Exercise Interventions Improved Sleep Quality through Regulating Intestinal Microbiota Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Exercise Intervention
2.3. Stool Collection
2.4. Extraction and Sequencing of Stool DNA
2.5. Analysis of Biological Information
2.6. Ethical and Data Protection Issues
3. Results
3.1. Demographic Characteristics
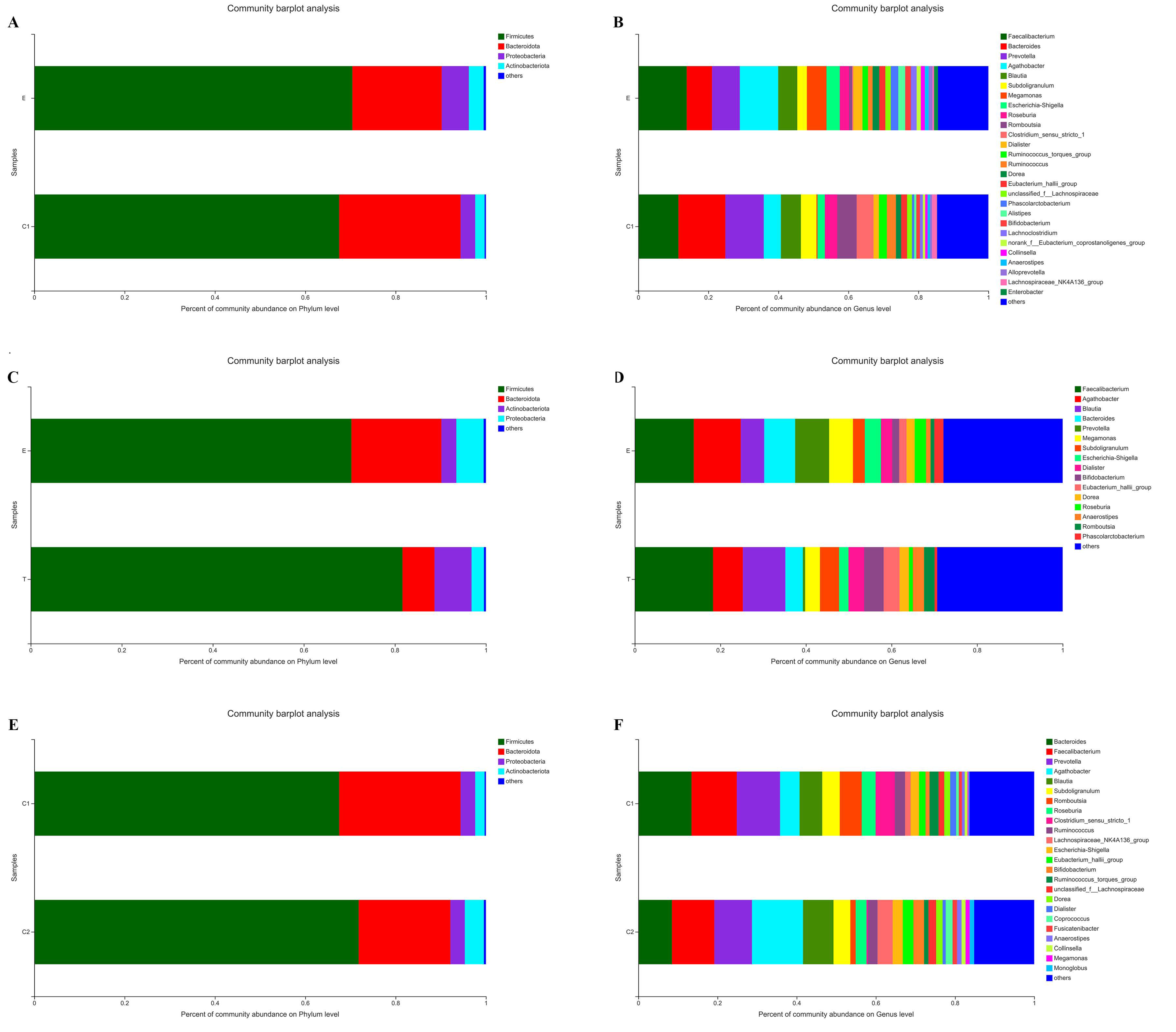
3.2. Species Composition Analysis
3.3. Alpha Diversity Analysis
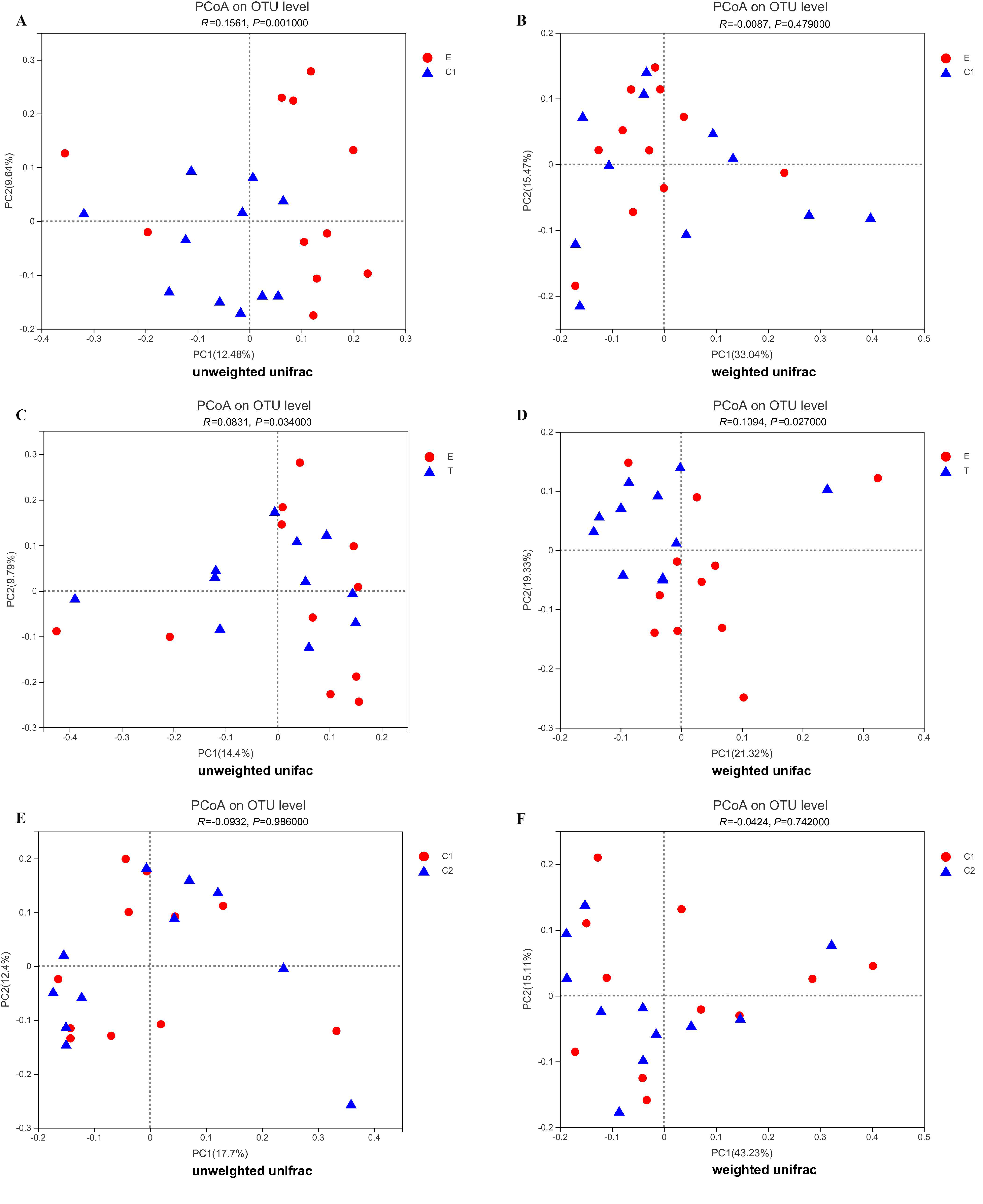
3.4. Analysis of Beta Diversity
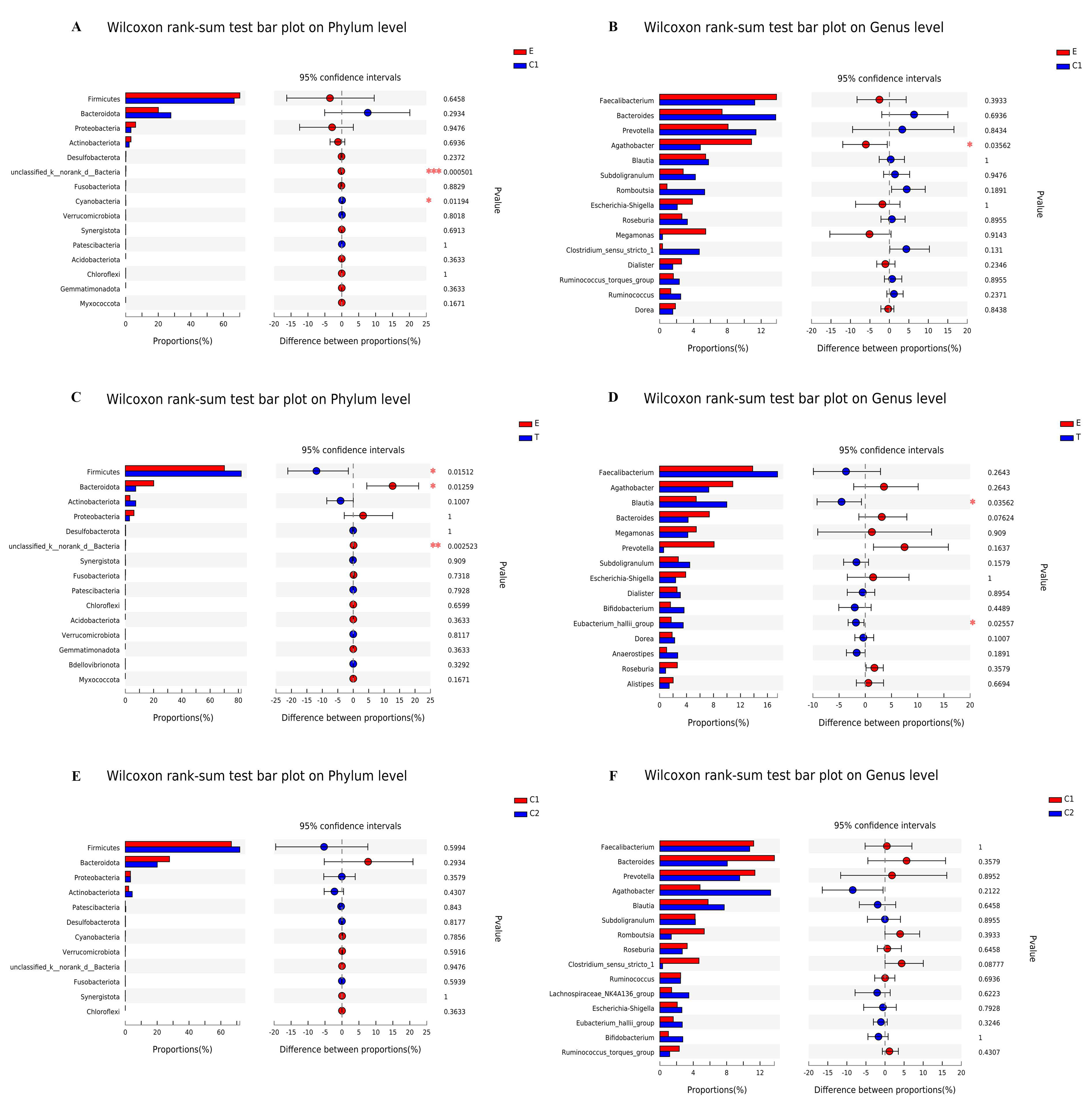
3.5. Analysis of Species Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, X.X.; Wu, Y.L.; Xu, S.J.; Zhu, R.P.; Jia, X.M.; Zhang, S.F.; Huang, K.; Zhu, P.; Hao, J.H.; Tao, F.B. Maternal anxiety during pregnancy and adverse birth outcomes: A systematic review and meta-analysis of prospective cohort studies. J. Affect. Disord. 2014, 159, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, Q.; Xiong, W.; Fan, X.; Guo, X.; Ma, J.; He, M.; Shen, Y.; Zhou, G.; Gong, P.; et al. Association between lifestyle factors and depressive symptoms among Chinese middle school students: A cross-sectional study. Psychol. Health Med. 2021, 26, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, A.M.; Albqoor, M.A. Prevalence and Correlates of Sleep Quality Among Jordanian University Students: A Cross-Sectional National Study. Eval. Health Prof. 2022, 45, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.B.; Kang, X.; Cai, Y. The gut microbiome as a target for adjuvant therapy in insomnia disorder. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101834. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.D.; Lin, W.F.; Chen, S.J.; Xiang, T.; Yang, Y.F.; Yin, Y.L.; Xu, G.H.; Liu, Z.H.; Liu, L.; Pan, J.Y.; et al. Gut Microbiota as an Objective Measurement for Auxiliary Diagnosis of Insomnia Disorder. Front. Microbiol. 2019, 10, 1770. [Google Scholar] [CrossRef]
- Yang, P.Y.; Ho, K.H.; Chen, H.C.; Chien, M.Y. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: A systematic review. J. Physiother. 2012, 58, 157–163. [Google Scholar] [CrossRef]
- McFadzean, R. Exercise Can Help Modulate Human Gut Microbiota. Honors Thesis, University of Colorado, Boulder, CO, USA, 2014. [Google Scholar]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017, 32, 300–313. [Google Scholar] [CrossRef]
- Maruvada, P.; Leone, V.; Kaplan, L.M.; Chang, E.B. The Human Microbiome and Obesity: Moving beyond Associations. Cell Host Microbe 2017, 22, 589–599. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Banerjee, A.; Pradhan, L.K.; Sahoo, P.K.; Jena, K.K.; Chauhan, N.R.; Chauhan, S.; Das, S.K. Unravelling the potential of gut microbiota in sustaining brain health and their current prospective towards development of neurotherapeutics. Arch. Microbiol. 2021, 203, 2895–2910. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA J. Am. Med. Assoc. 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, W.; Zhu, B.; Duan, R.; Yu, X.; Xu, W.; Wang, M.; Hua, W.; Yu, W.; Li, W.; et al. Prevalence and correlates of poor sleep quality among college students: A cross-sectional survey. Health Qual. Life Outcomes 2020, 18, 210. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O'Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O'Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Cataldi, S.; Bonavolontà, V.; Poli, L.; Clemente, F.M.; De Candia, M.; Carvutto, R.; Silva, A.F.; Badicu, G.; Greco, G.; Fischetti, F. The Relationship between Physical Activity, Physical Exercise, and Human Gut Microbiota in Healthy and Unhealthy Subjects: A Systematic Review. Biology 2022, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yuan, S.; Zhang, J. The interplay between sleep and gut microbiota. Brain Res. Bull. 2022, 180, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef]
- Farré, N.; Gozal, D. Sleep and the Microbiome: A Two-Way Relationship. Arch. Bronconeumol. 2019, 55, 7–8. [Google Scholar] [CrossRef]
- Frost, F. Einführung in das Mikrobiom. Die Inn. Med. 2022, 63, 1015–1021. [Google Scholar] [CrossRef]
- Matthewman, C.; Narin, A.; Huston, H.; Hopkins, C.E. Systems to model the personalized aspects of microbiome health and gut dysbiosis. Mol. Asp. Med. 2022, 101115. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Glavin, E.E.; Matthew, J.; Spaeth, A.M. Gender Differences in the Relationship Between Exercise, Sleep, and Mood in Young Adults. Health Educ. Behav. 2022, 49, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Hurdiel, R.; Watier, T.; Honn, K.; Pezé, T.; Zunquin, G.; Theunynck, D. Effects of a 12-week physical activities programme on sleep in female university students. Res. Sports Med. 2017, 25, 191–196. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Riemann, B.L.; Flatt, A.A.; Valentino, T.; Lustgarten, M.S. Self-reported sleep quality is associated with gut microbiome composition in young, healthy individuals: A pilot study. Sleep Med. 2020, 73, 76–81. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiology. Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Cryan, J.F.; O'Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Rosero, J.A.; Killer, J.; Sechovcová, H.; Mrázek, J.; Benada, O.; Fliegerová, K.; Havlík, J.; Kopečný, J. Reclassification of Eubacterium rectale (Hauduroy et al. 1937) Prévot 1938 in a new genus Agathobacter gen. nov. as Agathobacter rectalis comb. nov., and description of Agathobacter ruminis sp. nov., isolated from the rumen contents of sheep and cows. Int. J. Syst. Evol. Microbiol. 2016, 66, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Fermentation in the human large intestine: Its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011, 45, S120–S127. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Zhu, J.; Yang, T.; Guo, M.; Li, Q.; Chen, J.; Li, T. The Gut Microbiota and Associated Metabolites Are Altered in Sleep Disorder of Children with Autism Spectrum Disorders. Front. Psychiatry 2020, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.-X.; Fuentes-Broto, L. Chapter 8-Melatonin: A Multitasking Molecule. In Progress in Brain Research; Martini, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 181, pp. 127–151. [Google Scholar]
- Reiter, R.J. The melatonin rhythm: Both a clock and a calendar. Experientia 1993, 49, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Goldman, B.D. The circadian timing system and reproduction in mammals. Steroids 1999, 64, 679–685. [Google Scholar] [CrossRef]
- Center for Biologics Evaluation and Research. Guidance for Industry: Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information. 2016. Available online: https://www.fda.gov/media/82945/download (accessed on 12 June 2016).
- Engels, C.; Ruscheweyh, H.J.; Beerenwinkel, N.; Lacroix, C.; Schwab, C. The Common Gut Microbe Eubacterium hallii also Contributes to Intestinal Propionate Formation. Front. Microbiol. 2016, 7, 713. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Experimental Group (n = 11) | Control Group (n = 11) | x2/t/z | p Value |
|---|---|---|---|---|
| Age, Years | 22.55 ± 1.08 | 21.82 ± 1.27 | 1.384 | 0.182 |
| Gender, female/male | 7/4 | 11/0 | — | 0.090 |
| BMI | 17.65 ± 0.90 | 17.73 ± 1.19 | −0.156 | 0.878 |
| Smoking, yes/no | 2/9 | 0/11 | — | 0.090 |
| Drinking, yes/no | 2/9 | 1/10 | — | 0.090 |
| PSQI | ||||
| pre-exercise | 8.73 ± 1.66 | 4.64 ± 0.88 | 6.895 | <0.001 |
| post-exercise | 5.00 ± 1.41 * | — | — | — |
| Parameter | E | C1 | p Value |
|---|---|---|---|
| Sobs | 695.73 ± 130.22 | 437.09 ± 81.02 | <0.001 |
| Shannon | 3.85 ± 0.44 | 3.63 ± 0.51 | 0.27 |
| Simpson | 0.07 ± 0.04 | 0.07 ± 0.04 | 0.78 |
| Ace | 760.25 ± 146.67 | 588.35 ± 103.20 | 0.01 |
| Chao | 715.75 ± 134.95 | 569.54 ± 102.44 | 0.01 |
| Coverage | 1.00 ± 0.00 | 1.00 ± 0.00 | <0.001 |
| Parameter | E | T | p Value |
|---|---|---|---|
| Sobs | 695.73 ± 130.22 | 512.55 ± 88.59 | <0.001 |
| Shannon | 3.85 ± 0.44 | 3.90 ± 0.23 | 0.77 |
| Simpson | 0.07 ± 0.04 | 0.05 ± 0.01 | 0.26 |
| Ace | 760.25 ± 146.67 | 691.56 ± 146.48 | 0.29 |
| Chao | 715.75 ± 134.95 | 673.28 ± 126.53 | 0.46 |
| Coverage | 1.00 ± 0.00 | 1.00 ± 0.00 | <0.001 |
| Parameter | C1 | C2 | p Value |
|---|---|---|---|
| Sobs | 437.09 ± 81.02 | 411.64 ± 95.90 | 0.51 |
| Shannon | 3.63 ± 0.51 | 3.61 ± 0.37 | 0.95 |
| Simpson | 0.07 ± 0.04 | 0.07 ± 0.03 | 0.80 |
| Ace | 588.35 ± 103.20 | 563.19 ± 154.67 | 0.66 |
| Chao | 569.54 ± 102.44 | 551.30 ± 141.67 | 0.73 |
| Coverage | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Gong, F.; Wu, J.; You, D.; Zhao, Y.; Xu, L.; Cao, X.; Bao, F. Exercise Interventions Improved Sleep Quality through Regulating Intestinal Microbiota Composition. Int. J. Environ. Res. Public Health 2022, 19, 12385. https://doi.org/10.3390/ijerph191912385
Qiu L, Gong F, Wu J, You D, Zhao Y, Xu L, Cao X, Bao F. Exercise Interventions Improved Sleep Quality through Regulating Intestinal Microbiota Composition. International Journal of Environmental Research and Public Health. 2022; 19(19):12385. https://doi.org/10.3390/ijerph191912385
Chicago/Turabian StyleQiu, Liangwu, Fuhong Gong, Jiang Wu, Dingyun You, Yinzhou Zhao, Lianwu Xu, Xue Cao, and Fukai Bao. 2022. "Exercise Interventions Improved Sleep Quality through Regulating Intestinal Microbiota Composition" International Journal of Environmental Research and Public Health 19, no. 19: 12385. https://doi.org/10.3390/ijerph191912385
APA StyleQiu, L., Gong, F., Wu, J., You, D., Zhao, Y., Xu, L., Cao, X., & Bao, F. (2022). Exercise Interventions Improved Sleep Quality through Regulating Intestinal Microbiota Composition. International Journal of Environmental Research and Public Health, 19(19), 12385. https://doi.org/10.3390/ijerph191912385







