Effects of Carbonaceous Materials with Different Structures on Cadmium Fractions and Microecology in Cadmium-Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carbonaceous Materials and Soil
2.2. Experimental Design and Sample Collection
2.3. Characterization
2.4. Soil Sample Analysis
2.5. DNA Extraction and PCR Reaction
2.6. Sequencing Analysis and Statistical Analysis
3. Results
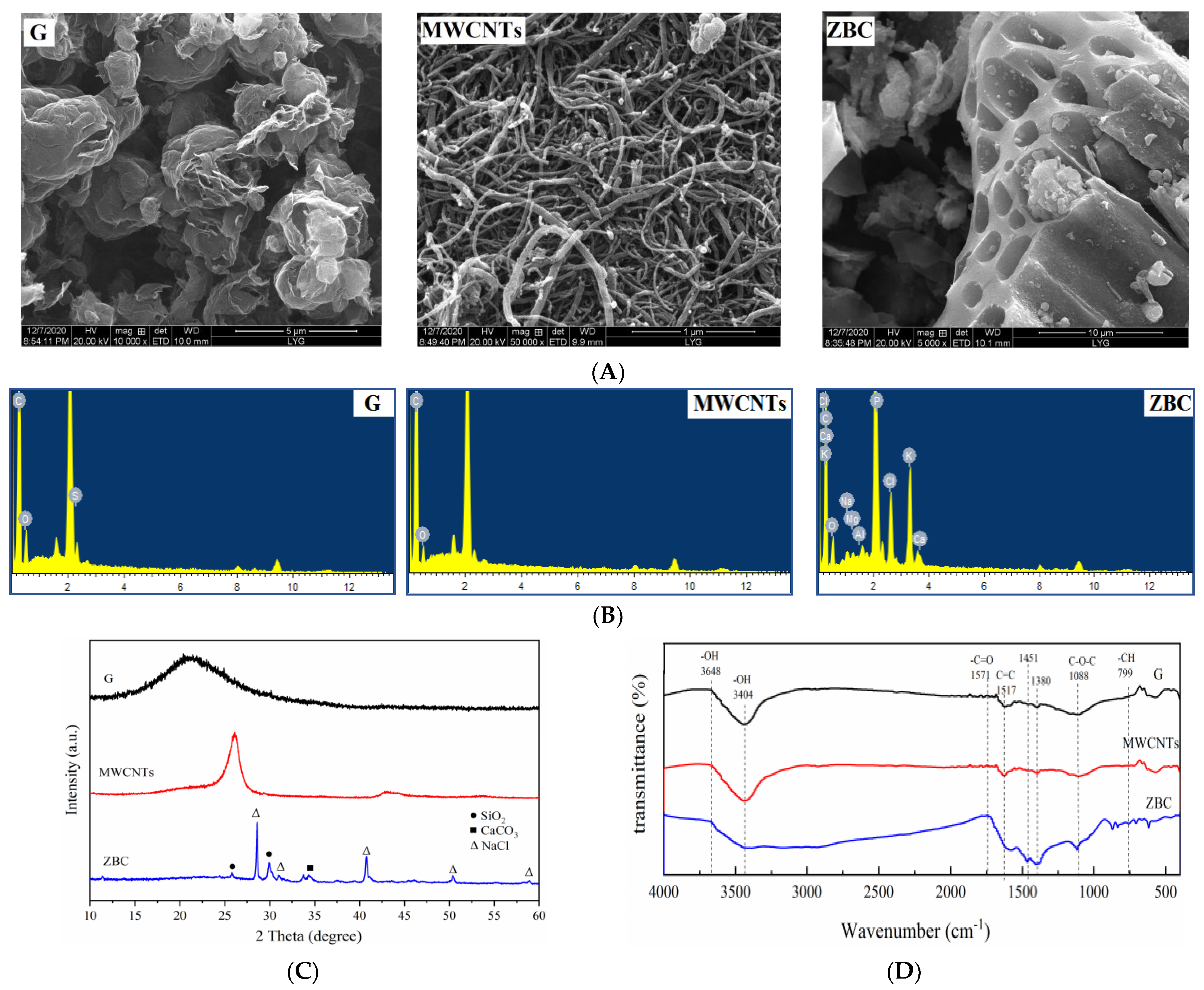
3.1. Characterization of Carbonaceous Amendments
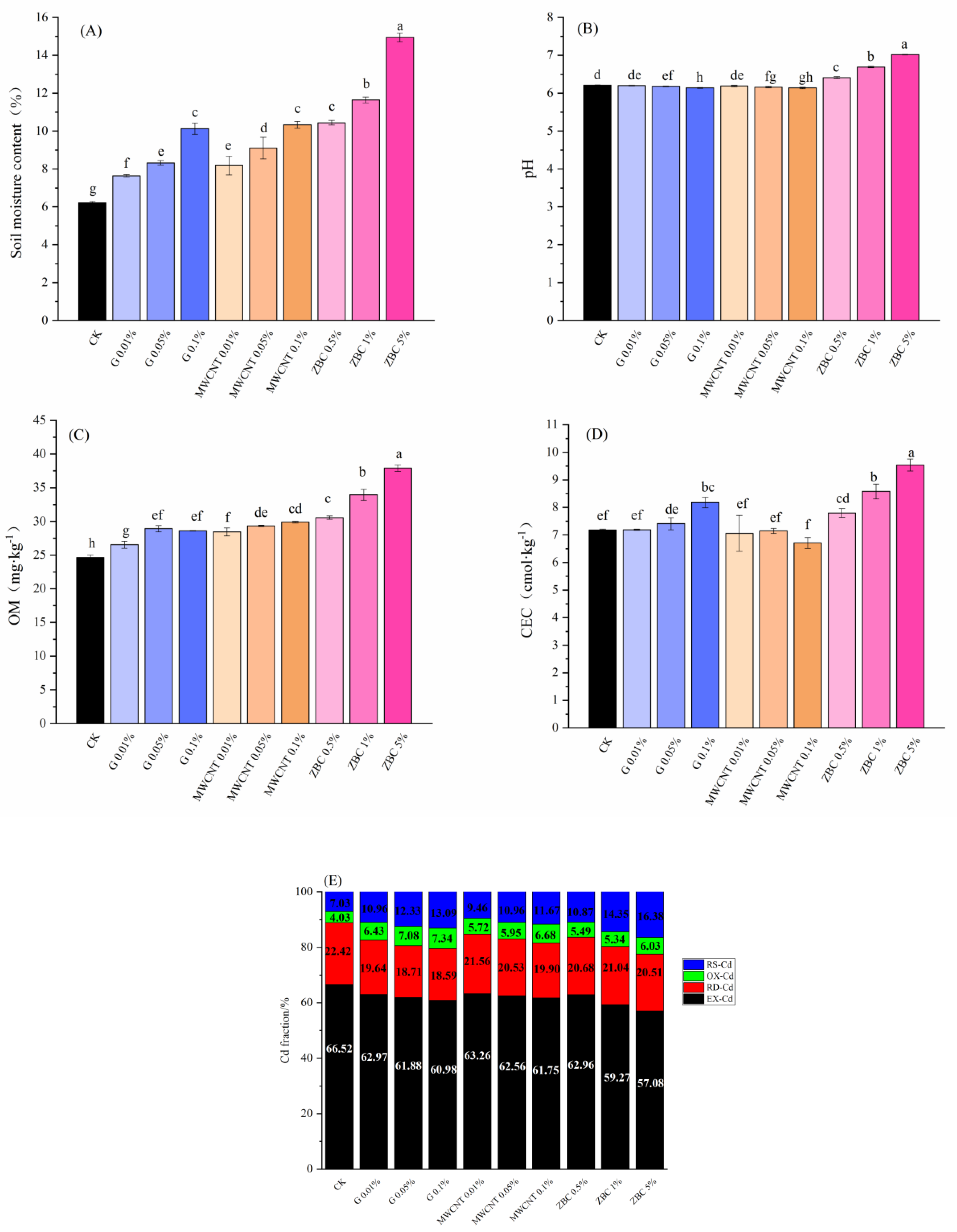
3.2. Effects of Carbonaceous Amendments on Basic Soil Properties and Cd Fractionation
3.3. Effect of Carbonaceous Amendments on Soil Microecology
3.3.1. Soil Nutrients
3.3.2. Soil Enzyme Activity
3.3.3. Effect of Carbonaceous Amendments on Soil Microbial Communities
Bacterial Community Diversity
Bacterial Community Composition and Structure
Correlation between Bacterial, Soil Nutrient, and Enzymatic Activities
Correlations between Environmental Parameters and Microbial Communities
4. Discussion
4.1. Effect of Carbonaceous Amendments on Cd Fractions
4.2. Effect of Carbonaceous Amendments on Soil Microecology
4.2.1. Soil Nutrients
4.2.2. Soil Enzyme Activities
4.2.3. Soil Microorganisms
Soil Microbial Diversity
Soil Microbial Composition and Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gorospe, J. Growing Greens and Soiled Soil: Trends in Heavy Metal Contamination in Vegetable Gardens of San Francisco. Ph.D. Thesis, San Jose State University, San Jose, CA, USA, 2012. [Google Scholar]
- Kumar, V.; Sharma, A.; Kaur, P.; Sidhu, G.P.S.; Bali, A.S.; Bhardwa, R.; Thukral, A.K.; Cerda, A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 2019, 216, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, Q.; Wu, M.; Lin, L.; Scholz, M. Review of remediation practices regarding cadmium-enriched farmland soil with particular reference to China. J. Environ. Manag. 2016, 181, 646–662. [Google Scholar] [CrossRef]
- Song, W.E.; Chen, S.B.; Liu, J.F.; Chen, L.; Song, N.N.; Li, N.; Liu, B. Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J. Integr. Agr. 2015, 14, 1845–1854. [Google Scholar] [CrossRef]
- Xu, X.H.; Zhao, Y.C.; Zhao, X.Y.; Wang, Y.D.; Deng, W.J. Sources of heavy metal pollution in agricultural soils of a rapidly industrializing area in the Yangtze Delta of China. Ecotox. Environ. Saf. 2014, 108, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Zheng, K.X.; Zhan, W.H.; Huang, L.Y.; Liu, Y.D.; Li, T.; Yang, Z.H.; Liao, Q.; Chen, R.H.; Zhang, C.S.; et al. Highly effective stabilization of Cd and Cu in two different soils and improvement of soil properties by multiple-modified biochar. Ecotox. Environ. Saf. 2021, 207, 111294. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Zhang, Y.H.; Zhan, J.; Tang, C.; Zhang, X.Z.; Huang, H.G.; Ye, D.H.; Wang, Y.D.; Li, T.X. A composite amendment benefits rice (Oryza sativa L.) safety and production in cadmium-contaminated soils by unique characteristics after oxidation modification. Sci. Total. Environ. 2021, 806, 2. [Google Scholar] [CrossRef]
- Song, D.; Xi, X.; Zheng, Q.; Liang, G.; Zhou, W.; Wang, X. Soil nutrient and microbial activity responses to two years after maize straw biochar application in a calcareous soil. Ecotoxicol. Environ. Saf. 2019, 180, 348–356. [Google Scholar] [CrossRef]
- Xu, D.Y.; Zhao, Y.; Sun, K.; Gao, B.; Wang, Z.Y.; Jin, J.; Zhang, Z.Y.; Wang, S.F.; Yan, Y.; Liu, X.T.; et al. Cadmium adsorption on plant- and manure-derived biochar and biochar-amended sandy soils: Impact of bulk and surface properties. Chemosphere 2014, 111, 320–326. [Google Scholar] [CrossRef]
- Abdelrhman, F.; Gao, J.Y.; Ali, U.; Wan, N.; Hu, H.Q. Assessment of goethite-combined/modified biochar for cadmium and arsenic remediation in alkaline paddy soil. Environ. Sci. Pollut. Res. 2022, 27, 40745–40754. [Google Scholar] [CrossRef]
- Rehman, M.Z.; Rizwan, M.; Hussain, A.; Saqib, M.; Ali, S.; Sohail, M.I.; Shafiq, M.; Hafeez, F. Alleviation of cadmium (Cd) toxicity and minimizing its uptake in wheat (Triticum aestivum) by using organic carbon sources in Cd spiked soil. Environ. Pollut. 2018, 241, 557–565. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C.H. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-Ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotox. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification-A critical review. Sci. Total Environ. 2017, 581, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Y.; Sima, J.; Zhao, L.; Masek, O.; Cao, X.D. Indispensable role of biochar-inherent mineral constituents in its environmental applications: A review. Bioresourse Technol. 2017, 241, 887–899. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Peng, T.Y.; Zhang, J.L.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.T.; Bolan, N.S.; Hou, D.Y. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef]
- Gong, X.M.; Huang, D.L.; Liu, Y.G.; Zeng, G.M.; Chen, S.; Wang, R.Z.; Xu, P.; Cheng Zhang, M.C.; Xue, W.J. Biochar facilitated the phytoremediation of cadmium contaminated sediments: Metal behavior, plant toxicity, and microbial activity. Sci. Total Environ. 2019, 666, 1126–1133. [Google Scholar] [CrossRef]
- ElNaggar, A.; Lee, S.S.; Rinklebe, F.M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- ElNaggar, A.; Lee, M.H.; Hur, J.; Lee, Y.H.; Igalavithana, A.D.; Shaheen, S.M.; Ryu, C.; Rinklebe, J.; Tsang, D.C.W.; Ok, Y.S. Biocharinduced metal immobilization and soil biogeochemical process: An integrated mechanistic approach. Sci. Total Environ. 2020, 698, 134112. [Google Scholar] [CrossRef]
- Mahesh, N.; Balakumar, S.; Shyamalagowri, S.; Manjunathan, J.; Pavithra, M.K.S.; Babu, P.S.; Kamaraj, M.; Govarthanan, M. Carbon-based adsorbents as proficient tools for the removal of heavy metals from aqueous solution: A state of art-review emphasizing recent progress and prospects. Environ. Res. 2022, 213, 113723. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.X.; Yu, S.J.; Fu, D.; Chen, J.R.; Wang, X.K. Environmental remediation of heavy metal ions by novel-nanomaterials:A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.D.; Zhang, C.; Li, W.Y.; Chen, C.Y.; Zhang, Z.; Chen, H.; Wang, J.; Li, Y.T.; Zhang, Y.L. Nano-FeS incorporated into stable lignin hydrogel: A novel strategy for cadmium removal from soil. Environ. Pollut. 2020, 264, 114739. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.S.; Imtiaz, M.; Huang, G.H.; Chhajro, M.A.; Liu, Y.H.; Fu, Q.L.; Zhu, J.; Ashraf, A.; Zafar, M.; Bashir, S. Immobilization of Pb and Cu in polluted soil by superphosphate, multi-walled carbon nanotube, rice straw and its derived biochar. Environ. Sci. Pollut. Res. 2016, 23, 15532–15543. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Yuan, X.; Wang, H.; Leng, L.J.; Li, H.; Wu, Z.B.; Jiang, L.B.; Xu, R.; Zeng, G.M. Implication of graphene oxide in Cd-contaminated soil: A case study of bacterial communities. J. Environ. Manag. 2018, 205, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Kim, M.J.; Ko, K.; Kim, J.H.; Kwon, H.A.; Hong, I.; Park, N.; Lee, S.W.; Mustafa, T.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2013, 9, 115–123. [Google Scholar]
- Nelson, D.W.; Sommers, L.E.; Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Sumner, M.E. Total carbon, organic carbon, and organic matter, methods of soil analysis. Part 3. Chem. Methods 1996, 5, 961–1010. [Google Scholar]
- Kahr, G.; Madsen, F.T. Determination of the cation exchange capacity and the surface area of bentonite, illite and kaolinite by methylene blue adsorption. Appl. Slay Sci. 1995, 9, 327–336. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods for Soil Agrochemistry; Chinese Agricultural Science and Technology Publishing House: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Yu, H.Y.; Liu, C.P.; Zhu, J.S.; Li, F.B.; Deng, D.M.; Wang, Q.; Liu, C.S. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. [Google Scholar] [CrossRef]
- Liu, X.; Gui, C.; Li, P.; Zhang, J.Y.; Zhong, H.; Wei, Y.S. Chemical forms and risk assessment of heavy metals in sludge-biochar produced by microwave-induced low temperature pyrolysis. RSC Adv. 2016, 6, 101960–101967. [Google Scholar] [CrossRef]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil Enzyme Activities and Biodiversity Measurements as Integrative Microbiological Indicators. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; American Society of Agronomy: Midson, WI, USA, 1996; Volume 49. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Al-Abed, S.R.; Jegadeesan, G.; Purandare, J.; Allen, D. Arsenic release from iron rich mineral processing waste: Influence of pH and redox potential. Chemosphere 2007, 66, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, H.T.; Li, M.; Zhang, Z.S. Removal of nitrogen and phosphorus pollutants from water by FeCl3-impregnated biochar. Ecol. Eng. 2020, 149, 105792. [Google Scholar]
- Li, Y.L.; Yu, H.; Liu, L.; Yu, H.B. Application of co-pyrolysis biochar for the adsorption and immobilization of heavy metals in contaminated environmental substrates. J. Hazard. Mater. 2021, 420, 126655. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, S.; Laird, D.A.; Wang, X.G.; Meng, Z.W. Adsorption behaviour and mechanisms of cadmium and nickel on rice straw biochars in single-and binary-metal systems. Chemosphere 2019, 218, 308–318. [Google Scholar] [CrossRef]
- Mohamed, I.; Ali, M.; Ahmed, N.; Abbas, M.H.H.; Abdelsalam, M.; Azab, A.; Fang, C. Cow manure-loaded biochar changes Cd fractionation and phytotoxicity potential for wheat in a natural acidic contaminated soil. Ecotox. Environ. Safe. 2018, 162, 348–353. [Google Scholar] [CrossRef]
- Fan, Q.; Sun, J.; Chu, L. Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef]
- Chen, H.B.; Yang, X.; Wang, H.L.; Sarkard, B.; Shaheen, S.M.; Gielen, G.; Bolan, N.; Guo, J.; Che, L.; Sun, H.L.; et al. Animal carcass- and wood-derived biochars improved nutrient bioavailability, enzyme activity, and plant growth in metal-phthalic acid ester co-contaminated soils: A trial for reclamation and improvement of degraded soils. J. Environ. Manag. 2020, 261, 110246. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Z.; Chen, L.; Sun, Y. Surface complexation modeling of adsorption of Cd(II) on graphene oxides. J. Mol. Liq. 2015, 209, 753–758. [Google Scholar] [CrossRef]
- Vithanage, M.; Herath, I.; Almaroai, Y.A.; Rajapaksha, A.U.; Huang, L.B.; Sung, J.K.; Lee, S.S.; Ok, Y.S. Effects of carbon nanotube and biochar on bioavailability of Pb, Cu and Sb in multi-metal contaminated soil. Environ. Geochem. Health 2018, 40, 565. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.Z.; Lin, X.M.; Che, L.; Ye, Z.Q.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J.L. Phosphorus sorption andavailability from biochars and soil/biochar mixtures. Clean-Soil Air Water 2014, 42, 626–634. [Google Scholar] [CrossRef]
- Cerozi, B.D.S.; Fitzsimmons, K. The effect of pH on phosphorus availability and speciation in an aquaponics nutrient solution. Bioresource Technol. 2016, 219, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xia, H.X.; Wu, J.; Yang, G.; Zhang, X.H.; Peng, H.; Yu, X.Y.; Li, L.; Xiao, H.; Qi, H. Shifts in the relative abundance of bacteria after wine-lees-derived biochar intervention in multi metal-contaminated paddy soil. Sci. Total Environ. 2017, 599–600, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Kang, F.; Xiang, Y.; Jiao, Y. Effects of humic acid-modified magnetic Fe3O4/MgAl-layered double hydroxide on the plant growth, soil enzyme activity, and metal availability. Ecotoxicol. Environ. Saf. 2019, 182, 109424. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Li, D.; Tang, B.; Man, S.; Jia, Y.; Xu, H. Vermicompost and biochar as bio-conditioners to immobilize heavy metal and improve soil fertility on cadmium contaminated soil under acid rain stress. Sci. Total Environ. 2018, 621, 1057. [Google Scholar] [CrossRef]
- Tiwari, A. A novel nanocomposite matrix based on silylated chitosan and multiwall carban nanotubes for the immobilization of urease. J. Inorg. Organomet. Polym. Mater. 2009, 19, 361–366. [Google Scholar] [CrossRef]
- Wei, X.L.; Ge, Z.Q. Effect of graphene oxide on conformation and activity of catalase. Carbon 2013, 60, 401–409. [Google Scholar] [CrossRef]
- Qu, Y.; Ma, Q.; Deng, J.; Shen, W.L.; Zhang, X.W.; He, Z.L.; Nostrand, J.D.V.; Zhou, J.t.; Zhou, J.Z. Responses of microbial communities to single-walled carbon nanotubes in phenol wastewater treatment systems. Environ. Sci. Technol. 2015, 49, 4627–4635. [Google Scholar] [CrossRef]
- Yabe, S.; Sakai, Y.; Abe, K.; Yokota, A. Diversity of Ktedonobacteria with Actinomycetes-Like morphology in terrestrial environments. Microbes Environ. 2017, 32, 61–70. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cui, J.T.; Yang, L.; Zhao, C.A.; Wang, T.Y.; Yan, L.; Liu, S.X. Phosphorus-Release Dynamics by Phosphate Solubilizing Actinomycetes and its Enhancement of Growth and Yields in Maize. Int. J. Agric. Biol. 2018, 20, 437–444. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Z.; Ma, C.; Zhu, J.; Wang, P.; Zhu, Y.; Liu, Z. Effects of Carbonaceous Materials with Different Structures on Cadmium Fractions and Microecology in Cadmium-Contaminated Soils. Int. J. Environ. Res. Public Health 2022, 19, 12381. https://doi.org/10.3390/ijerph191912381
Long Z, Ma C, Zhu J, Wang P, Zhu Y, Liu Z. Effects of Carbonaceous Materials with Different Structures on Cadmium Fractions and Microecology in Cadmium-Contaminated Soils. International Journal of Environmental Research and Public Health. 2022; 19(19):12381. https://doi.org/10.3390/ijerph191912381
Chicago/Turabian StyleLong, Zihan, Chunya Ma, Jian Zhu, Ping Wang, Yelin Zhu, and Zhiming Liu. 2022. "Effects of Carbonaceous Materials with Different Structures on Cadmium Fractions and Microecology in Cadmium-Contaminated Soils" International Journal of Environmental Research and Public Health 19, no. 19: 12381. https://doi.org/10.3390/ijerph191912381
APA StyleLong, Z., Ma, C., Zhu, J., Wang, P., Zhu, Y., & Liu, Z. (2022). Effects of Carbonaceous Materials with Different Structures on Cadmium Fractions and Microecology in Cadmium-Contaminated Soils. International Journal of Environmental Research and Public Health, 19(19), 12381. https://doi.org/10.3390/ijerph191912381




