Pharmacoepidemiological Research on N-Nitrosodimethylamine-Contaminated Ranitidine Use and Long-Term Cancer Risk: A Population-Based Longitudinal Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
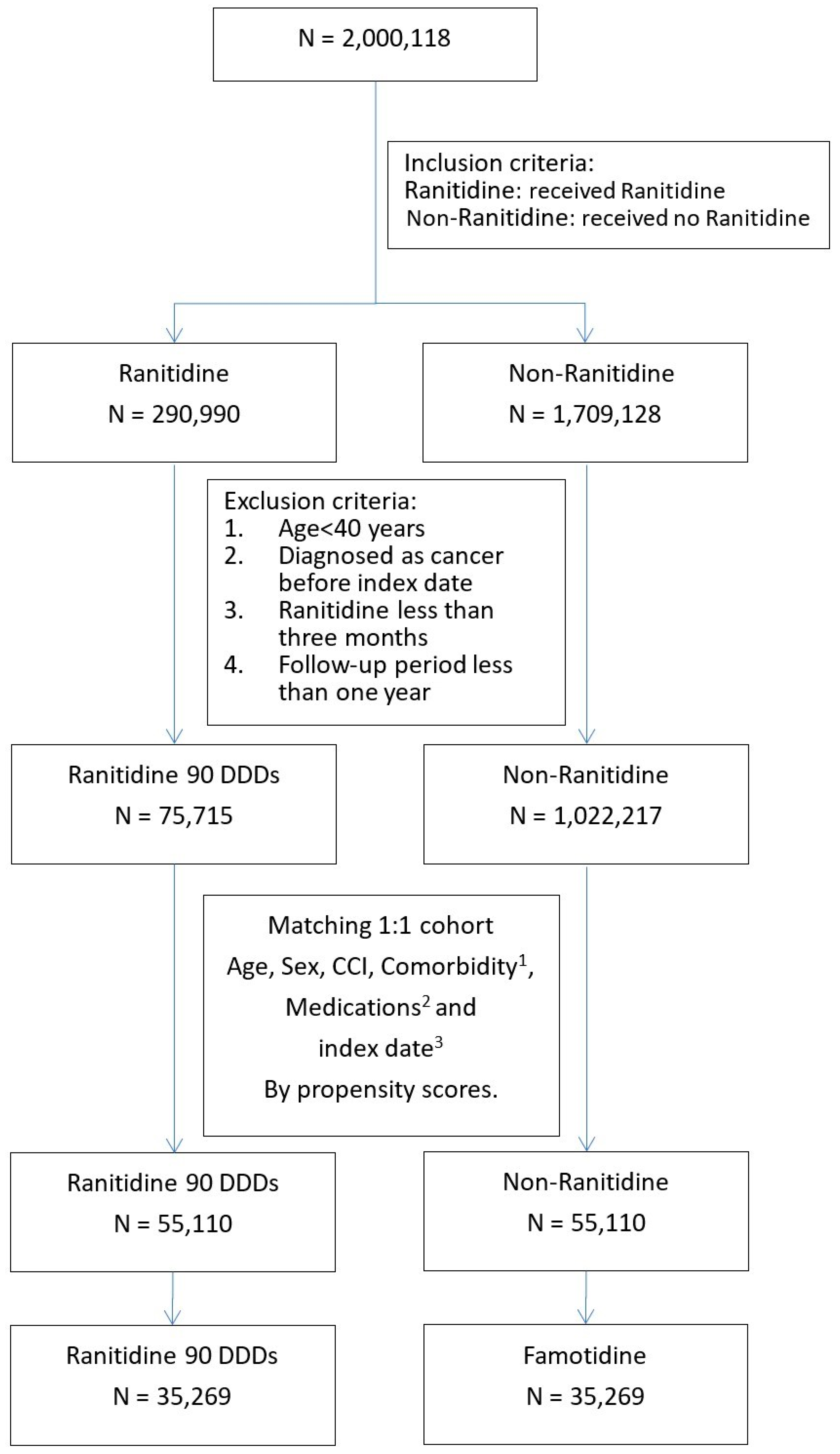
2.2. Study Design and Study Participants
2.3. Potential Confounders
2.4. Covariate Assessment
2.5. Main Outcome Measurements
2.6. Exposure Definition and Follow-Up
2.7. Statistical Analysis
3. Results
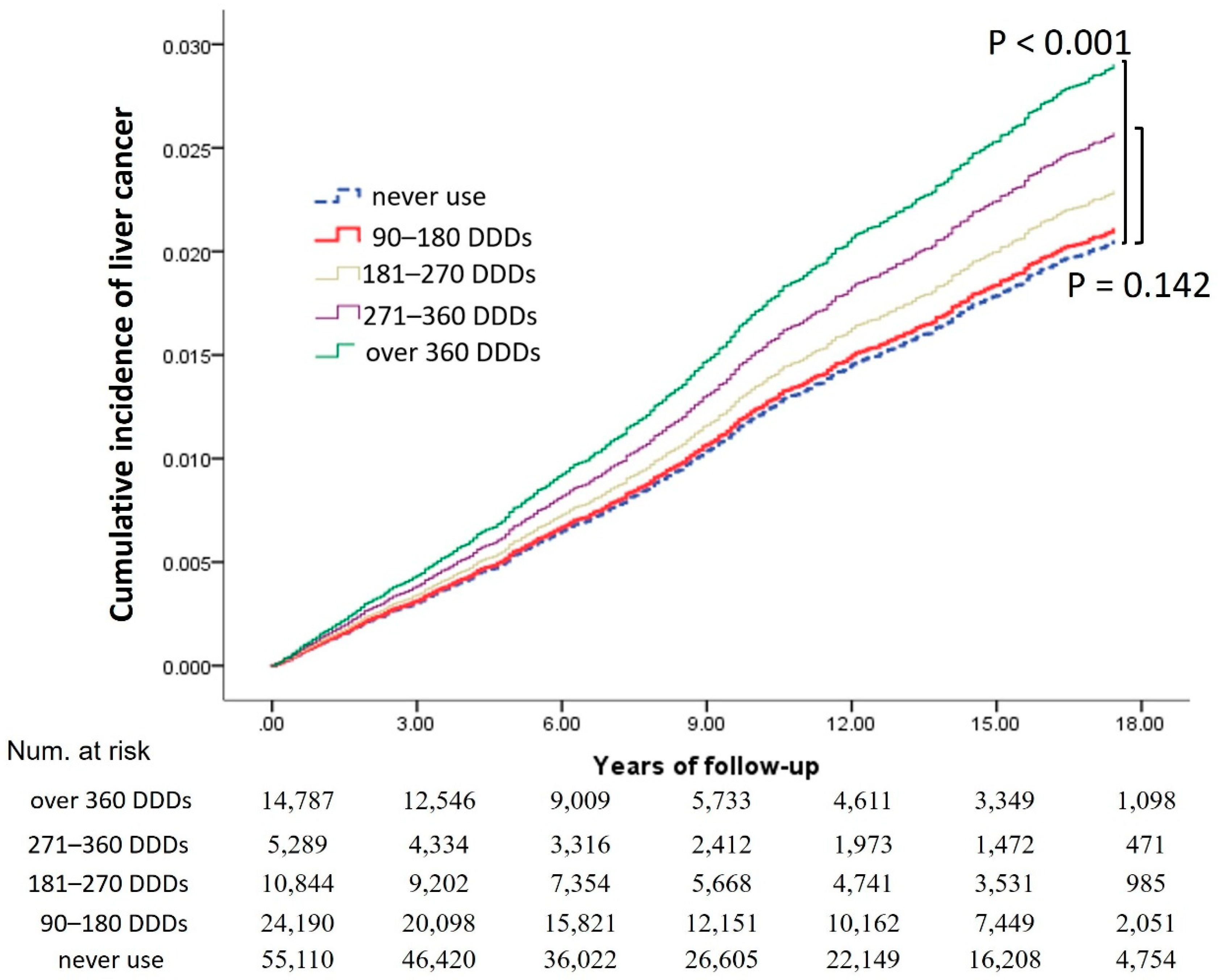
3.1. Ranitidine Duration Effect on Cancer Development
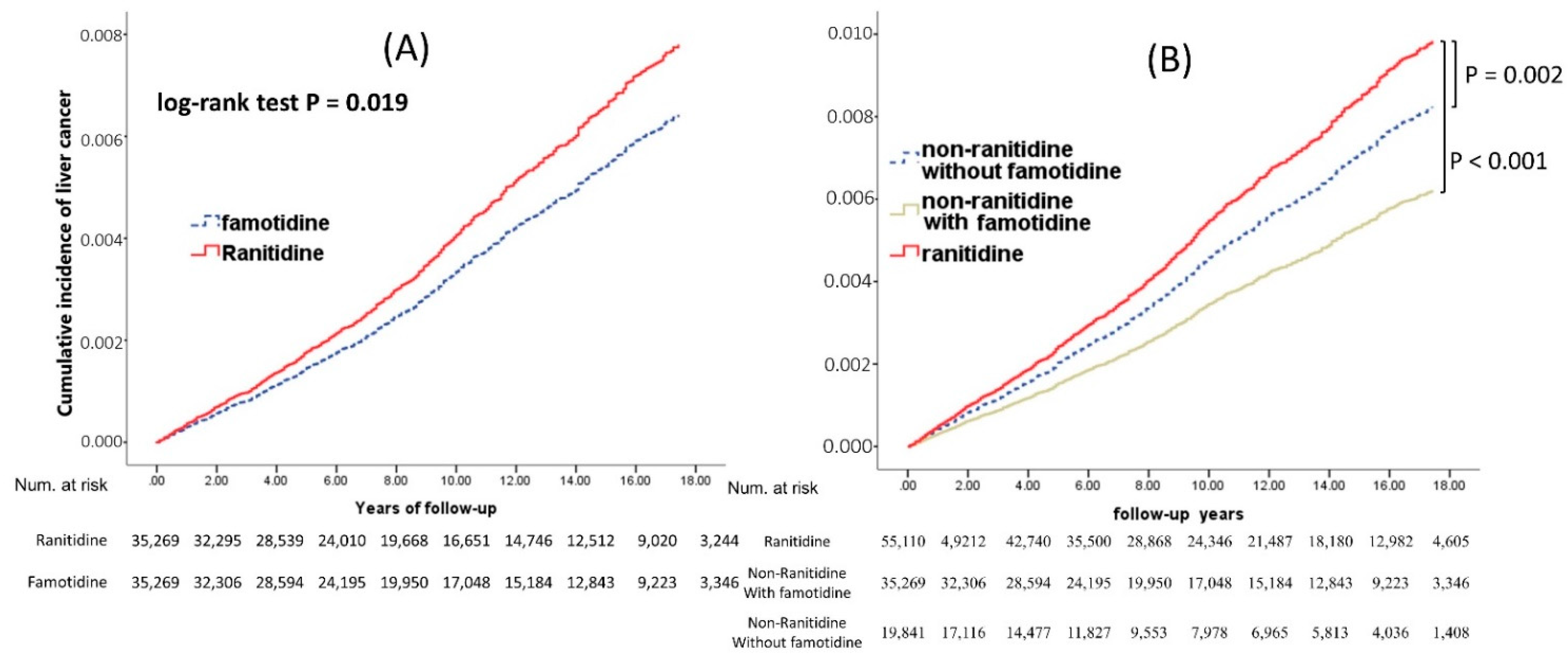
3.2. Comparison between Ranitidine and Famotidine for the Association with Patient Outcomes
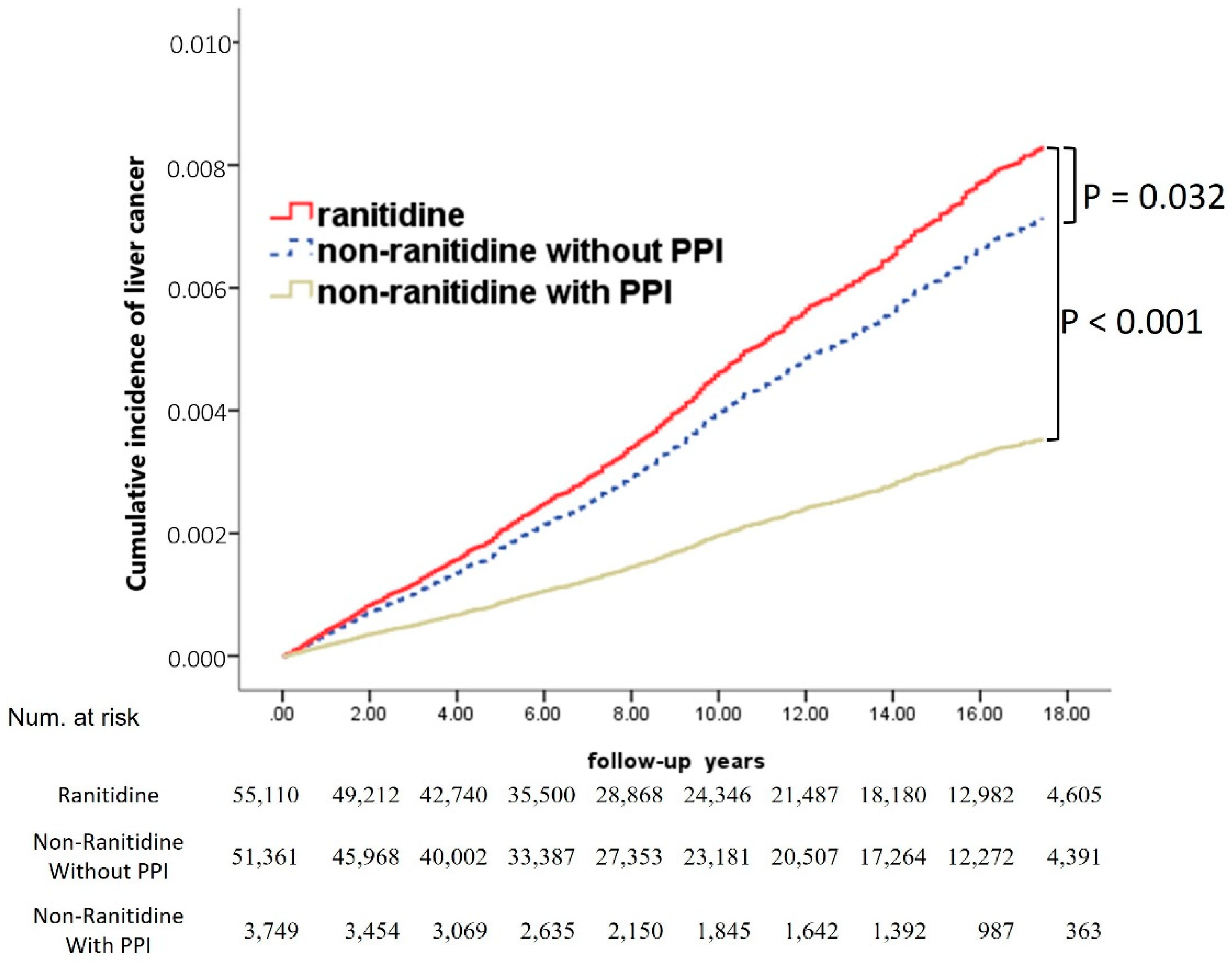
3.3. Comparison between Ranitidine and PPIs for Their Association with Liver Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipsy, R.J.; Fennerty, B.; Fagan, T.C. Clinical review of histamine2 receptor antagonists. Arch. Intern. Med 1990, 150, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, N.S.; Straus, S.M.; Mantel-Teeuwisse, A.K.; Domergue, F.; Egberts, T.C.; Leufkens, H.G.; De Bruin, M.L. Traceability of biopharmaceuticals in spontaneous reporting systems: A cross-sectional study in the FDA Adverse Event Reporting System (FAERS) and EudraVigilance databases. Drug Saf. 2013, 36, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Tricker, A.R.; Preussmann, R. Carcinogenic N-nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential. Mutat. Res. Genet. Toxicol. 1991, 259, 277–289. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Updates and Press Announcements on NDMA in Zantac (Ranitidine). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcementsndma-zantac-ranitidine (accessed on 16 April 2020).
- European Medicines Agency. EMA Reviewing Medicines Containing Valsartan from Zhejiang Huahai Following the Detection of an Impurity. 2018. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2018/07/news_detail_002984.jsp&mid=WC0b01ac058004d5c1 (accessed on 24 August 2018).
- Mahase, E. FDA recalls ranitidine medicines over potential cancer causing impurity. BMJ 2019, 367, I5832. [Google Scholar] [CrossRef]
- Medicines and Healthcare Products Regulatory Agency. Class 2 Medicines Recall: Zantac Injection 50 mg/2 mL, Zantac Syrup 150 mg/10 mL, Zantac Tablets 150 mg, Zantac Tablets 300 mg (EL (19)A 24). 8 October 2019. Available online: https://www.gov.uk/drug-devic-alerts/class-2-medicines-recall-zantac-injection-50mg-2ml-zantac-syrup-150mg-10ml-zantac-tablets-150mg-zantac-tablets-300mg-el-19-a-24 (accessed on 22 June 2020).
- Lijinsky, W.; Reuber, M.D. Carcinogenesis in rats by nitrosodimethylamine and other nitrosomethylalkylamines at low doses. Cancer Lett. 1984, 22, 83–88. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Overall Evaluations of Carcinogenicity: And Updating of IARC Monographs, Volume 1 to 42. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans: Supplement 7; IARC: Lyon, France, 1987; Volume 7, pp. 1–440. [Google Scholar]
- Brambilla, G.; Cavanna, M.; Faggin, P.; Maura, A.; Pino, A.; Ricci, R.; Robbiano, L. Genotoxic effects in rodents given high oral doses of ranitidine and sodium nitrite. Carcinogenesis 1983, 4, 1281–1285. [Google Scholar] [CrossRef]
- Loh, Y.H.; Jakszyn, P.; Luben, R.N.; Mulligan, A.A.; Mitrou, P.N.; Khaw, K.T. N-Nitroso compounds and cancer incidence: The European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. Am. J. Clin. Nutr. 2011, 93, 1053–1061. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, P.P.; Zhao, J.; Green, R.; Sun, Z.; Roebothan, B.; Squires, J.; Buehler, S.; Dicks, E.; Zhao, J.; et al. Dietary N-nitroso compounds and risk of colorectal cancer: A case-control study in Newfoundland and Labrador and Ontario, Canada. Br. J. Nutr. 2014, 111, 1109–1117. [Google Scholar] [CrossRef]
- Habel, L.A.; Levin, T.R.; Friedman, G.D. Cimetidine use and risk of breast, prostate, and other cancers. Pharmacoepidemiol. Drug Saf. 2000, 9, 149–155. [Google Scholar] [CrossRef]
- Iwagami, M.; Kumazawa, R.; Miyamoto, Y.; Ito, Y.; Ishimaru, M.; Morita, K.; Hamada, S.; Tamiya, N.; Yasunaga, H. Risk of Cancer in Association with Ranitidine and Nizatidine vs Other H2 Blockers: Analysis of the Japan Medical Data Center Claims Database 2005–2018. Drug Saf. 2021, 44, 361–371. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, J.-H.; Seo, G.H.; Park, H. Risk of Cancer Following the Use of N-Nitrosodimethylamine (NDMA) Contaminated Ranitidine Products: A Nationwide Cohort Study in South Korea. J. Clin. Med. 2021, 10, 153. [Google Scholar] [CrossRef]
- Kantor, E.D.; O’Connell, K.; Du, M.; Mendelsohn, R.B.; Liang, P.S.; Braunstein, L.Z. Ranitidine use and cancer risk: Results from UK Biobank. Gastroenterology 2021, 160, 1856–1859. [Google Scholar] [CrossRef]
- Cardwell, C.R.; McDowell, R.D.; Hughes, C.M.; Hicks, B.; Murchie, P. Exposure to Ranitidine and Risk of Bladder Cancer: A Nested Case-Control Study. Am. J. Gastroenterol. 2021, 116, 1612–1619. [Google Scholar] [CrossRef]
- McGwin, G. The Associa-tion between Ranitidine Use and Gas-trointestinal Cancers. Cancers 2021, 13, 24. [Google Scholar] [CrossRef]
- Song, P.; Wu, L.; Guan, W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: A meta-analysis. Nutrients 2015, 7, 9872–9895. [Google Scholar] [CrossRef]
- Hidajat, M.; McElvenny, D.M.; Ritchie, P.; Darnton, A.; Mueller, W.; Van Tongeren, M.; De Vocht, F. Lifetime exposure to rubber dusts, fumes and N-nitrosamines and cancer mortality in a cohort of British rubber workers with 49 years follow-up. Occup. Environ. Med. 2019, 76, 250–258. [Google Scholar] [CrossRef]
- Tran-Duy, A.; Spaetgens, B.; Hoes, A.W.; de Wit, N.J.; Stehouwer, C.D. Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1706–1719. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.; Chen, L.; Wong, I.C.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2018, 67, 28–35. [Google Scholar] [CrossRef]
- Li, D.K.; Yan, P.; Abou-Samra, A.B.; Chung, R.T.; Butt, A.A. Proton pump inhibitors are associated with accelerated development of cirrhosis, hepatic decompensation and hepatocellular carcinoma in noncirrhotic patients with chronic hepatitis C infection: Results from ERCHIVES. Aliment. Pharmacol. Ther. 2018, 47, 246–258. [Google Scholar] [CrossRef]
- Lai, S.W.; Liao, K.F.; Lai, H.C.; Lin, C.L.; Sung, F.C. Proton pump inhibitors and risk of hepatocellular carcinoma: A case-control study in Taiwan. Acta Gastro-Enterol. Belg. 2013, 76, 348–350. [Google Scholar]
- Kao, W.-Y.; Su, C.-W.; Tan, E.C.-H.; Lee, P.-C.; Chen, P.-H.; Tang, J.-H.; Huang, Y.-H.; Huo, T.-L.; Chang, C.-C.; Hou, M.-C.; et al. Proton pump inhibitors and risk of hepatocellular carcinoma in patients with chronic hepatitis B or C. Hepatology 2019, 69, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeong, S.; Park, S.J.; Chang, J.; Choi, S.; Cho, Y.; Ahn, J.C.; Lee, G.; Son, J.S.; Park, S.M. Association between Proton Pump Inhibitor Use and Risk of Hepatocellular Carcinoma: A Korean Nationally Representative Cohort Study. J. Clin. Med. 2022, 11, 2865. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. ATC/DDD Index 2020. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 22 June 2020).
- Wesdorp, I.C.; Dekker, W.; Klinkenberg-Knol, E.C. Treatment of reflux oesophagitis with ranitidine. Gut 1983, 24, 921–924. [Google Scholar] [CrossRef]
- Ashton, M.G.; Holdsworth, C.D.; Ryan, F.P.; Moore, M. Healing of gastric ulcers after one, two, and three months of ranitidine. Br. Med. J. (Clin. Res. Ed.) 1982, 284, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Dusetzina, S.B.; Farley, J.F. Revisiting the washout period in the incident user study design: Why 6–12 months may not be sufficient. J. Comp. Eff. Res. 2015, 4, 27–35. [Google Scholar] [CrossRef]
- Greifer, N. Estimating Effects After Matching. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Available online: https://cran.r-project.org/web/packages/MatchIt/vignettes/estimating-effects.html (accessed on 1 August 2022).
- Abadie, A.; Spiess, J. Robust post-matching inference. J. Am. Stat. Assoc. 2022, 117, 983–995. [Google Scholar] [CrossRef]
- Higbee, J.D.; Lefler, J.S.; Burnett, R.T.; Ezzati, M.; Marshall, J.D.; Kim, S.-Y.; Bechle, M.; Robinson, A.L.; Pope, C.A., III. Estimating long-term pollution exposure effects through inverse probability weighting methods with Cox proportional hazards models. Environ. Epidemiol. 2020, 4, e085. [Google Scholar] [CrossRef]
- Davison, A.C.; Hinkley, D.V. Bootstrap Methods and Their Application; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Knekt, P.; Järvinen, R.; Dich, J.; Hakulinen, T. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: A follow-up study. Int. J. Cancer 1999, 80, 852–856. [Google Scholar] [CrossRef]
- Bartsch, H.; KO’Neill, I. Ninth International Meeting on N-Nitroso Compounds: Exposures, Mechanisms, and Relevance to Human Cancer. Cancer Res. 1988, 48, 4711–4714. [Google Scholar]
- Thorens, J.; Froehlich, F.; Schwizer, W.; Saraga, E.; Bille, J.; Gyr, K.; Duroux, P.; Nicolet, M.; Pignatelli, B.; Blum, A.L.; et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: A prospective randomised double blind study. Gut 1996, 39, 54–59. [Google Scholar] [CrossRef]
- Laine, L.; Ahnen, D.; McClain, C.; Solcia, E.; Walsh, J.H. potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment. Pharmacol. Ther. 2000, 14, 651–668. [Google Scholar] [CrossRef]
- Tao, X.; Wang, N.; Qin, W. Gut microbiota and hepatocellular carcinoma. Gastrointest. Tumors 2015, 2, 33–40. [Google Scholar] [CrossRef]
- Tannenbaum, S.R. N-nitroso compounds: A perspective on human exposure. Lancet 1983, 1, 629–632. [Google Scholar] [CrossRef]
- Mitacek, E.J.; Brunnemann, K.D.; Suttajit, M.; Martin, N.; Limsila, T.; Ohshima, H.; Caplan, L.S. Exposure to N-nitroso compounds in a population of high liver cancer regions in Thailand: Volatile nitrosamine (VNA) levels in Thai food. Food Chem. Toxicol. 1999, 37, 297–305. [Google Scholar] [CrossRef]
- Jirillo, E.; Caccavo, D.; Magrone, T.; Piccigallo, E.; Amati, L.; Lembo, A.; Kalis, C.; Gumenscheimer, M. The role of the liver in the response to LPS: Experimental and clinical findings. J. Endotoxin Res. 2002, 8, 319–327. [Google Scholar] [CrossRef]
- Darnaud, M.; Faivre, J.; Moniaux, N. Targeting gut flora to prevent progression of hepatocellular carcinoma. J. Hepatol. 2013, 58, 385–387. [Google Scholar] [CrossRef]
- Orlando, L.A.; Lenard, L.; Orlando, R.C. Chronic hypergastrinemia: Causes and consequences. Dig. Dis. Sci. 2007, 52, 2482–2489. [Google Scholar] [CrossRef]
- Moore, T.C.; Jepeal, L.I.; Boylan, M.O.; Singh, S.K.; Boyd, N.; Beer, D.G.; Chang, A.J.; Wolfe, M.M. Gastrin stimulates receptor-mediated proliferation of human esophageal adenocarcinoma cells. Regul. Pept. 2004, 120, 195–203. [Google Scholar] [CrossRef]
- Salas, M.; Hotman, A.; Stricker, B.H. Confounding by indication: An example of variation in the use of epidemiologic terminology. Am. J. Epidemiol. 1999, 149, 981–983. [Google Scholar] [CrossRef]
- Ray, W.A. Evaluating medication effects outside of clinical trials: New-user designs. Am. J. Epidemiol. 2003, 158, 915–920. [Google Scholar] [CrossRef]
- Perrio, M.; Waller, P.C.; Shakir, S.A. An analysis of the exclusion criteria used in observational pharmacoepidemiological studies. Pharmacoepidemiol. Drug Saf. 2007, 16, 329–336. [Google Scholar] [CrossRef]
- Horwitz, R.I.; Feinstein, A.R. The problem of “protopathic bias” in case-control studies. Am. J. Med. 1980, 68, 255–258. [Google Scholar] [CrossRef]
- Stürmer, T.; Joshi, M.; Glynn, R.J.; Avorn, J.; Rothman, K.J.; Schneeweiss, S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J. Clin. Epidemiol. 2006, 59, 437.e1–437.e24. [Google Scholar] [CrossRef] [PubMed]
- Hallas, J.; Pottegård, A. Use of self-controlled designs in pharmacoepidemiology. J. Intern. Med. 2014, 275, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Peikes, D.N.; Moreno, L.; Orzol, S.M. Propensity score matching: A note of caution for evaluators of social programs. Am. Stat. 2008, 62, 222–231. [Google Scholar] [CrossRef]
- Swenberg, J.A.; Bedell, M.A.; Billings, K.C.; Umbenhauer, D.R.; Pegg, A.E. Cell-specific differences in O6-alkylguanine DNA repair activity during continuous exposure to carcinogen. Proc. Natl. Acad. Sci. USA 1982, 79, 5499–5502. [Google Scholar] [CrossRef]
- Bedell, M.A.; Lewis, J.G.; Billings, K.C.; Swenberg, J.A. Cell specificity in hepatocarcinogenesis: 06-methylguanine preferentially accumulates in target cell DNA during continuous exposure of rats to 1,2-dimethylhydrazine. Cancer Res. 1982, 42, 3079–3083. [Google Scholar]
- Dumenco, L.L.; Allay, E.; Norton, K.; Gerson, S.L. The prevention of thymic lymphomas in transgenic mice by human O6-alkylguanine-DNA alkyltransferase. Science 1993, 259, 219–222. [Google Scholar] [CrossRef]
- Nakatsuru, Y.; Matsukuma, S.; Nemoto, N.; Sugano, H.; Sekiguchi, M.; Ishikawa, T. O6-methylguanine-DNA methyltransferase protects against nitrosamine-induced hepatocarcinogenesis. Proc. Natl. Acad. Sci. USA 1993, 90, 6468–6472. [Google Scholar] [CrossRef]
- Zaidi, N.H.; Pretlow, T.P.; O’Riordan, M.A.; Dumenco, L.L.; Allay, E.; Gerson, S.L. Transgenic expression of human MGMT protects against azoxymethane-induced aberrant crypt foci and G to A mutations in the K-ras oncogene of mouse colon. Carcinogenesis 1995, 16, 451–456. [Google Scholar] [CrossRef]
- Souliotis, V.L.; Henneman, J.R.; Reed, C.D.; Chhabra, S.K.; Diwan, B.A.; Anderson, L.M.; Kyrtopoulos, S.A. DNA adducts and liver DNA replication in rats during chronic exposure to N-nitrosodimethylamine (NDMA) and their relationships to the dose-dependence of NDMA hepatocarcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2002, 500, 75–87. [Google Scholar] [CrossRef]

| Characteristics | Untreated n = 55,110 | % | Ranitidine n = 55,110 | % | p-Value | |
|---|---|---|---|---|---|---|
| Sex | Female | 28,794 | 52.2% | 28,794 | 52.2% | 1.000 |
| male | 26,316 | 47.8% | 26,316 | 47.8% | ||
| Age (mean ± SD) | 66.8 ± 14.1 | 66.8 ± 14.1 | 1.000 | |||
| CCI | 0–1 | 12,444 | 22.6% | 12,444 | 22.6% | 1.000 |
| 2–3 | 16,205 | 29.4% | 16,205 | 29.4% | ||
| 4–5 | 12,799 | 23.2% | 12,799 | 23.2% | ||
| >5 | 13,662 | 24.8% | 13,662 | 24.8% | ||
| HCD | No | 22,204 | 40.3% | 22,204 | 40.3% | 1.000 |
| Yes | 32,906 | 59.7% | 32,906 | 59.7% | ||
| Hyperlipidemia | No | 26,072 | 47.3% | 26,072 | 47.3% | 1.000 |
| Yes | 29,038 | 52.7% | 29,038 | 52.7% | ||
| DM | No | 37,598 | 68.2% | 37,598 | 68.2% | 1.000 |
| Yes | 17,512 | 31.8% | 17,512 | 31.8% | ||
| CKD | No | 49,704 | 90.2% | 49,704 | 90.2% | 1.000 |
| Yes | 5406 | 9.8% | 5406 | 9.8% | ||
| Aspirin | No | 25,810 | 46.8% | 25,810 | 46.8% | 1.000 |
| Yes | 29,300 | 53.2% | 29,300 | 53.2% | ||
| Statins | No | 32,089 | 58.2% | 32,089 | 58.2% | 1.000 |
| Yes | 23,021 | 41.8% | 23,021 | 41.8% | ||
| ACEIs | No | 31,214 | 56.6% | 31,214 | 56.6% | 1.000 |
| Yes | 23,896 | 43.4% | 23,896 | 43.4% | ||
| β-Blockers | No | 16,047 | 29.1% | 16,047 | 29.1% | 1.000 |
| Yes | 39,063 | 70.9% | 39,063 | 70.9% | ||
| Famotidine | No | 19,841 | 36.0% | 19,841 | 36.0% | 1.000 |
| Yes | 35,269 | 64.0% | 35,269 | 64.0% | ||
| Spironolactone | No | 48,320 | 87.7% | 48,320 | 87.7% | 1.000 |
| Yes | 6790 | 12.3% | 6790 | 12.3% | ||
| Glucocorticoids | No | 5748 | 10.4% | 5748 | 10.4% | 1.000 |
| Yes | 49,362 | 89.6% | 49,362 | 89.6% | ||
| SSRIs | No | 44,749 | 81.2% | 44,749 | 81.2% | 1.000 |
| Yes | 10,361 | 18.8% | 10,361 | 18.8% | ||
| Antiviral therapy | No | 54,271 | 98.5% | 54,271 | 98.5% | 1.000 |
| Yes | 839 | 1.5% | 839 | 1.5% | ||
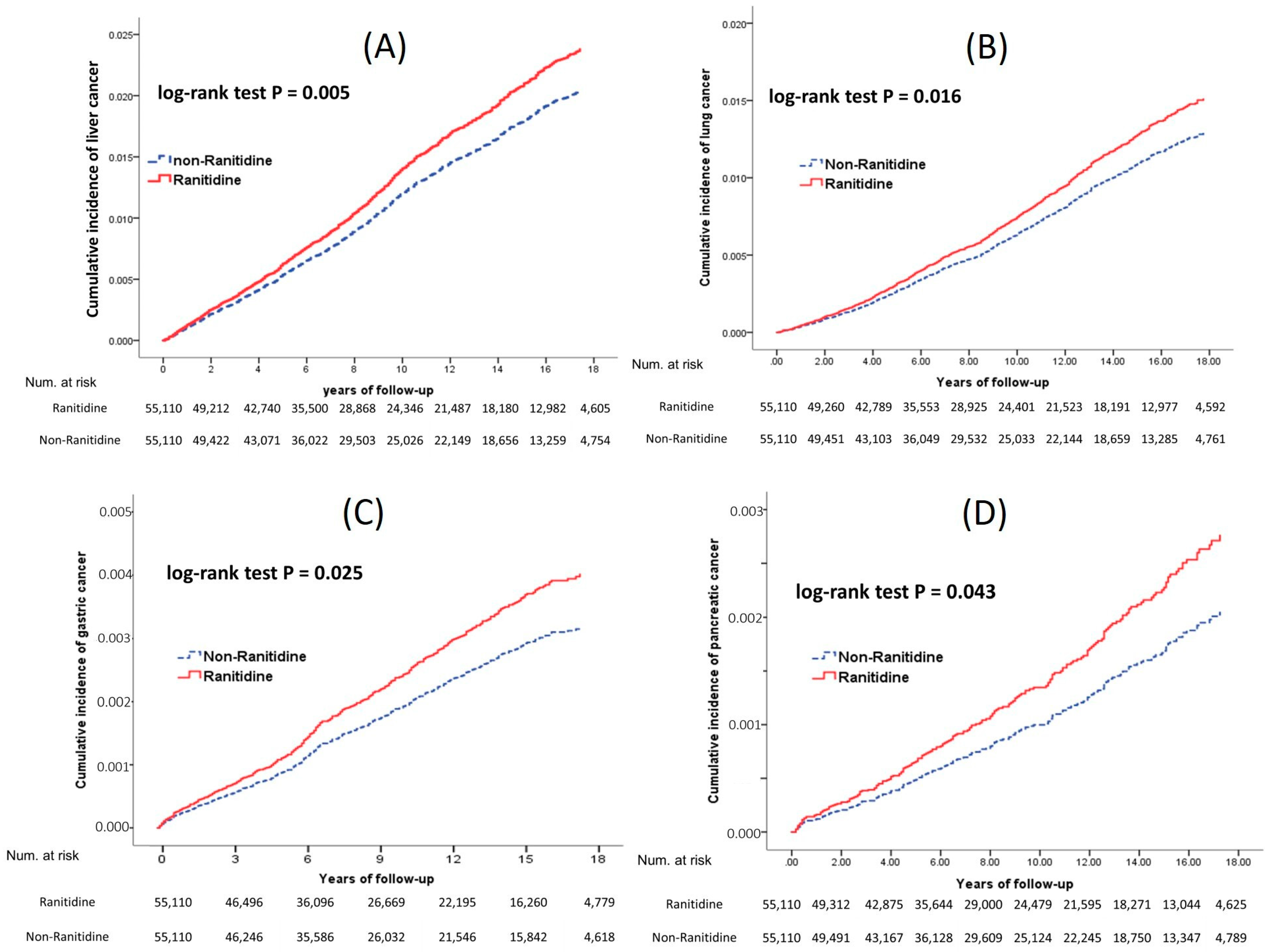
| Cancers | Untreated | Incidence Rate * | (95% CI) | Ranitidine | Incidence Rate * | (95% CI) |
|---|---|---|---|---|---|---|
| Liver cancer | 619 | 1.16 | (1.07–1.25) | 711 | 1.35 | (1.25–1.45) |
| Oral Cancer | 191 | 0.36 | (0.30–0.41) | 161 | 0.30 | (0.26–0.36) |
| Esophageal cancer | 82 | 0.15 | (0.12–0.19) | 101 | 0.19 | (0.16–0.23) |
| Gastric cancer | 210 | 0.39 | (0.34–0.44) | 255 | 0.48 | (0.43–0.55) |
| Colon cancer | 527 | 0.99 | (0.90–1.07) | 492 | 0.93 | (0.85–1.02) |
| Rectal cancer | 286 | 0.53 | (0.47–0.60) | 304 | 0.58 | (0.51–0.64) |
| Pancreas cancer | 93 | 0.17 | (0.14–0.21) | 121 | 0.23 | (0.19–0.27) |
| Lung cancer | 575 | 1.07 | (0.98–1.16) | 649 | 1.23 | (1.14–1.33) |
| Bone cancer | 3 | 0.01 | (0.00–0.02) | 4 | 0.01 | (0.00–0.02) |
| Bladder cancer | 177 | 0.33 | (0.28–0.38) | 181 | 0.34 | (0.30–0.40) |
| Renal cancer | 154 | 0.29 | (0.25–0.33) | 181 | 0.34 | (0.30–0.40) |
| Thyroid cancer | 96 | 0.18 | (0.14–0.21) | 90 | 0.17 | (0.14–0.21) |
| Skin cancer | 203 | 0.38 | (0.33–0.43) | 189 | 0.36 | (0.31–0.41) |
| Breast cancer (female) | 407 | 0.76 | (0.69–0.83) | 455 | 0.86 | (0.79–0.95) |
| Uterine cancer | 67 | 0.12 | (0.09–0.15) | 53 | 0.10 | (0.08–0.13) |
| Cervix cancer | 166 | 0.31 | (0.27–0.36) | 155 | 0.29 | (0.25–0.34) |
| Ovarian cancer | 54 | 0.10 | (0.08–0.13) | 42 | 0.08 | (0.06–0.11) |
| Prostate cancer | 306 | 0.57 | (0.51–0.63) | 313 | 0.59 | (0.53–0.66) |
| All cancers | 4399 | 8.49 | (8.24–8.73) | 4682 | 9.19 | (8.93–9.45) |
| Liver Cancer | p | Gastric Cancer | p | Lung Cancer | p | Pancreatic Cancer | p | |
|---|---|---|---|---|---|---|---|---|
| Never used | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 90–180 DDDs * | 1.03 (0.89–1.18) | 0.690 | 1.26 (1.00–1.59) | 0.049 | 1.25 (1.09–1.44) | 0.002 | 1.64 (1.19–2.26) | 0.003 |
| 181–270 DDDs | 1.12 (0.93–1.34) | 0.220 | 1.13 (0.82–1.54) | 0.452 | 1.09 (0.90–1.32) | 0.403 | 1.10 (0.69–1.77) | 0.682 |
| 271–360 DDDs | 1.26 (0.99–1.61) | 0.064 | 1.27 (0.84–1.93) | 0.252 | 1.31 (1.02–1.68) | 0.032 | 0.92 (0.45–1.89) | 0.816 |
| Over 360 DDDs | 1.42 (1.22–1.66) | <0.001 | 1.33 (1.02–1.74) | 0.037 | 1.04 (0.87–1.24) | 0.658 | 1.22 (0.80–1.85) | 0.358 |
| Cancers | Famotidine | % | Ranitidine | % | Total | p-Value | HR (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Liver cancer | 380 | 1.1% | 442 | 1.3% | 822 | 0.032 | 1.22(1.06–1.40) | 0.005 |
| Oral cancer | 125 | 0.4% | 107 | 0.3% | 232 | 0.237 | 0.87(0.67–1.12) | 0.286 |
| Esophageal cancer | 52 | 0.1% | 60 | 0.2% | 112 | 0.451 | 1.19(0.82–1.72) | 0.364 |
| Gastric cancer | 142 | 0.4% | 165 | 0.5% | 307 | 0.208 | 1.19(0.95–1.49) | 0.122 |
| Colon cancer | 365 | 1.0% | 309 | 0.9% | 674 | 0.033 | 0.86(0.74–1.01) | 0.059 |
| Rectal cancer | 193 | 0.5% | 195 | 0.6% | 388 | 0.919 | 1.03(0.84–1.26) | 0.768 |
| Pancreas cancer | 66 | 0.2% | 80 | 0.2% | 146 | 0.281 | 1.25(0.90–1.73) | 0.186 |
| Lung cancer | 356 | 1.0% | 400 | 1.1% | 756 | 0.116 | 1.15(1.00–1.33) | 0.052 |
| Bone cancer * | n/a | n/a | n/a | n/a | n/a | n/a | 1.51(0.25–9.03) | 0.652 |
| Bladder cancer | 116 | 0.3% | 117 | 0.3% | 233 | 0.948 | 1.03(0.80–1.33) | 0.830 |
| Renal cancer | 98 | 0.3% | 128 | 0.4% | 226 | 0.053 | 1.33(1.02–1.73) | 0.034 |
| Thyroid cancer | 71 | 0.2% | 63 | 0.2% | 134 | 0.545 | 0.89(0.64–1.25) | 0.514 |
| Skin cancer | 137 | 0.4% | 137 | 0.4% | 274 | 1.000 | 1.02(0.81–1.30) | 0.842 |
| All cancers | 2924 | 8.3% | 3052 | 8.7% | 5976 | 0.086 | 1.07(1.02–1.12) | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-H.; Chen, I.-I.; Chen, C.-H.; Tseng, Y.-T. Pharmacoepidemiological Research on N-Nitrosodimethylamine-Contaminated Ranitidine Use and Long-Term Cancer Risk: A Population-Based Longitudinal Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 12469. https://doi.org/10.3390/ijerph191912469
Wang C-H, Chen I-I, Chen C-H, Tseng Y-T. Pharmacoepidemiological Research on N-Nitrosodimethylamine-Contaminated Ranitidine Use and Long-Term Cancer Risk: A Population-Based Longitudinal Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(19):12469. https://doi.org/10.3390/ijerph191912469
Chicago/Turabian StyleWang, Chun-Hsiang, I-I Chen, Chung-Hung Chen, and Yuan-Tsung Tseng. 2022. "Pharmacoepidemiological Research on N-Nitrosodimethylamine-Contaminated Ranitidine Use and Long-Term Cancer Risk: A Population-Based Longitudinal Cohort Study" International Journal of Environmental Research and Public Health 19, no. 19: 12469. https://doi.org/10.3390/ijerph191912469
APA StyleWang, C.-H., Chen, I.-I., Chen, C.-H., & Tseng, Y.-T. (2022). Pharmacoepidemiological Research on N-Nitrosodimethylamine-Contaminated Ranitidine Use and Long-Term Cancer Risk: A Population-Based Longitudinal Cohort Study. International Journal of Environmental Research and Public Health, 19(19), 12469. https://doi.org/10.3390/ijerph191912469





