J. Am. Soc. Echocardiogr. 2008, 21, 861–867. [Google Scholar] [CrossRef]
- Hilberath, J.N.; Burrage, P.S.; Shernan, S.K.; Varelmann, D.J.; Wilusz, K.; Fox, J.A.; Eltzschig, H.K.; Epstein, L.M.; Nowak-Machen, M. Rescue transoesophageal echocardiography for refractory haemodynamic instability during transvenous lead extraction. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 926–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regoli, F.; D’Ambrosio, G.; Caputo, M.L.; Svab, S.; Conte, G.; Moccetti, T.; Klersy, C.; Cassina, T.; Demertzis, S.; Auricchio, A. New-onset pericardial effusion during transvenous lead extraction: Incidence, causative mechanisms, and associated factors. J. Interv. Card. Electrophysiol. 2018, 51, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Hayıroğlu, M.İ.; Çınar, T.; Çinier, G.; Yüksel, G.; Pay, L.; Keskin, K.; Coşkun, C.; Ayan, G.; Çiçek, V.; Tekkeşin, A.İ. Prognostic value of serum albumin for long-term mortality in patients witch chamber permanent pacemakers. Biomark Med. 2022, 16, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Hayıroğlu, M.İ.; Çınar, T.; Çinier, G.; Pay, L.; Yumurtaş, A.Ç.; Tezen, O.; Eren, S.; Kolak, Z.; Çetin, T.; Cicek, V.; et al. Evaluating systemic immune-inflammation index in patients with implantable cardioverter defibrillator for heart failure with reduced ejection fraction. Pacing Clin. Electrophysiol. 2022, 45, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Agboola, K.M.; Lee, J.M.; Liu, X.; Novak, E.; Cuculich, P.S.; Cooper, D.H.; Noheria, A. Interaction of cardiac implantable electronic device and patent foramen ovale in ischemic stroke: A case-only study. Pacing Clin. Electrophysiol. 2019, 42, 341–348. [Google Scholar] [CrossRef]
- Lee, J.Z.; Agasthi, P.; Pasha, A.K.; Tarin, C.; Tseng, A.S.; Diehl, N.N.; Hodge, D.O.; DeSimone, C.V.; Killu, A.M.; Brady, P.A.; et al. Stroke in patients with cardiovascular implantable electronic device infection undergoing transvenous lead removal. Multicent. Study Heart Rhythm 2018, 15, 1593–1600. [Google Scholar] [CrossRef] [Green Version]





| Patient Characteristics | Flying Ghosts (Ghosts Shifting Spontaneously to Pulmonary Vascular Bed) | Stable Ghosts (Ghosts Remaining Attached to Cardiovascular Wall) | Ghosts Absent during and after TLE |
|---|---|---|---|
| Group/number of patients | 1: N = 171 (15.50%) | 2: N = 322 (29.19%) | 3: N = 610 (55.31%) |
| Form of result presentation | mean ± SD/n (%) p (1 vs. 2) | mean ± SD/n (%) p (2 vs. 3) | mean ± SD/n (%) p (1 vs. 3) |
| Patient age during TLE [years] | 66.06 ± 16.20 p = 0.912 | 65.73 ± 15.29 p < 0.006 | 68.87 ± 13.38 p < 0.047 |
| Patient age at first system implantation [years] | 53.53 ± 18.63 p = 0.044 | 56.21 ± 17.39 p = 0.028 | 59.37 ± 14.81 p < 0.001 |
| Female | 70 (40.94) p = 0.654 | 140 (43.48) p = 0.260 | 232 (38.03) p = 0.267 |
| Etiology: ischemic heart disease | 109 (63.72) p = 0.990 | 204 (63.35) p = 0.421 | 404 (66.23) p = 0.545 |
| NYHA III & IV | 31 (18.13) p = 0.799 | 54 (16.77) p = 0.854 | 98 (16.07) p = 0.592 |
| LVEF average [%] | 48.43 ± 15.07 p = 0.610 | 49.04 ± 14.86 p = 0.335 | 47.93 ± 15.82 p = 0.819 |
| LVEF category: significantly decreased (<30%) | 24 (14.04) p = 0.714 | 40 (12.42) p = 0.101 | 102 (16.72) p = 0.468 |
| Permanent atrial fibrillation | 39 (22.81) p = 0.937 | 71 (22.05) p = 0.499 | 148 (24.26) p = 0.770 |
| Congestive heart failure | 58 (33.92) p = 0.085 | 84 (26.09) p = 0.652 | 169 (27.71) p = 0.137 |
| Renal failure (any) | 34 (19.88) p = 0.451 | 75 (23.19) p = 0.313 | 162 (26.56) p = 0.377 |
| Charlson comorbidity index | 5.175 ±4.185 p = 0.710 | 4.708 ± 3.746 p = 0.099 | 5.090 ± 3.671 p = 0.434 |
| Univariable Regression | Multivariable Regression | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Flying ghosts | ||||||
| Patient age at first system implantation [by year] | 0.977 | 0.967–0.987 | <0.001 | 0.984 | 0.970–0.997 | 0.019 |
| Systemic infection | 0.526 | 0.301–0.920 | 0.024 | 0.539 | 0.289–1.006 | 0.052 |
| Number of CIED-related procedures before TLE | 1.227 | 1.046–1.439 | 0.012 | 1.081 | 0.832–1.403 | 0.560 |
| Dwell time of the oldest target lead in the patient before TLE [by year] | 1.060 | 1.035–1.085 | <0.001 | 0.988 | 0.946–1.033 | 0.607 |
| Any form of scar tissue on the lead(s) | 10.34 | 5.625–19.02 | <0.001 | 7.106 | 3.424–14.75 | <0.001 |
| Number of separate scars | 1.685 | 1.457–1.949 | <0.001 | 1.372 | 1.076–1.749 | 0.011 |
| Lead adhesion to heart structures (any) | 2.811 | 1.961–4.028 | <0.001 | 0.517 | 0.281–0.953 | 0.034 |
| Stable ghosts | ||||||
| Patient age at first system implantation [by year] | 0.989 | 0.985–0.993 | <0.001 | 0.989 | 0.980–0.999 | 0.030 |
| Charlson comorbidity index | 0.976 | 0.940–1.013 | 0.197 | |||
| Abandoned lead before TLE | 1.883 | 1.207–2.938 | 0.005 | 1.152 | 0.653–2.032 | 0.623 |
| Number of leads in the heart before TLE | 1.554 | 1.280–1.887 | <0.001 | 1.450 | 1.141–1.842 | 0.002 |
| Number of CIED-related procedures before lead extraction | 1.127 | 0.981–1.296 | 0.092 | 0.940 | 0.786–1.124 | 0.499 |
| All forms of scar tissue on the lead(s) | 2.304 | 1.708–3.106 | <0.001 | 1.940 | 1.293–2.909 | <0.001 |
| Number of separate scars | 1.424 | 1.260–1.609 | <0.001 | 1.147 | 0.939–1.403 | 0.179 |
| Lead adhesion to heart structures (any) | 1.699 | 1.252–2.307 | <0.001 | 1.045 | 0.658–1.660 | 0.851 |
| Long Term and 30-Days Survival after TLE | Flying Ghosts (Ghosts Shifting Spontaneously to Pulmonary Vascular Bed) | Stable Ghosts (Ghosts Remaining Attached to Cardiovascular Wall) | Ghosts Absent during and after TLE |
|---|---|---|---|
| Group/number of patients | 1: N = 171 | 2: N = 322 | 3: N = 610 |
| Long-term survival of entire group of patients after TLE during mean 1264 ± 644.7 (2–2466) days follow-up. Log rank p for all model = 0.186 | |||
| Follow-up (mean ± SD) [days] | 1048 ± 417.5 p < 0.001 | 1511 ± 588.0 p = 0.567 | 1544 ± 555.0 p < 0.001 |
| Alive during follow-up (entire group) (n, %) | 143 (83.63) Log rank test p = 0.914 | 242 (75.16) Log rank test p = 0.121 | 437 (71.64) Log rank test p = 0.202 |
| Died during follow-up (entire group) (n, %) | 28 (16.37) Log rank test p = 0.914 | 80 (24.84) Log rank test p = 0.121 | 173 (28.36) Log rank test p = 0.202 |
| Long-term survival after TLE in the subgroup of non-infectious and infectious patients. Log rank p for all model = 0.036 | |||
| Non-infectious; died/alive (n, %) | 19/131 (12.67) Log rank test p = 0.430 | 41/200 (17.01) Log rank test p = 0.014 | 114/360 (24.05) Log rank test p = 0.259 |
| Infectious (all); died/alive (n, %) | 9/12 (42,86) Chi2 (vs. non-infectious) p = 0.001 | 39/42 (48.15) Chi2 (vs. non-infectious) p < 0.001 | 59/77 (43.38) Chi2 (vs. non-infectious) p < 0.001 |
| Long-term survival after TLE in subgroup of patients with pocket infection. Log rank p for all model = 0.786 | |||
| Local (pocket) infection; died/alive (n, %) | 2/4 (33.33) Log rank test p = 1.000 | 8/10 (44.44) Log rank test p = 0.463 | 11/27 (28.95) Log rank test p = 0.917 |
| Long term survival after TLE in subgroup of patients with systemic. Log rank p for all model = 0.948 | |||
| Systemic infection; died/alive (n, %) | 7/8 (46.67) Log rank test p = 0.789 | 31/32 (49.21) Log rank test p = 0.839 | 48/50 (48.98) Log rank test p = 0.812 |
| 30-days survival after TLE in entire group of patients | |||
| Entire group; died/alive (n, %) | 2/169 (1.17) Chi2 (1 vs. 2) p = 0.660 | 7/315 (2.17) Chi2 (2 vs. 32) p = 0.747 | 10/600 (1.64) Chi2 (1 vs. 3) p = 0.929 |
| 30-days survival after TLE in subgroup of non-infectious patients | |||
| Non-infectious; died/alive (n, %) | 2/148 (1.33) Chi2 (1 vs. 2) p = 0.972 | 2/239 (0.83) Chi2 (2 vs. 3) p = 0.679 | 4/470 (0.84) Chi2 (1 vs. 3) p = 0.956 |
| Infectious (all); died/alive (n, %) | 0/21 (0.00) Chi2 (vs. non-infectious) p = 0.581 | 5/76 (6.17) Chi2 (vs. non-infectious) p = 0.016 | 6/130 (4.41) Chi2 (vs. non-infectious) p = 0.012 |
| 30-days survival after TLE in subgroup of patients with pocket infection | |||
| Local (pocket) infection; died/alive (n, %) | 0/6 (0.00) | 0/18 (0.00) | 0/38 (0.00) |
| Long term survival after TLE in subgroup of patients with systemic infection | |||
| Systemic infection; died/alive (n, %) | 0/15 (0.00) Chi2 (1 vs. 2) p = 0.588 | 5/58 (7.94) Chi2 (2 vs. 3) p = 0.900 | 6/92 (6.12) Chi2 (1 vs. 3) p = 0.714 |
| Univariable Cox Regression | Multivariable Cox Regression | |||||
|---|---|---|---|---|---|---|
| HR | 95% | p | HR | 95% | p | |
| All patients | ||||||
| Female gender | 0.441 | 0.336–0.579 | <0.001 | 0.621 | 0.463–0.833 | <0.001 |
| Patient age during TLE [by year] | 1.049 | 1.038–1.061 | <0.001 | 1.040 | 1.025–1.054 | <0.001 |
| NYHA FC class [by one] | 2.914 | 2.410–3.523 | <0.001 | 1.526 | 1.197–1.946 | <0.001 |
| LVEF [by 1p%] | 0.966 | 0.958–0.973 | <0.001 | 0.987 | 0.981–0.978 | 0.009 |
| Renal failure (any) | 3.269 | 2.587–4.131 | <0.001 | 1.599 | 1.243–2.058 | 0.001 |
| Ischemic heart disease | 1.457 | 1.125–1.887 | 0.004 | 1.014 | 0.758–1.355 | 0.927 |
| Permanent atrial fibrillation | 2.372 | 1.866–3.015 | <0.001 | 1.443 | 1.122–1.856 | 0.004 |
| Charlson comorbidity index | 1.142 | 1.111–1.174 | <0.001 | 1.044 | 1.010–1.080 | 0.012 |
| Flying ghosts (1st group) | 0.795 | 0.537–1.178 | 0.252 | |||
| Stable ghosts (2nd group) | 0.881 | 0.680–1.141 | 0.336 | |||
| Ghosts absent (3rd group) | 1.223 | 0.962–1.555 | 0.100 | |||
| ICD presence before TLE | 1.156 | 0.884–1.512 | 0.292 | |||
| Device type: CRTP/CRTD | 3.125 | 2.382–4.100 | <0.001 | 1.702 | 1.248–2.322 | <0.001 |
| Systemic infection | 2.854 | 2.220–3.667 | <0.001 | 1.894 | 1.460–2.457 | <0.001 |
| Isolated pocket infection | 1.281 | 0.829–1.979 | 0.265 | |||
| Infectious patients | ||||||
| Female gender | 0.485 | 0.301–0.780 | 0.003 | 0.578 | 0.347–0.964 | 0.036 |
| Patient age during TLE [by year] | 1.027 | 1.009–1.045 | 0.003 | 1.037 | 1.014–1.061 | 0.002 |
| NYHA FC class [by one] | 2.278 | 1.687–3.075 | <0.001 | 1.659 | 1.118–2.462 | 0.012 |
| LVEF [by 1p%] | 0.969 | 0.957–0.981 | <0.001 | 0.988 | 0.972–1.004 | 0.133 |
| Renal failure (any) | 2.512 | 1.729–3.649 | <0.001 | 1.686 | 1.136–2.503 | 0.009 |
| Ischemic heart disease | 0.938 | 0.620–1.421 | 0.764 | |||
| Permanent atrial fibrillation | 1.642 | 1.106–2.438 | 0.014 | 1.010 | 0.657–1.552 | 0.965 |
| Charlson comorbidity index | 1.096 | 1.047–1.147 | <0.001 | 1.025 | 0.972–1.082 | 0.363 |
| Flying ghosts (1st group) | 1.089 | 0.548–2.163 | 0.807 | |||
| Stable ghosts (2nd group) | 1.041 | 0.705–1.536 | 0.841 | |||
| Ghosts absent (3rd group) | 0.940 | 0.645–1.369 | 0.747 | |||
| Device type: ICD | 1.018 | 0.666–1.556 | 0.935 | |||
| Device type: CRTP/CRTD | 2.783 | 1.861–4.161 | <0.001 | 1.766 | 1.087–2.868 | 0.022 |
| Isolated pocket infection | 0.560 | 0.351–0.894 | 0.015 | 1.083 | 0.581–2.018 | 0.801 |
| Vegetations presence | 1.620 | 1.094–2.399 | 0.016 | 1.405 | 0.835–2.365 | 0.201 |
| Non-infectious patients | ||||||
| Female gender | 0.478 | 0.342–0.669 | <0.001 | 0.638 | 0.443–0.919 | 0.016 |
| Patient age during TLE [by year] | 1.056 | 1.040–1.071 | <0.001 | 1.043 | 1.024–1.062 | 0.000 |
| NYHA FC class [by one] | 3.140 | 2.439–4.043 | <0.001 | 1.619 | 1.165–2.249 | 0.004 |
| LVEF [by 1p%] | 0.965 | 0.956–0.975 | <0.001 | 0.988 | 0.975–1.000 | 0.048 |
| Renal failure (any) | 3.356 | 2.483–4.535 | <0.001 | 1.489 | 1.072–2.068 | 0.017 |
| Ischemic heart disease | 1.602 | 1.151–2.230 | 0.005 | 0.882 | 0.618–1.258 | 0.488 |
| Permanent atrial fibrillation | 2.815 | 2.078–3.814 | <0.001 | 1.693 | 1.236–2.320 | 0.001 |
| Charlson comorbidity index | 1.148 | 1.108–1.190 | <0.001 | 1.058 | 1.013–1.105 | 0.011 |
| Flying ghosts (1st group) | 0.822 | 0.509–1.330 | 0.425 | |||
| Stable ghosts (2nd group) | 0.721 | 0.508–1.024 | 0.068 | 1.103 | 0.633–1.923 | 0.728 |
| Ghosts absent (3rd group) | 1.428 | 1.043–1.954 | 0.026 | 1.352 | 0.821–2.226 | 0.236 |
| Device type: ICD | 1.162 | 0.820–1.645 | 0.398 | |||
| Device type: CRTP/CRTD | 2.758 | 1.895–4.015 | <0.001 | 1.684 | 1.126–2.520 | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Tułecki, Ł.; Stefańczyk, P.; Kutarski, A. “Ghost”, a Well-Known but Not Fully Explained Echocardiographic Finding during Transvenous Lead Extraction: Clinical Significance. Int. J. Environ. Res. Public Health 2022, 19, 12542. https://doi.org/10.3390/ijerph191912542
Nowosielecka D, Jacheć W, Polewczyk A, Tułecki Ł, Stefańczyk P, Kutarski A. “Ghost”, a Well-Known but Not Fully Explained Echocardiographic Finding during Transvenous Lead Extraction: Clinical Significance. International Journal of Environmental Research and Public Health. 2022; 19(19):12542. https://doi.org/10.3390/ijerph191912542
Chicago/Turabian StyleNowosielecka, Dorota, Wojciech Jacheć, Anna Polewczyk, Łukasz Tułecki, Paweł Stefańczyk, and Andrzej Kutarski. 2022. "“Ghost”, a Well-Known but Not Fully Explained Echocardiographic Finding during Transvenous Lead Extraction: Clinical Significance" International Journal of Environmental Research and Public Health 19, no. 19: 12542. https://doi.org/10.3390/ijerph191912542





