Adsorption of Magenta Dye on PbO Doped MgZnO: Interpretation of Statistical Physics Parameters Using Double-Layer Models
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. Preparation of PbO@MgZnO
2.3. Characterization
2.4. Mathematical Modeling for the MD Adsorption
3. Results and Discussion
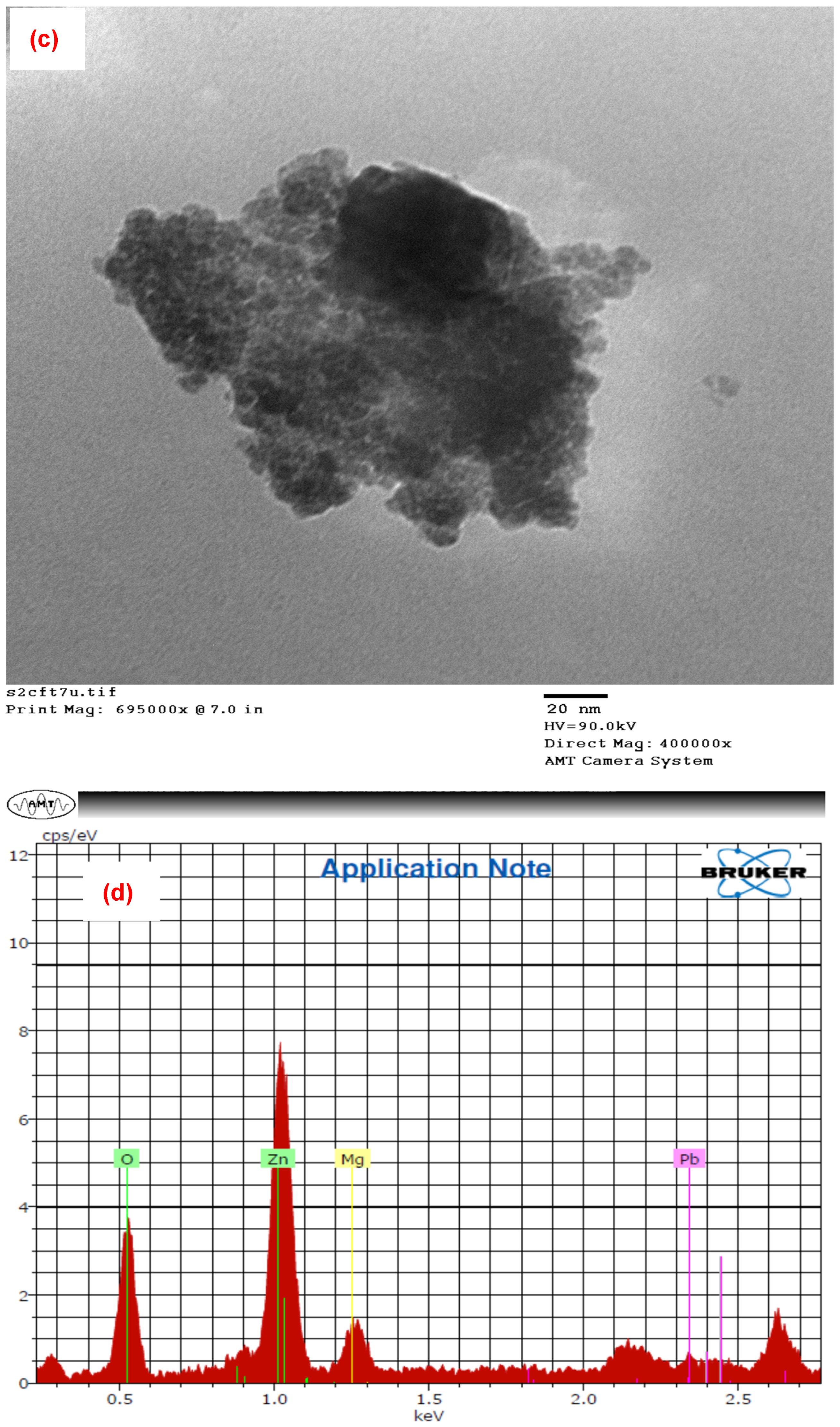
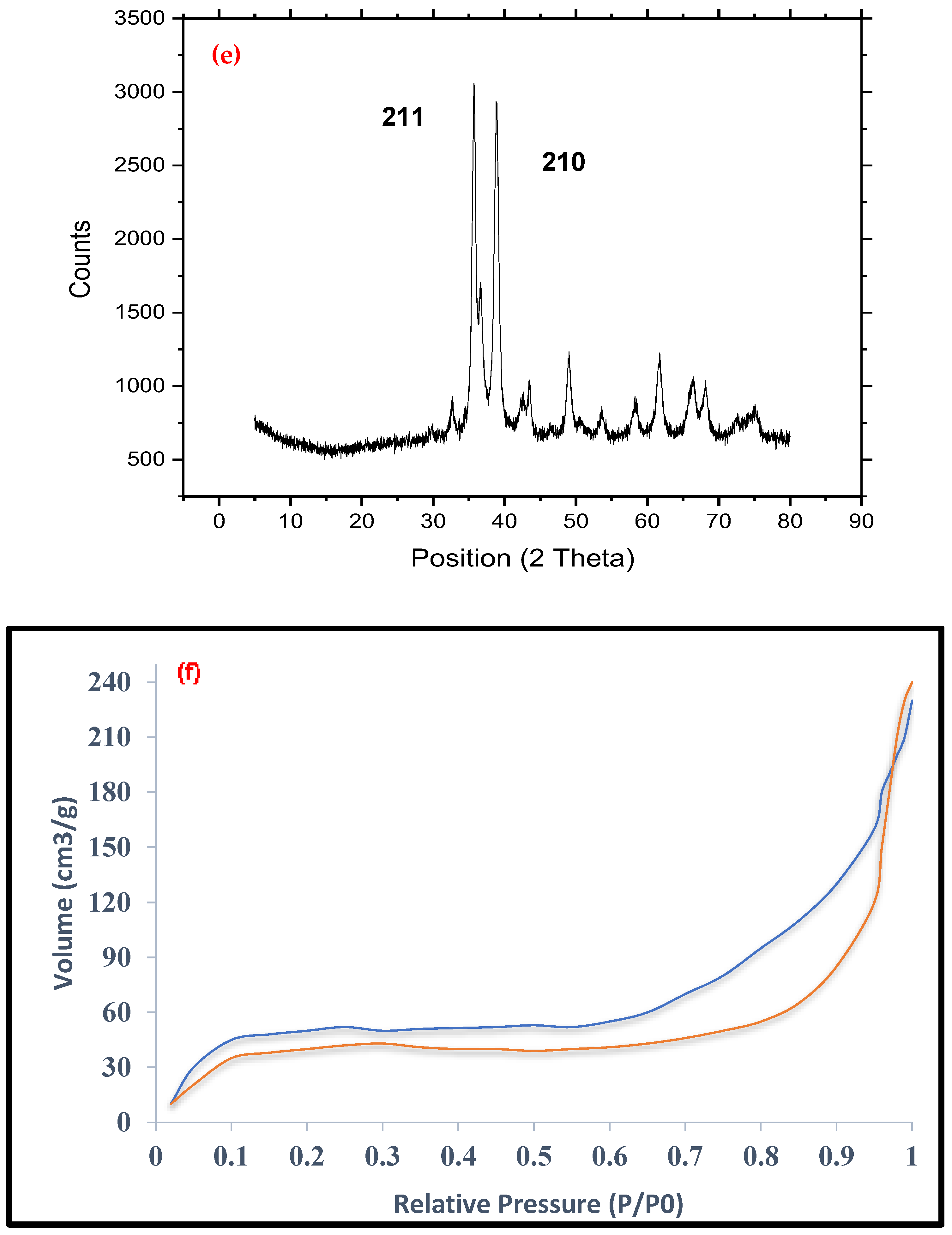
3.1. Properties of PbO@MgZnO
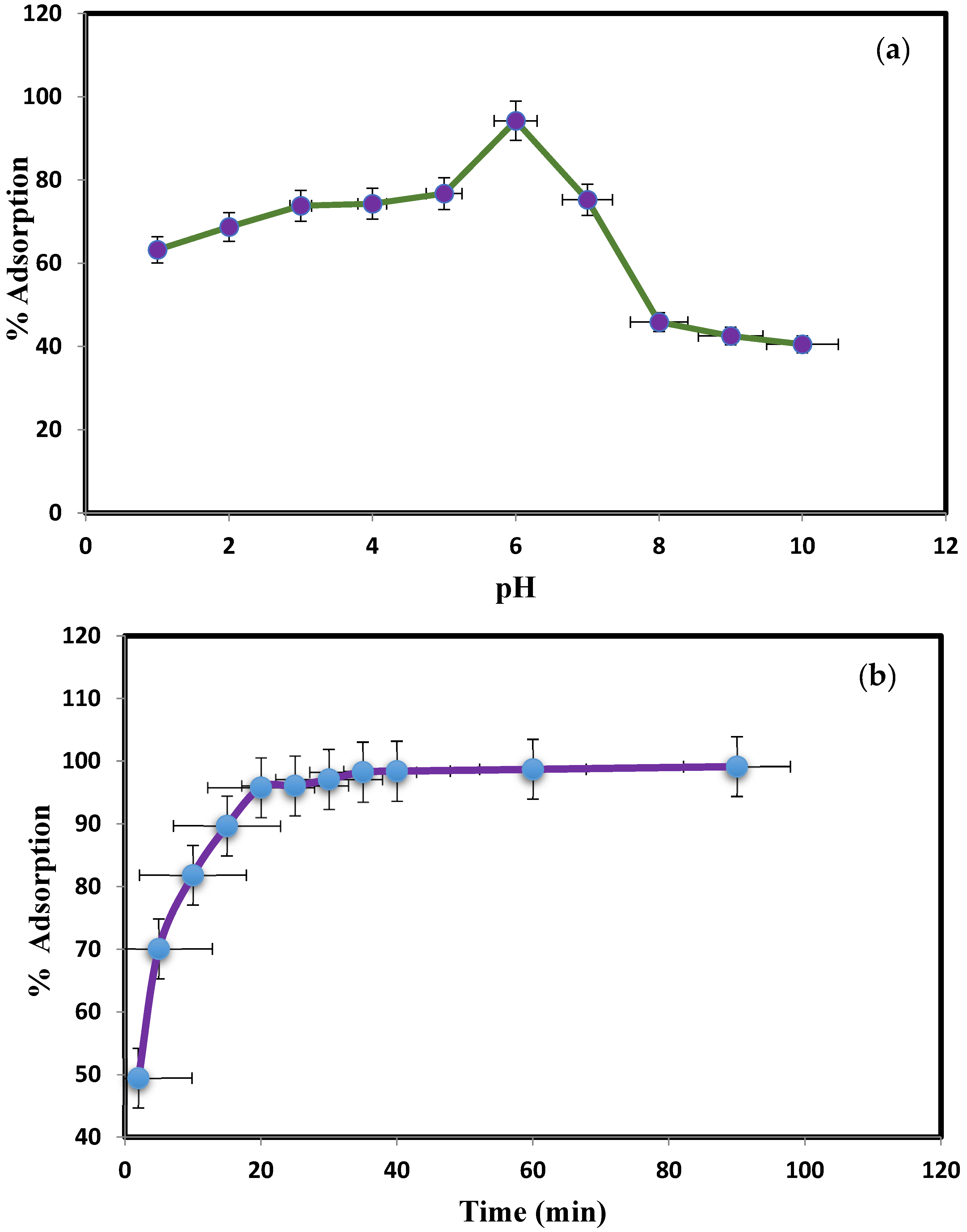
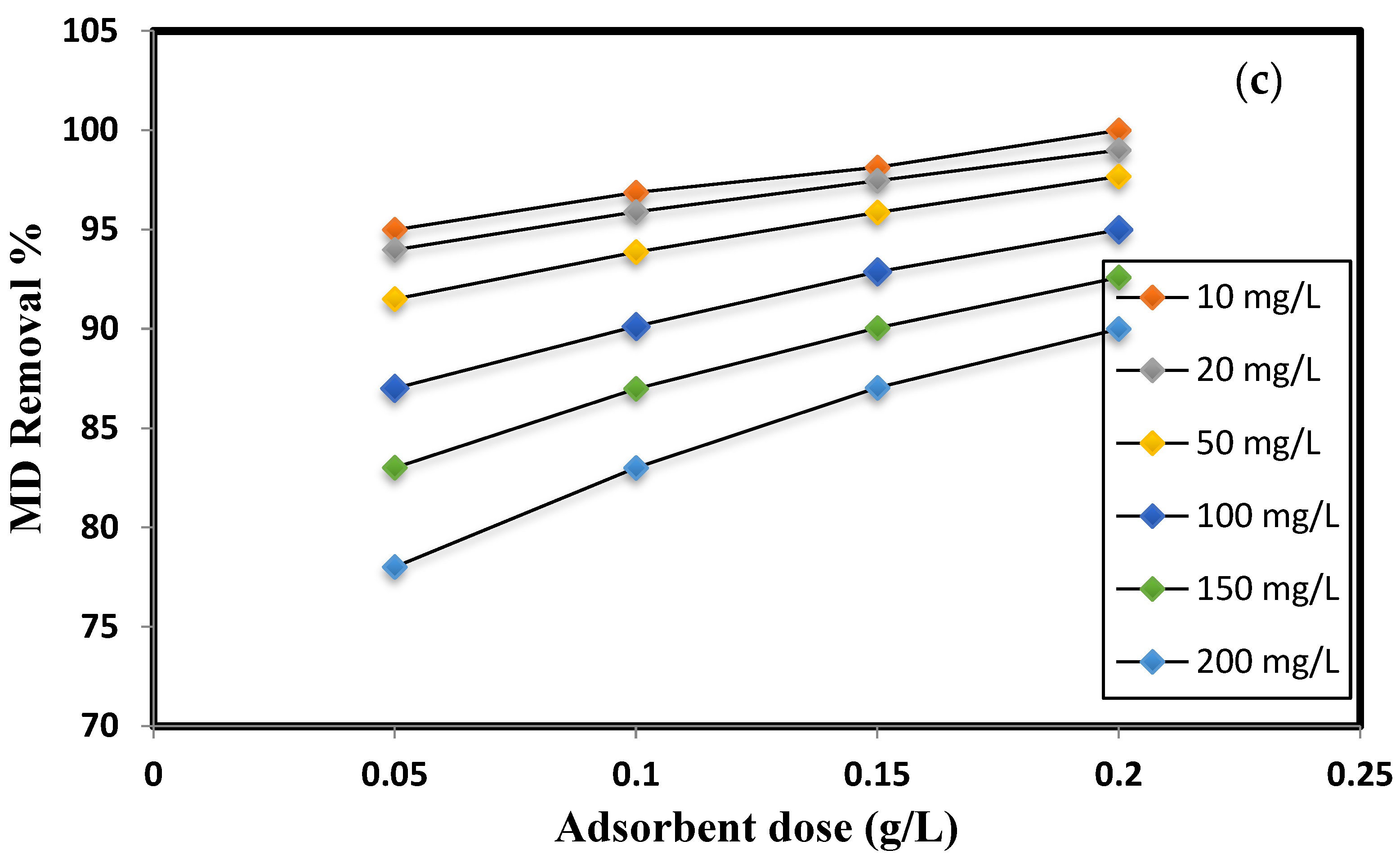
3.2. Effect of pH Variation, Contact Time, and Adsorbent Dose
3.3. Rate of Adsorption and Thermodynamic Parameter
4. Interpretation of the Steric Parameters
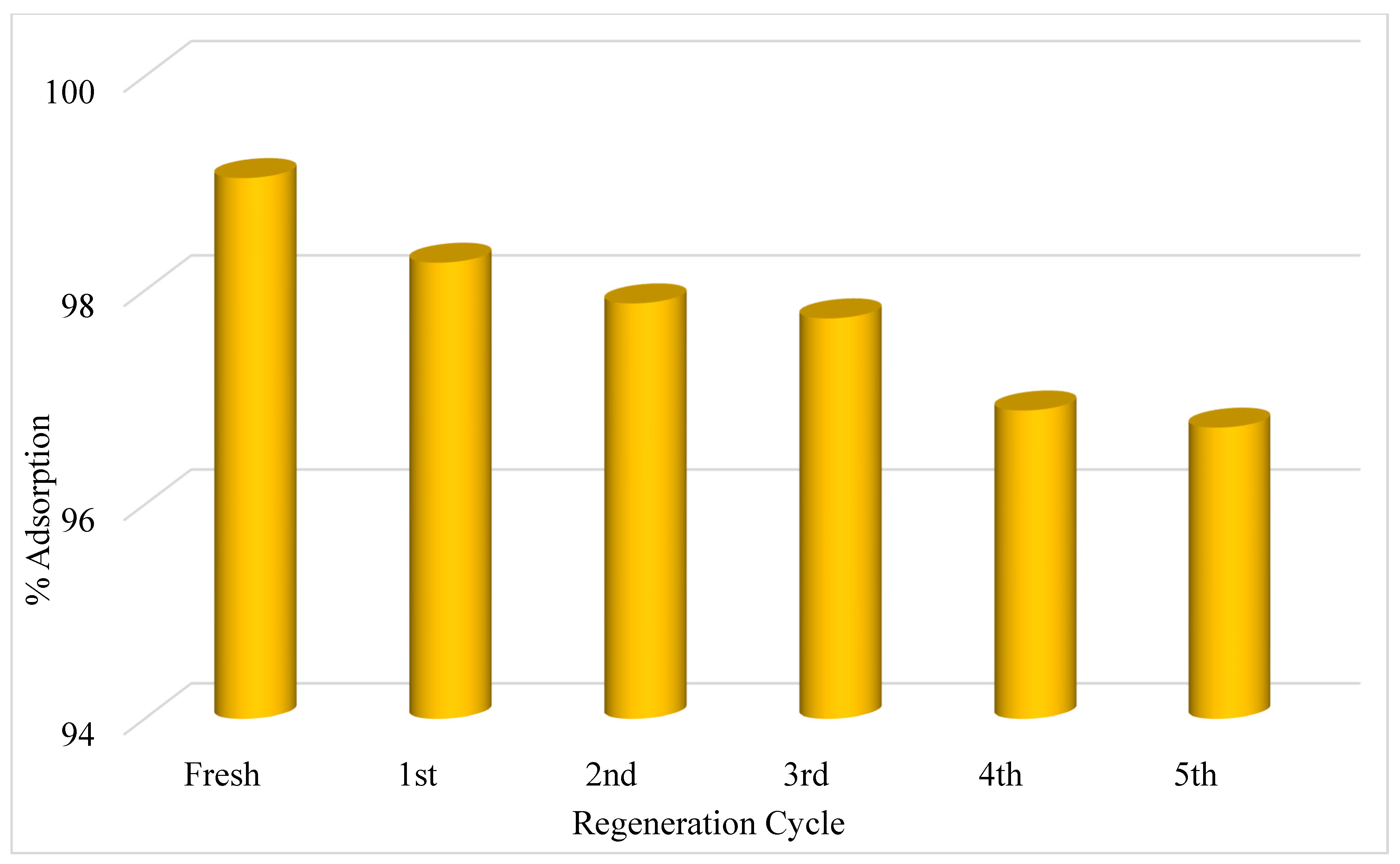
5. Reuse of PbO@MgZnO
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atrous, M.; Bouzid, M.; Lima, E.C.; Silas, P.; Bonilla-petriciolet, A.; Ben, A. Adsorption of dyes acid red 1 and acid green 25 on grafted clay: Modeling and statistical physics interpretation. J. Mol. Liquids 2019, 294, 111610. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Synthesis of pearl necklace-like ZIF-8@ chitosan/PVA nanofiber with synergistic effect for recycling aqueous dye removal. Carbohydr. Polym. 2020, 227, 115364. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Baig, N.; Ihsanullah; Sajid, M.; Saleh, T.A. Graphene-based adsorbents for the removal of toxic organic pollutants: A review. J. Environ. Manag. 2019, 244, 370–382. [Google Scholar] [CrossRef]
- Fegade, U.; Kolate, S.; Dhake, R.; Inamuddin, I.; Altalhi, T.; Kanchi, S. Adsorption of Congo Red on Pb doped FexOy: Experimental study and theoretical modeling via double-layer statistical physics models. Water Sci. Technol. 2021, 83, 1714–1727. [Google Scholar] [CrossRef]
- Pakzad, K.; Alinezhad, H.; Nasrollahzadeh, M. Green synthesis of Ni@Fe3O4 and CuO nanoparticles using Euphorbia maculata extract as photocatalysts for the degradation of organic pollutants under UV-irradiation. Ceram. Int. 2019, 45, 17173–17182. [Google Scholar] [CrossRef]
- Saxena, R.; Saxena, M.; Lochab, A. Recent Progress in Nanomaterials for Adsorptive Removal of Organic Contaminants from Wastewater. Chem. Select. 2020, 5, 335–353. [Google Scholar] [CrossRef]
- Jethave, G.; Fegade, U. Design, and synthesis of Zn0.3Fe0.45O3 nanoparticle for efficient removal of Congo red dye and its kinetic and isotherm investigation. Int. J. Ind. Chem. 2018, 9, 85–97. [Google Scholar] [CrossRef]
- Kondalkar, M.; Fegade, U.; Attarde, S.; Ingle, S. Experimental investigation on phosphate adsorption, mechanism and desorption properties of Mn-Zn-Ti oxide tri-metal alloy nanocomposite. J. Dispers. Sci. Technol. 2018, 11, 1635–1643. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Shi, X.; Nawar, S.; Werner, J.G.; Huang, G.; Ye, M.; Weitz, D.A.; Solovev, A.A.; Mei, Y. Hydrogel microcapsules with photocatalytic nanoparticles for removal of organic pollutants. Environ. Sci. Nano. 2020, 7, 656–664. [Google Scholar] [CrossRef]
- Majee, B.P.; Bhawna; Mishra, A.K. Bi-functional ZnO nanoparticles as a reusable SERS substrate for nano-molar detection of organic pollutants. Mater. Res. Express. 2019, 6, 1250j1. [Google Scholar] [CrossRef]
- Donga, C.; Mishra, S.B.; Abd-El-Aziz, A.S.; Mishra, A.K. Advances in Graphene-Based Magnetic and Graphene-Based/TiO2 Nanoparticles in the Removal of Heavy Metals and Organic Pollutants from Industrial Wastewater. J. Inorg. Organomet. Polym. Mater. 2020, 31, 1–18. [Google Scholar] [CrossRef]
- Jethave, G.; Fegade, U.; Rathod, R.; Pawar, J. Dye Pollutants removal from Wastewater using Metal Oxide Nanoparticle embedded Activated Carbon: An Immobilization study. J. Dispers. Sci. Technol. 2019, 40, 563–573. [Google Scholar] [CrossRef]
- Jethave, G.; Fegade, U.; Attarde, S.; Ingle, S. Facile synthesis of Lead Doped Zinc-Aluminum Oxide Nanoparticles (LD-ZAO-NPs) for efficient adsorption of anionic dye: Kinetic, isotherm and thermodynamic behaviors. J. Ind. Eng. Chem. 2017, 53, 294–306. [Google Scholar] [CrossRef]
- Singh, V.; Bansal, P. Fabrication, and characterization of needle-shaped CuO nanoparticles and their application as photocatalyst for degradation of organic pollutants. Mater. Lett. 2020, 261, 126929. [Google Scholar] [CrossRef]
- Khan, S.B. Metal nanoparticles containing chitosan wrapped cellulose nanocomposites for catalytic hydrogen production and reduction of environmental pollutants. Carbohydr. Polym. 2020, 242, 116286. [Google Scholar] [CrossRef]
- Binaeian, E.; Zadvarzi, S.B.; Yuan, D. Anionic dye uptake via composite using chitosan-polyacrylamide hydrogel as a matrix containing TiO2 nanoparticles; comprehensive adsorption studies. Int. J. Biol. Macromol. 2020, 162, 150–162. [Google Scholar] [CrossRef]
- Khan, S.B.; Khan, M.S.J.; Kamal, T.; Asiri, A.M.; Bakhsh, E.M. Polymer supported metallic nanoparticles as a solid catalyst for the removal of organic pollutants. Cellulose 2020, 27, 5907–5921. [Google Scholar] [CrossRef]
- Abbasi, S. Adsorption of Dye Organic Pollutant Using Magnetic ZnO Embedded on the Surface of Graphene Oxide. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1924–1934. [Google Scholar] [CrossRef]
- Kondalkar, M.; Fegade, U.; Attarde, S.; Ingle, S. Phosphate removal, mechanism, and adsorption properties of Fe-Mn-Zn oxide trimetal alloy nanocomposite fabricated via co-precipitation method. Sep. Sci. Technol. 2019, 54, 2682–2694. [Google Scholar] [CrossRef]
- Jethave, G.; Fegade, U.; Attarde, S.; Ingle, S. Decontamination study of Eriochrome Black-T from wastewater by using AlTiPbO Nanoparticles (ATPO-NPs) for Sustainable Clean Environment. J. Water Environ. Nanotechnol. 2019, 4, 263–274. [Google Scholar]
- Sellaoui, L.; Franco, D.; Ghalla, H.; Georgian, J.; Netto, M.S.; Dotto, G.L.; Bonilla-Petriciolet, A.; Belmabrouk, H.; Bajahzar, A. Insights of the adsorption mechanism of methylene blue on Brazilian berries seeds: Experiments, phenomenological modeling and DFT calculations. Chem. Eng. J. 2020, 394, 125011. [Google Scholar] [CrossRef]
- Fegade, U.; Jethave, G.; Hong, W.G.; Khan, I.; Marwani, H.M.; Inamuddin; Wu, R.-J.; Dhake, R. Multifunctional Zn0.3Al0.4O4.5 MDystals: An Efficient Photocatalyst for Formaldehyde Degradation and EBT Adsorption. Arabian J. Chem. 2020, 13, 8262–8270. [Google Scholar] [CrossRef]
- Fegade, U.; Jethave, G.; Su, K.Y.; Huang, W.R.; Wu, R.J. An multifunction Zn0.3Mn0.4O4 nanospheres for carbon dioxide reduction to methane via photocatalysis and reused after five cycles for phosphate adsorption. J. Environ. Chem. Eng. 2018, 6, 1918–1925. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Activated carbon/metal-organic framework composite as a bio-based novel green adsorbent: Preparation and mathematical pollutant removal modeling. J. Mol. Liq. 2019, 277, 310–322. [Google Scholar] [CrossRef]
- Muthukumaran, C.; Sivakumar, V.M.; Thirumarimurugan, M. Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nano adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 63, 354–362. [Google Scholar] [CrossRef]
- Sun, P.; Hui, C.; Khan, R.A.; Guo, X.; Yang, S.; Zhao, Y. Mechanistic links between magnetic nanoparticles and recovery potential and enhanced capacity for crystal violet of nanoparticles coated kaolin. J. Clean. Prod. 2017, 164, 695–702. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Khan, Z.M.; Sultan, M.; Naz, I. Green Synthesis of Phytogenic Magnetic Nanoparticles and Their Applications in the Adsorptive Removal of Crystal Violet from Aqueous Solution. Arab. J. Sci. Eng. 2018, 43, 6245–6259. [Google Scholar] [CrossRef]
- Abobakr, S.M.; Abdo, N.I.; Mansour, R.A. Remediation of Contaminated Water With Crystal Violet Dye by Using Magnetite Nanoparticles: Synthesis, Characterization and Adsorption Mechanism Studies. J. Env. Stud. 2020, 6, 10. [Google Scholar]
- Kifayatullah, H.M.; Tahir, H.; Shah, A.R. Modeling and optimization of ultrasound-assisted adsorption of crystal violet dye by grapheme oxide nanoparticles using response surface methodology. Int. J. Environ. Anal. Chem. 2020, 1–17. [Google Scholar] [CrossRef]
- Gautam, D.; Hooda, S. Magnetic Graphene Oxide/Chitin Nanocomposites for Efficient Adsorption of Methylene Blue and Crystal Violet from Aqueous Solutions. J. Chem. Eng. Data 2020, 65, 4052–4062. [Google Scholar] [CrossRef]
- Fegade, U.; Marek, J.; Patil, R.; Attarde, S.; Kuwar, A. A selective fluorescent receptor for the determination of nickel(II) in semi-aqueous media. J. Lumin. 2014, 146, 234–238. [Google Scholar] [CrossRef]
- Fegade, U.; Sharma, H.; Singh, N.; Ingle, S.; Attarde, S.; Kuwar, A. An amide based dipodal Zn2+ complex for multications recognition: Nanomolar detection. J. Lumin. 2014, 149, 190–195. [Google Scholar] [CrossRef]
- Fegade, U.; Tayade, S.; Chaitanya, G.K.; Attarde, S.; Kuwar, A. Fluorescent, and Chromogenic Receptor Bearing Amine and Hydroxyl Functionality for Iron (III) Detection in Aqueous Solution. J. Fluoresc. 2014, 24, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Fegade, U.; Sharma, H.; Attarde, S.; Singh, N.; Kuwar, A. Urea Based Dipodal Fluorescence Receptor for Sensing of Fe3+ Ion in Semi-Aqueous Medium. J. Fluoresc. 2014, 24, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Patil, R.; Fegade, U.; Sahoo, S.K.; Singh, N.; Bendre, R.; Kuwar, A. A novel phthalazine-based highly selective chromogenic and fluorogenic chemosensor for Co2+ in semi-aqueous medium: Application in cancer cell imaging. Photochem. Photobiol. Sci. 2015, 14, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Fegade, U.; Sahoo, S.K.; Patil, S.; Kaur, R.; Singh, N.; Bendre, R.; Kuwar, A. A novel chromogenic and fluorogenic chemosensor for detection of trace water in methanol. Sens. Actuators B 2015, 210, 324–327. [Google Scholar] [CrossRef]
- Patil, R.; Fegade, U.; Kaur, R.; Sahoo, S.K.; Singh, N.; Kuwar, A. Highly sensitive and selective determination of Hg2+ by using 3-((2-(1H-benzo[d]imidazol-2-yl)phenylamino)methyl)benzene-1,2-diol as fluorescent chemosensor and its application in a real water sample. Supramol. Chem. 2015, 27, 527–532. [Google Scholar] [CrossRef]
- Bhosale, J.; Fegade, U.; Bondhopadhyay, B.; Kaur, S.; Singh, N.; Basuc, A.; Dabure, R.; Kuwar, R.B.A. Pyrrole-coupled salicylamide-based fluorescence “turn on” probe for highly selective recognition of Zn2+ ions in mixed aqueous media: Application in living cell imaging. J. Mol. Recognit. 2015, 28, 369–375. [Google Scholar] [CrossRef]
- Fegade, U.; Kolate, S.; Gokulakrishnan, K.; Ramalingan, C.; Altalhi, T.; Inamuddin; Kanchi, S. A Selective Ratiometric Receptor 2-((E)-(3-(prop-1-en-2-yl)phenylimino)methyl)-4-nitrophenol for the Detection of Cu2+ ions Supported By DFT Studies. J. Fluoresc. 2021, 31, 625–634. [Google Scholar] [CrossRef]
- Fegade, U.; Jethave, G.; Attarde, S.; Kolate, S.; Inamuddin; Altalhi, T.; Kanchid, S. Statistical Physics Modelling of EBT Adsorption on Pb(II) doped Zinc Oxide Nanoparticles: Kinetics, Isotherm and Reused Study. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Pawar, S.; Fegade, U.; Bhardwaj, V.K.; Singh, N.; Bendre, R. Anil Kuwar, 2-((E)-(2-aminophenylimino)methyl)-6-isopropyl-3-methylphenol based fluorescent receptor for dual Ni2+ and Cu2+ recognition: Nanomolar detection. Polyhedron 2015, 87, 79–85. [Google Scholar] [CrossRef]
- Jethave, G.; Fegade, U.; Attarde, S.; Ingle, S.; Ghaedi, M.; Sabzehmeidani, M.M. Exploration of the adsorption capability by doping Pb@ZnFe2O4 nanocomposites (NCs) for decontamination of dye from textile wastewater. Heliyon 2019, 5, e02412. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Comparative sorption kinetic studies of dye and aromatic compounds onto fly ash. J. Environ. Sci. Heal. - Part A 1999, 34, 1179–1204. [Google Scholar] [CrossRef]
- van Tran, T.; Cao, V.D.; Nguyen, V.H.; Hoang, B.N.; Vo, D.V.N.; Nguyen, T.D.; Bach, L.G. MIL-53 (Fe) derived magnetic porous carbon as a robust adsorbent for the removal of phenolic compounds under the optimized conditions. J. Environ. Chem. Eng. 2019, 8, 102902. [Google Scholar] [CrossRef]
- van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Bach, L.G.; Vo, D.V.N.; Hong, S.S.; Phan, T.Q.T.; Nguyen, T.D. Tunable Synthesis of Mesoporous Carbons from Fe3O(BDC)3 for Chloramphenicol Antibiotic Remediation. Nanomaterials 2019, 9, 237. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.; Heller, W. The Adsorption of cis- and trans-Azobenzene. J. Am. Chem. Soc. 1939, 61, 2228–2230. [Google Scholar] [CrossRef]
- Dada, A.O. Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low-cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef] [Green Version]
- Deniz, F. Adsorption Properties of Low-Cost Biomaterial Derived from Prunus amygdalus L. for Dye Removal from Water. Sci. World J. 2013, 9, 961671. [Google Scholar] [CrossRef]
- Abbasi, S.; Mehri-Saddat; Ekrami-Kakhki; Tahari, M. Modeling and predicting the photodecomposition of methylene blue via ZnO–SnO2 hybrids using design of experiments (DOE). J. Mater. Sci. Mater. Electr. 2017, 28, 15306–15312. [Google Scholar] [CrossRef]
- Rahimi, B.; Jafari, N.; Farrokhzadeh, A.A.H.; Ebrahimi, A. The influence of ZnO nanoparticles amount on the optimization of photodegradation of methyl orange using decorated MWCNTs. Prog. Ind. Eco. An Inter. J. 2019, 13, 3–15. [Google Scholar] [CrossRef]
- Barakat, M.A.E.; Kumar, R.; Seliem, M.K.; Selim, A.Q.; Mobarak, M.; Anastopoulos, I.; Giannakoudakis, D.; Barczak, M.; Bonilla-Petricioletand, A.; Mohamed, E.A. Exfoliated Clay decorated with Magnetic Iron Nanoparticles for crystal Violet Adsorption: Modeling and Physicochemical Interpretation. Nanomaterials 2020, 10, 1454. [Google Scholar] [CrossRef]
- Basha, I.A.; Nagalakshmi, R.; Shanthi, T. Removal of Congo red and magenta dyes from industrial waste water by thorn apple leaf powder. Int. J. Chem. Sci 2016, 14, 57–64. [Google Scholar]
- Gao, R.; Shen, X.; Wang, L. Adsorption of basic Magenta on graphene oxide modified sugarcane bagasse. BioResources 2019, 14, 8100–8113. [Google Scholar]
- Sivarajasekar, N.; Baskar, R. Adsorption of Basic Magenta II onto H2SO4 activated immature Gossypium hirsutum seeds: Kinetics, isotherms, mass transfer, thermodynamics and process design. Arabian J. Chem. 2019, 12, 1322–1337. [Google Scholar] [CrossRef]
- Devi, S.; Murugappan, A. Adsorption of basic magenta using fresh water algae and brown marine seaweed: Characterization studies and error analysis. J. Eng. Sci. Technol. 2016, 11, 1421–1436. [Google Scholar]
- Niu, Y.; Jia, R.; Liu, C.; Han, X.; Chang, C.; Chen, J. Optimization of basic magenta adsorption onto Fe/Cu nanocomposites synthesized by sweet potato leaf extract using response surface methodology. Korean J. Chem. Eng. 2021, 38, 1556–1565. [Google Scholar] [CrossRef]
- Alorabi, A.Q.; Hassan, M.S.; Alam, M.M.; Zabin, S.A.; Alsenani, N.I.; Baghdadi, N.E. Natural clay as a low-cost adsorbent for crystal violet dye removal and antimicrobial activity. Nanomaterials 2021, 11, 2789. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Debnath, A. Reactive orange 12 dye adsorption onto magnetically separable CaFe2O4 nanoparticles synthesized by simple chemical route: Kinetic, isotherm and neural network modeling. Water Pract. Technol. 2021, 16, 1141–1158. [Google Scholar] [CrossRef]
- Hussain, S.; Kamran, M.; Khan, S.A.; Shaheen, K.; Shah, Z.; Suo, H.; Khan, Q.; Shah, A.B.; Rehman, W.U.; Al-Ghamdi, Y.O.; et al. Adsorption, kinetics and thermodynamics studies of methyl orange dye sequestration through chitosan composites films. Int. J. Biol. Macromol. 2021, 168, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Hii, H.T. Adsorption isotherm and kinetic models for removal of methyl orange and remazol brilliant blue r by coconut shell activated carbon. Trop. Aquat. Soil Pollut. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Aniagor, C.O.; Afifi, M.A.; Hashem, A. Modelling of basic blue-9 dye sorption onto hydrolyzed polyacrylonitrile grafted starch composite. Carbohydr. Polym. Technol. Appl. 2021, 2, 100141. [Google Scholar] [CrossRef]
- Grabi, H.; Lemlikchi, W.; Derridj, F.; Lemlikchi, S.; Trari, M. Efficient native biosorbent derived from agricultural waste precursor for anionic dye adsorption in synthetic wastewater. Biomass Convers. Biorefinery 2021, 1–18. [Google Scholar] [CrossRef]
- Oueslati, K.; Naifar, A.; Sakly, A.; Kyzas, G.Z.; Lamine, A.B. Statistical and physical interpretation of dye adsorption onto low-cost biomass by using simulation methods. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 128969. [Google Scholar] [CrossRef]
- Elsherif, K.; El-Dali, A.; Alkarewi, A.; Ewlad-Ahmed, A.; Treban, A. Adsorption of crystal violet dye onto olive leaves powder: Equilibrium and kinetic studies. Chem. Int. 2021, 7, 79–89. [Google Scholar]
- Raj, A.; Yadav, A.; Rawat, A.P.; Singh, A.K.; Kumar, S.; Pandey, A.K.; Sirohi, R.; Pandey, A. Kinetic and thermodynamic investigations of sewage sludge biochar in removal of Remazol Brilliant Blue R dye from aqueous solution and evaluation of residual dyes cytotoxicity. Environ. Technol. Innov. 2021, 23, 101556. [Google Scholar] [CrossRef]
- Bensalah, J.; Habsaoui, A.; Dagdag, O.; Lebkiri, A.; Ismi, I.; Rifi, E.H.; Warad, I.; Zarrouk, A. Adsorption of a cationic dye (Safranin) by artificial cationic resins AmberliteŪIRC-50: Equilibrium, kinetic and thermodynamic study. Chem. Data Collect. 2021, 35, 100756. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Ajayi, O.A.; Popoola, L.T. Application of Taguchi design approach to parametric optimization of adsorption of crystal violet dye by activated carbon from poultry litter. Sci. Afr. 2021, 13, e00850. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, S.; Sharma, V.; Mishra, P.K.; Ekielski, A.; Sharma, V.; Kumar, V. Methylene blue dye adsorption from wastewater using hydroxyapatite/gold nanocomposite: Kinetic and thermodynamics studies. Nanomaterials 2021, 11, 1403. [Google Scholar] [CrossRef]
- Banisheykholeslami, F.; Hosseini, M.; Darzi, G.N. Design of PAMAM grafted chitosan dendrimers biosorbent for removal of anionic dyes: Adsorption isotherms, kinetics and thermodynamics studies. Int. J. Biol. Macromol. 2021, 177, 306–316. [Google Scholar] [CrossRef] [PubMed]



| Kinetic Model Parameters | ||||||
|---|---|---|---|---|---|---|
| Concentration (ppm) | 10 | 20 | 50 | 100 | 150 | 200 |
| Kadgmg−1 min−1 | 0.0415 | 0.0136 | 0.0026 | 0.0018 | 0.0012 | 0.0013 |
| qe (mg/g) | 10.64 | 21.74 | 52.63 | 100.00 | 166.67 | 333.33 |
| R2 | 0.999 | 0.999 | 0.998 | 0.998 | 0.999 | 0.999 |
| Langmuir | Freundlich | ||
|---|---|---|---|
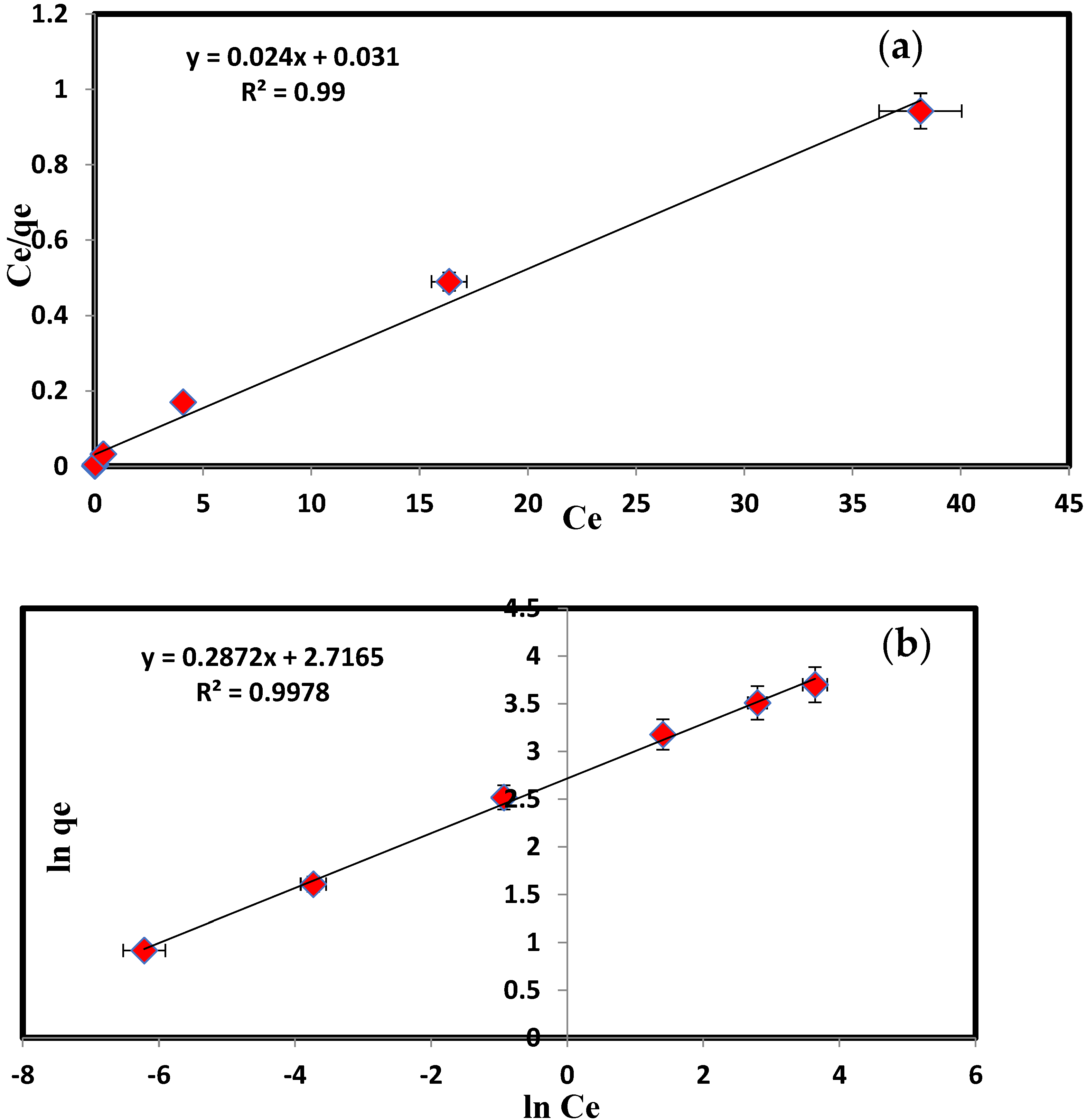
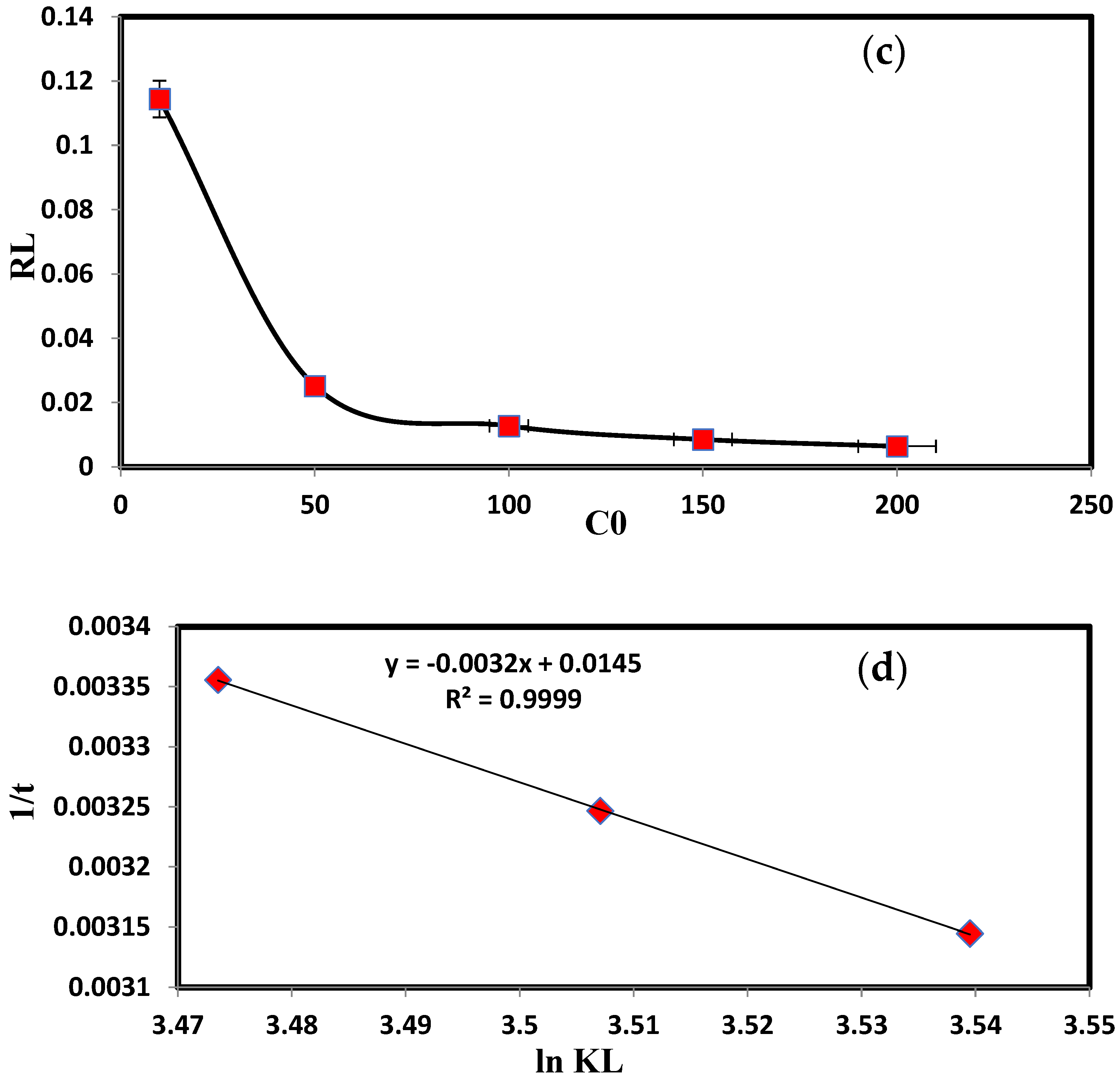
| KL | 32.25 | log Kf | 2.176 |
| qmax = KL/αL | 333.33 | 1/n | 0.287 |
| R2 | 0.99 | R2 | 0.997 |
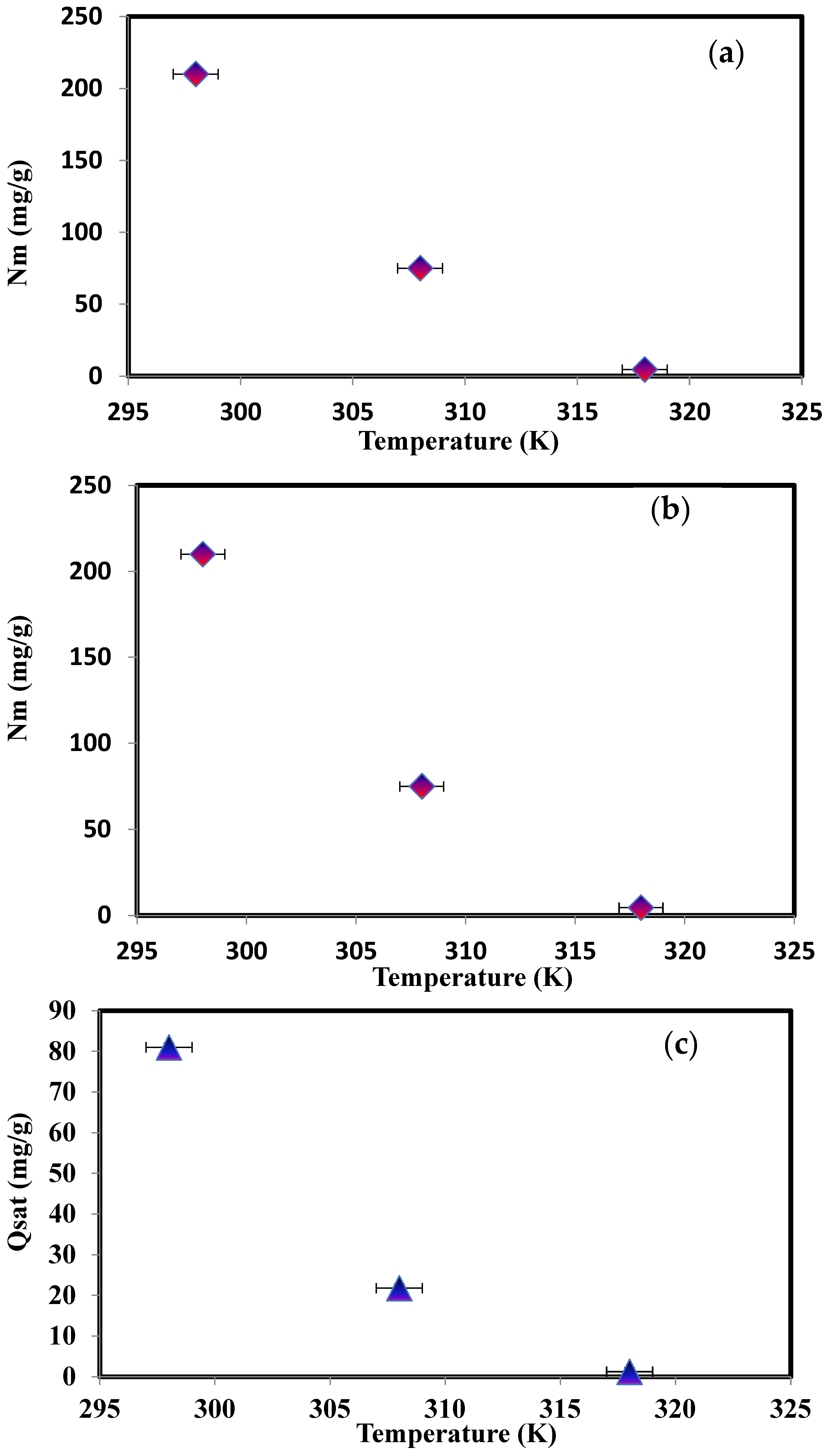
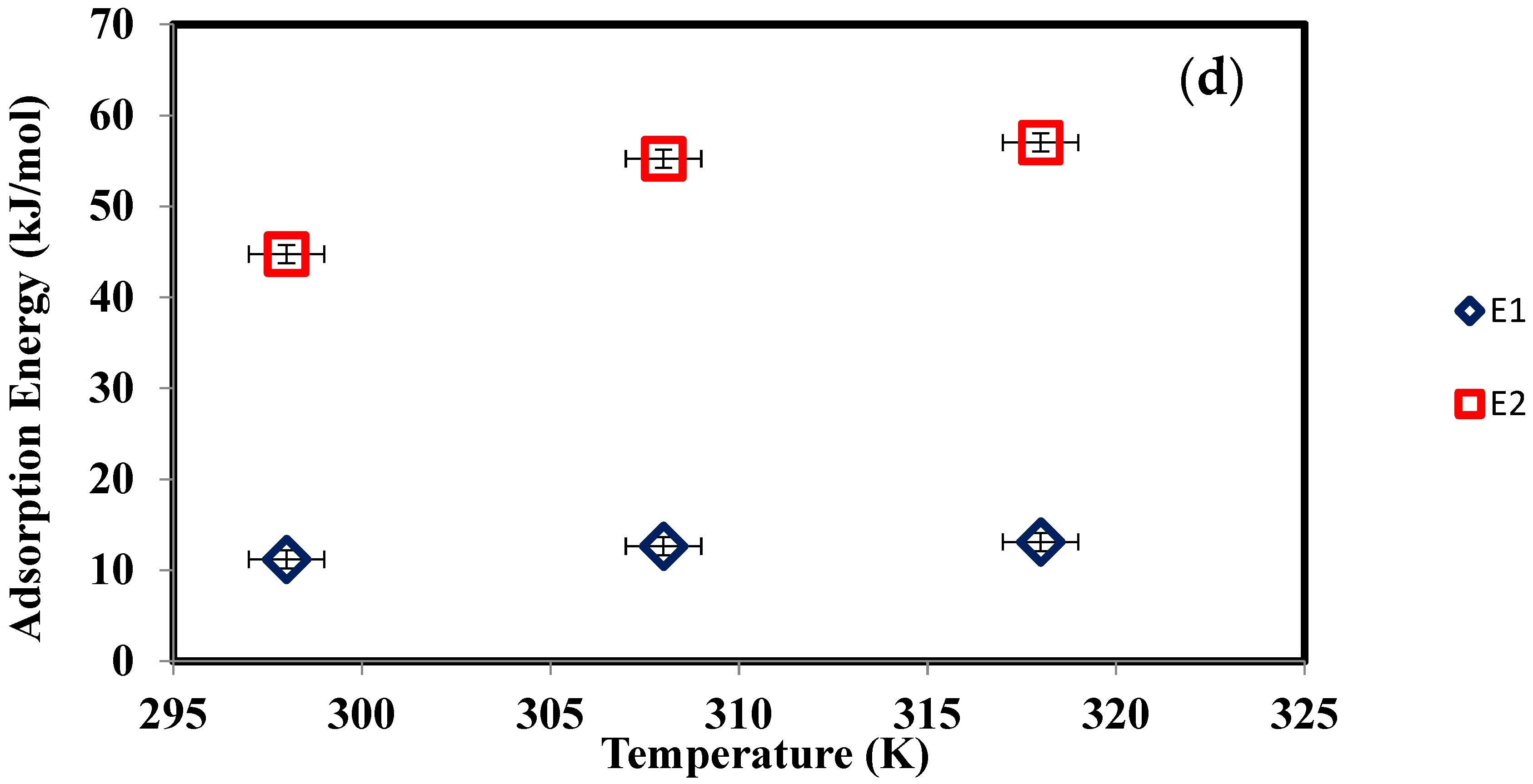
| Temperature (K) | Nm (mg/g) | n | Nt = 1 + N2 | ε1 (kJ/mol) | ε2 (kJ/mol) | Qsat (mg/g) |
|---|---|---|---|---|---|---|
| 298 | 210 | 0.385 | 1.002 | 11.195 | 44.744 | 81.011 |
| 308 | 75 | 0.290 | 1.001 | 12.643 | 55.225 | 21.771 |
| 318 | 4.5 | 0.280 | 1.0001 | 13.107 | 57.018 | 1.260 |
| Temperature | 298 | 308 | 318 | |||
|---|---|---|---|---|---|---|
| (K) | R2 | RMSE | R2 | RMSE | R2 | RMSE |
| Model 1 | 0.988 | 0.6168 | 0.995 | 1.8782 | 0.992 | 9.094 |
| Model 2 | 0.983 | 0.6899 | 0.996 | 1.3724 | 0.994 | 0.836 |
| Model 3 | 0.997 | 1.4534 | 0.998 | 5.415 | 0.998 | 4.157 |
| Adsorbent | Qmax (mg/g) | Reference |
|---|---|---|
| Thorn apple leaf powder | 1.059 | [57] |
| Graphene oxide modified sugarcane bagasse | 145 | [58] |
| H2SO4 activated immature Gossypium Hirsutum seeds | 86.24 | [59] |
| Gracilaria edulis algae | 1250 | [60] |
| Lyngbya wollei algae | 333 | [60] |
| Fe/Cu nanocomposites | 235 | [61] |
| Natural Clay | 198 | [62] |
| PbO@MgZnO | 333 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altalhi, T.; Jethave, G.; Fegade, U.; Mersal, G.A.M.; Ibrahim, M.M.; Mahmoud, M.H.H.; Kumeria, T.; Isai, K.A.; Sonawane, M. Adsorption of Magenta Dye on PbO Doped MgZnO: Interpretation of Statistical Physics Parameters Using Double-Layer Models. Int. J. Environ. Res. Public Health 2022, 19, 12199. https://doi.org/10.3390/ijerph191912199
Altalhi T, Jethave G, Fegade U, Mersal GAM, Ibrahim MM, Mahmoud MHH, Kumeria T, Isai KA, Sonawane M. Adsorption of Magenta Dye on PbO Doped MgZnO: Interpretation of Statistical Physics Parameters Using Double-Layer Models. International Journal of Environmental Research and Public Health. 2022; 19(19):12199. https://doi.org/10.3390/ijerph191912199
Chicago/Turabian StyleAltalhi, Tariq, Ganesh Jethave, Umesh Fegade, Gaber A. M. Mersal, Mohamed M. Ibrahim, M.H.H. Mahmoud, Tushar Kumeria, Kalpesh A. Isai, and Milind Sonawane. 2022. "Adsorption of Magenta Dye on PbO Doped MgZnO: Interpretation of Statistical Physics Parameters Using Double-Layer Models" International Journal of Environmental Research and Public Health 19, no. 19: 12199. https://doi.org/10.3390/ijerph191912199
APA StyleAltalhi, T., Jethave, G., Fegade, U., Mersal, G. A. M., Ibrahim, M. M., Mahmoud, M. H. H., Kumeria, T., Isai, K. A., & Sonawane, M. (2022). Adsorption of Magenta Dye on PbO Doped MgZnO: Interpretation of Statistical Physics Parameters Using Double-Layer Models. International Journal of Environmental Research and Public Health, 19(19), 12199. https://doi.org/10.3390/ijerph191912199







