Effect of pH-Dependent Homo/Heteronuclear CAHB on Adsorption and Desorption Behaviors of Ionizable Organic Compounds on Carbonaceous Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorbents, Chemicals, and Characterization
2.2. Bath Adsorption and Desorption Experiments at Different pHs
2.3. Data Analysis
2.4. DFT Computational Details
3. Results and Discussion
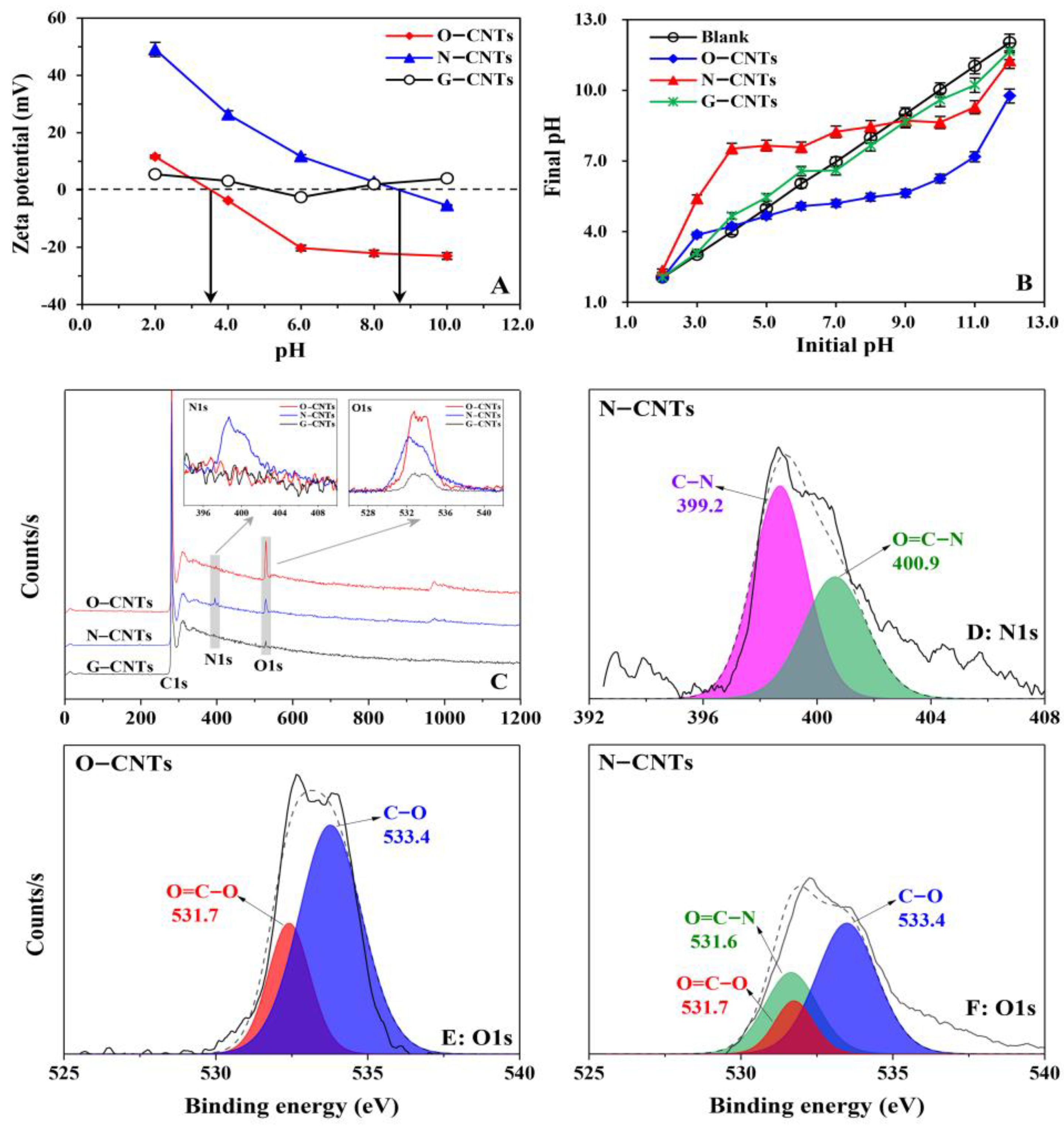
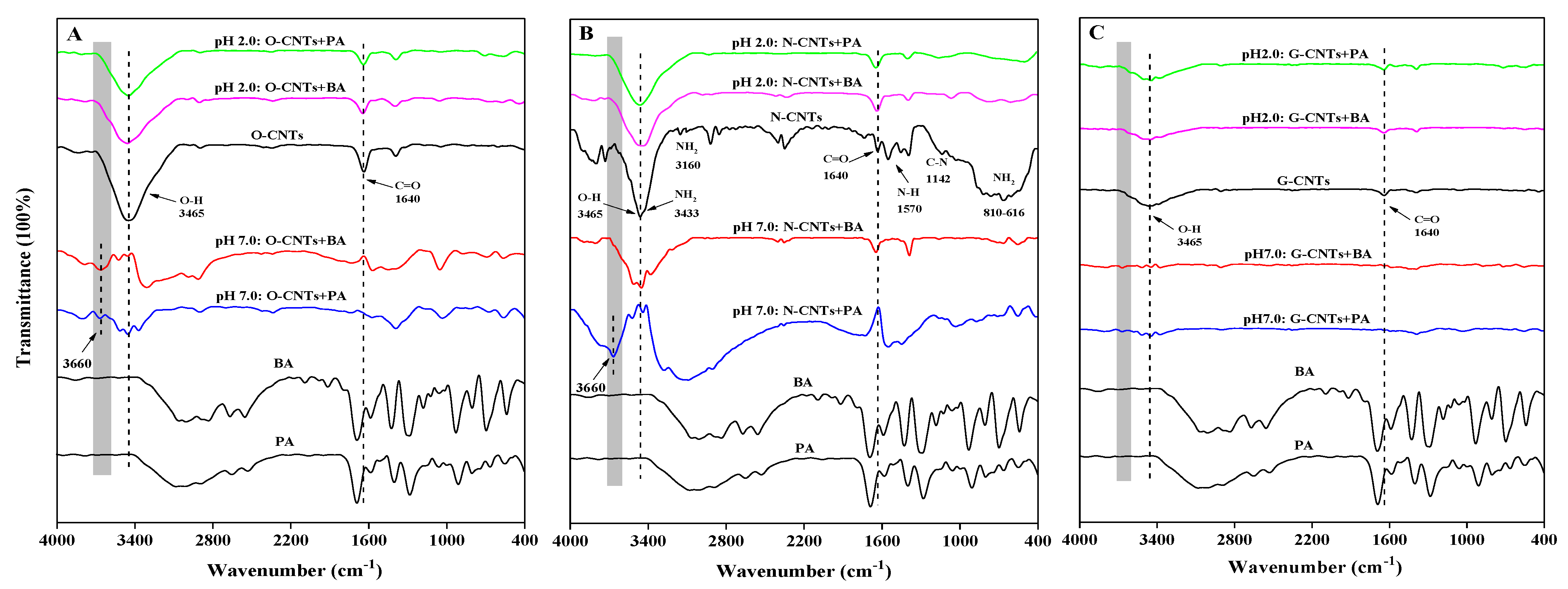
3.1. Characterization of CNTs
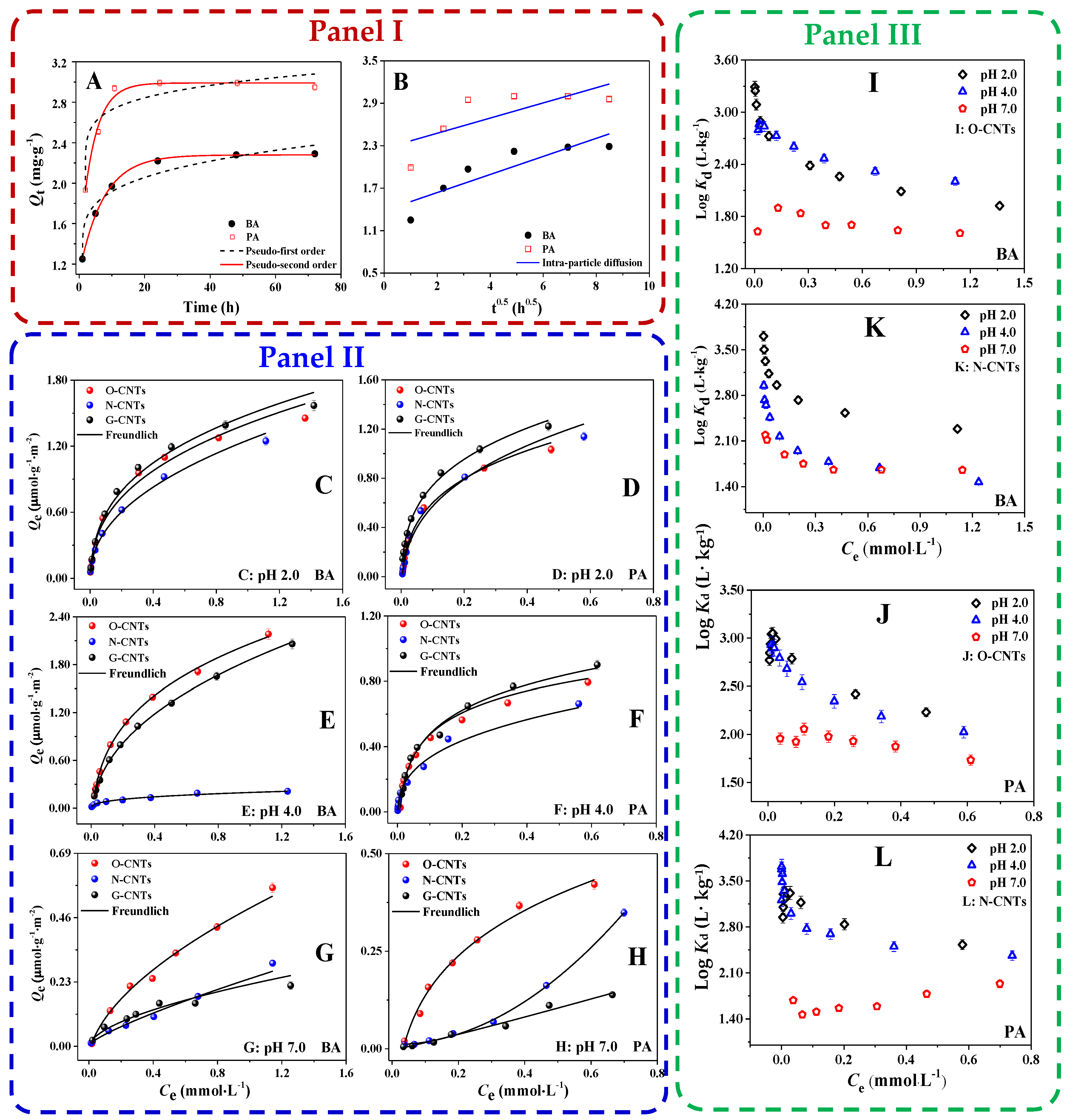
3.2. Adsorption Kinetics of BA and PA on CNTs
3.3. Adsorption Isotherms of BA and PA on CNTs-Effect of pH
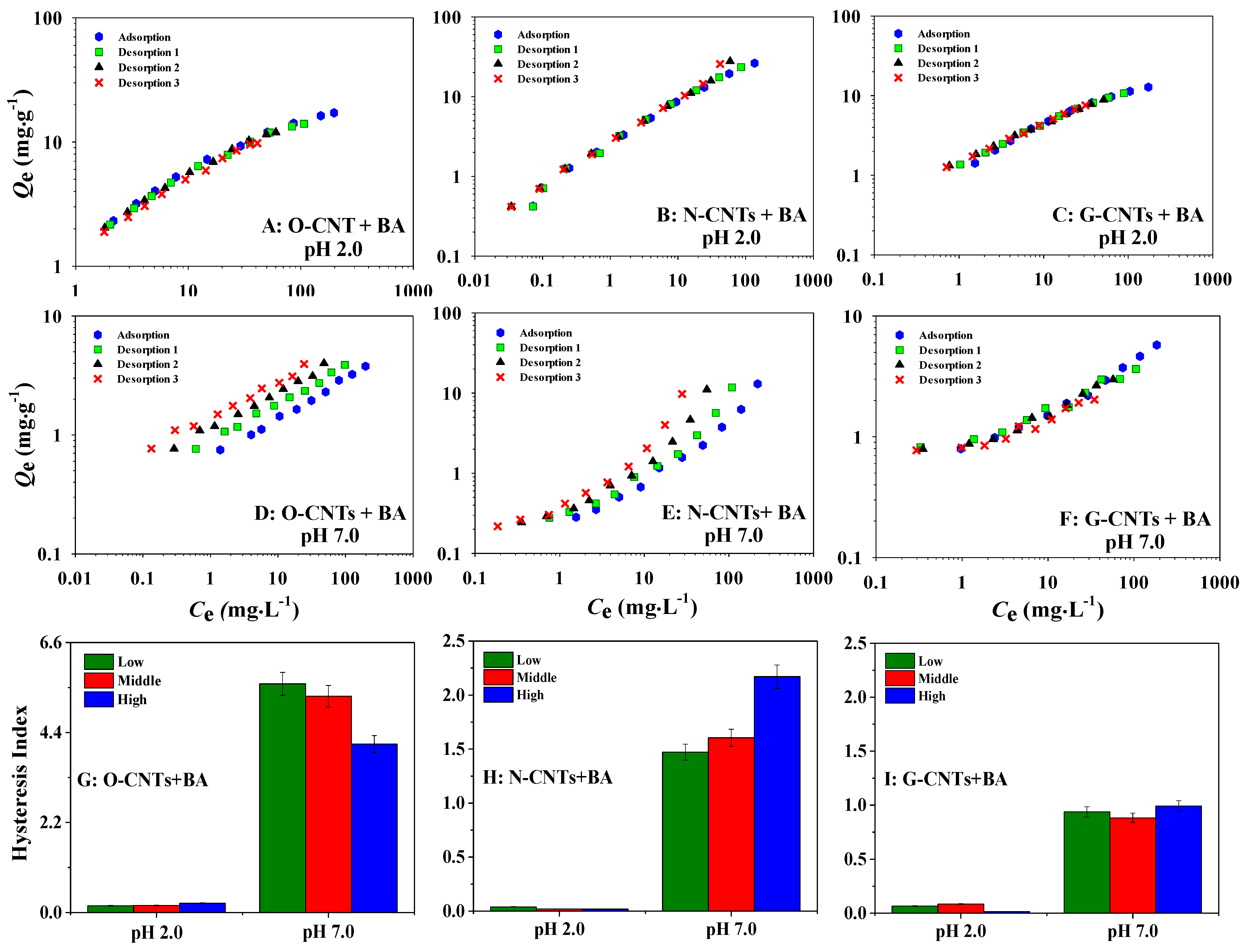
3.4. Desorption of BA and PA on CNTs at Different PHs
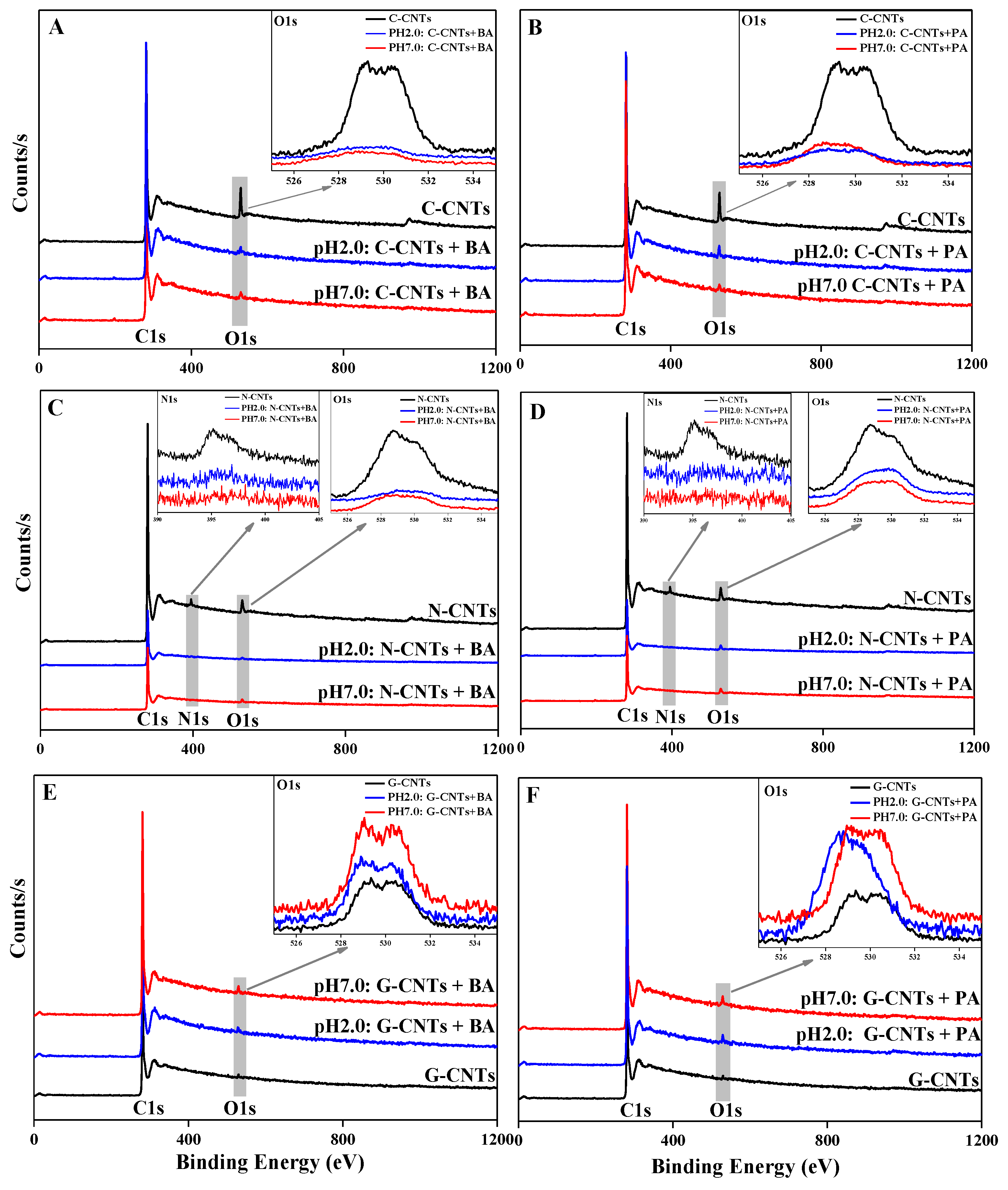
3.5. XPS, FTIR, and DFT Analysis of Homonuclear/Heteronuclear CAHB
4. Conclusions and Environmental Significance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, G.; Arp, H.; Aumeier, B.; Bucheli, T.; Chefetz, B.; Chen, W.; Droge, S.; Endo, S.; Escher, B.; Hale, S.; et al. Sorption and Mobility of Charged Organic Compounds: How to Confront and Overcome Limitations in Their Assessment. Environ. Sci. Technol. 2022, 56, 4702–4710. [Google Scholar] [CrossRef] [PubMed]
- Manjarres-Lopez, D.P.; Andrades, M.S.; Sanchez-Gonzalez, S.; Rodriguez-Cruz, M.S.; Sanchez-Martin, M.J.; Herrero-Hernandez, E. Assessment of pesticide residues in waters and soils of a vineyard region and its temporal evolution. Environ. Pollut. 2021, 284, 117463. [Google Scholar] [CrossRef] [PubMed]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Yang, Q.; Jiang, L.; Li, G. Occurrence and distribution of Pharmaceuticals and Personal Care Products (PPCPs) in wastewater related riverbank groundwater. Sci. Total Environ. 2022, 821, 153372. [Google Scholar] [CrossRef]
- Vitale, C.M.; Di Guardo, A. A review of the predictive models estimating association of neutral and ionizable organic chemicals with dissolved organic carbon. Sci. Total Environ. 2019, 666, 1022–1032. [Google Scholar] [CrossRef]
- Zhou, J.; Saeidi, N.; Wick, L.Y.; Kopinke, F.D.; Georgi, A. Adsorption of polar and ionic organic compounds on activated carbon: Surface chemistry matters. Sci. Total Environ. 2021, 794, 148508. [Google Scholar] [CrossRef]
- Karlsson, M.V.; Carter, L.J.; Agatz, A.; Boxall, A.B.A. Novel Approach for Characterizing pH-Dependent Uptake of Ionizable Chemicals in Aquatic Organisms. Environ. Sci. Technol. 2017, 51, 6965–6971. [Google Scholar] [CrossRef]
- Bueno, M.J.M.; Valverde, M.G.; Gomez-Ramos, M.M.; Andujar, J.A.S.; Barcelo, D.; Fernandez-Alba, A.R. Fate, modeling, and human health risk of organic contaminants present in tomato plants irrigated with reclaimed water under real-world field conditions. Sci. Total Environ. 2022, 806, 150909. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, Y.; Yang, X.; Liu, D.; Shan, S.; Cui, F.; Xing, B. Understanding the pH-dependent adsorption of ionizable compounds on graphene oxide using molecular dynamics simulations. Environ. Sci. Nano 2017, 4, 1935–1943. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y.; Zhang, D.; Pan, B. pH-dependent KOW provides new insights in understanding the adsorption mechanism of ionizable organic chemicals on carbonaceous materials. Sci. Total Environ. 2018, 618, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Zhang, H.; Ghosh, S.; Pan, B. Fast and slow adsorption of carbamazepine on biochar as affected by carbon structure and mineral composition. Sci. Total Environ. 2017, 579, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, G.; Gharasoo, M.; Huffer, T.; Hofmann, T. Deep Learning Neural Network Approach for Predicting the Sorption of Ionizable and Polar Organic Pollutants to a Wide Range of Carbonaceous Materials. Environ. Sci. Technol. 2020, 54, 4583–4591. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ji, W. Sorption of ionizable organic chemicals to carbonaceous adsorbents: Solution pH change and contributions of different species. Sci. Total Environ. 2019, 647, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, L.; Zhang, T.; Yu, H.; Zhan, Y.; Yang, Y.; Zeng, H.; Chen, S.; Peng, D. Adsorption behavior and mechanism of sulfonamides on phosphonic chelating cellulose under different pH effects. Bioresour. Technol. 2019, 288, 121510. [Google Scholar] [CrossRef]
- Liang, D.; Yu, F.; Zhu, K.; Zhang, Z.; Tang, J.; Xie, Q.; Liu, J.; Xie, F. Quaternary ammonium salts targeted regulate the surface charge distribution of activated carbon: A study of their binding modes and modification effects. Environ. Res. 2022, 214, 11410. [Google Scholar] [CrossRef]
- Roman, M.; Gutierrez, L.; Van Dijk, L.H.; Vanoppen, M.; Post, J.W.; Wols, B.A.; Cornelissen, E.R.; Verliefde, A.R.D. Effect of pH on the transport and adsorption of organic micropollutants in ion-exchange membranes in electrodialysis-based desalination. Sep. Purif. Technol. 2020, 252, 117487. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, J.; Zheng, H.; Li, X.; Wang, Y.; Li, X.; Xing, B. Adsorption, desorption and coadsorption behaviors of sulfamerazine, Pb(II) and benzoic acid on carbon nanotubes and nano-silica. Sci. Total Environ. 2020, 738, 139685. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Yang, C.; Liu, S.; Tan, X.; He, Y.; Liu, N.; Zhou, L.; Cai, X.; Wen, J. Effects of heteroaggregation with metal oxides and clays on tetracycline adsorption by graphene oxide. Sci. Total Environ. 2020, 719, 137283. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption Mechanisms of Organic Chemicals on Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, T.; Yang, K.; Wu, F. The relationship between humic acid (HA) adsorption on and stabilizing multiwalled carbon nanotubes (MWNTs) in water: Effects of HA, MWNT and solution properties. J. Hazard. Mater. 2012, 241–242, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shan, L.; Lv, Q.; Cai, S.; Quan, G.; Yan, J. Production of hierarchically porous carbon from natural biomass waste for efficient organic contaminants adsorption. J. Clean. Prod. 2020, 263, 121352. [Google Scholar] [CrossRef]
- Ni, J.; Pignatello, J.J.; Xing, B. Adsorption of aromatic carboxylate ions to black carbon (biochar) is accompanied by proton exchange with water. Environ. Sci. Technol. 2011, 45, 9240–9248. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gamiz, B.; Wang, Y.; Pignatello, J.J.; Xing, B. Competitive sorption used to probe strong hydrogen bonding sites for weak organic acids on carbon nanotubes. Environ. Sci. Technol. 2015, 49, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pignatello, J.J.; Wang, Y.; Xing, B. New insight into adsorption mechanism of ionizable compounds on carbon nanotubes. Environ. Sci. Technol. 2013, 47, 8334–8341. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Li, X.; Zhang, Z.; Liu, F.; Deng, Y.; Zhang, X.; Li, A.; He, L.; Xing, B. High Adsorption of Sulfamethoxazole by an Amine-Modified Polystyrene-Divinylbenzene Resin and Its Mechanistic Insight. Environ. Sci. Technol. 2016, 50, 10015–10023. [Google Scholar] [CrossRef]
- Zhao, J.; Chu, G.; Pan, B.; Zhou, Y.; Wu, M.; Liu, Y.; Duan, W.; Lang, D.; Zhao, Q.; Xing, B. Homo-Conjugation of Low Molecular Weight Organic Acids Competes with Their Complexation with Cu(II). Environ. Sci. Technol. 2018, 52, 5173–5181. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Tang, H.; Li, H.; Pan, B. New insights on the understanding of the high adsorption of bisphenol compounds on reduced graphene oxide at high pH values via charge assisted hydrogen bond. J. Hazard. Mater. 2019, 371, 513–520. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. Towards an unified hydrogen-bond theory. J. Mol. Struct. 2000, 552, 1–15. [Google Scholar] [CrossRef]
- Gilli, P.; Pretto, L.; Bertolasi, V.; Gilli, G. Predicting Hydrogen-Bond Strengths from Acid−Base Molecular Properties. The pKa Slide Rule: Toward the Solution of a Long-Lasting Problem. Acc. Chem. Res. 2009, 42, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Pignatello, J.J. Effects of Post-Pyrolysis Air Oxidation of Biomass Chars on Adsorption of Neutral and Ionizable Compounds. Environ. Sci. Technol. 2016, 50, 6276–6283. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, A.; Calzada, J.; Nunez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef] [PubMed]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.L.; Ricchetti, L.B.; Pittman, C.U.; Anderson, R.; Mlsna, T.E. Rapid removal of salicylic acid, 4-nitroaniline, benzoic acid and phthalic acid from wastewater using magnetized fast pyrolysis biochar from waste Douglas fir. Chem. Eng. J. 2017, 319, 75–88. [Google Scholar] [CrossRef]
- Dai, J.; Xiao, X.; Duan, S.; Liu, J.; He, J.; Lei, J.; Wang, L. Synthesis of novel microporous nanocomposites of ZIF-8 on multiwalled carbon nanotubes for adsorptive removing benzoic acid from water. Chem. Eng. J. 2018, 331, 64–74. [Google Scholar] [CrossRef]
- Hannon, P.R.; Flaws, J.A. The effects of phthalates on the ovary. Front. Endocrinol. 2015, 6, 8. [Google Scholar] [CrossRef]
- Shuai, W.; Gu, C.; Fang, G.; Zhou, D.; Gao, J. Effects of iron (hydr)oxides on the degradation of diethyl phthalate ester in heterogeneous (photo)-Fenton reactions. J. Environ. Sci. 2019, 80, 5–13. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water—A critical review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Khan, N.A.; Jung, B.K.; Hasan, Z.; Jhung, S.H. Adsorption and removal of phthalic acid and diethyl phthalate from water with zeolitic imidazolate and metal-organic frameworks. J. Hazard. Mater. 2015, 282, 194–200. [Google Scholar] [CrossRef]
- Tielens, F.; Folliet, N.; Bondaz, L.; Etemovic, S.; Babonneau, F.; Gervais, C.; Azaïs, T. Molecular Picture of the Adsorption of Ibuprofen and Benzoic Acid on Hydrated Amorphous Silica through DFT-D Calculations Combined with Solid-State NMR Experiments. J. Phys. Chem. C 2017, 121, 17339–17347. [Google Scholar] [CrossRef]
- Xu, Y.; Jang, J.; Gye, M.C. The Xenopus laevis teratogenesis assay for developmental toxicity of phthalate plasticizers and alternatives. Environ. Pollut. 2022, 300, 118985. [Google Scholar] [CrossRef] [PubMed]
- Vuković, G.D.; Marinković, A.D.; Škapin, S.D.; Ristić, M.Đ.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of lead from water by amino modified multi-walled carbon nanotubes. Chem. Eng. J. 2011, 173, 855–865. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Sheng, G.; Hu, J.; Tan, X.; Wang, X. Effect of surfactants on Pb(II) adsorption from aqueous solutions using oxidized multiwall carbon nanotubes. Chem. Eng. J. 2011, 166, 551–558. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; ZA, A.L.; Alsuhybani, M.; Algamdi, M. Excellent adsorptive performance of a new nanocomposite for removal of toxic Pb(II) from aqueous environment: Adsorption mechanism and modeling analysis. J. Hazard. Mater. 2020, 389, 121896. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Fan, H.; Zhuo, Q.; Liu, X.; Wu, Z. Covalent incorporation of aminated carbon nanotubes into epoxy resin network. High Perform. Polym. 2014, 26, 892–899. [Google Scholar] [CrossRef]
- Ramanathan, T.; Fisher, F.T.; Ruoff, R.S.; Brinson, L.C. Amino-Functionalized Carbon Nanotubes for Binding to Polymers and Biological Systems. Chem. Mater. 2005, 17, 1290–1295. [Google Scholar] [CrossRef]
- Ehsan, S.P.; Hossein, A.; Mohamad, E.; Hooman, F.; Seyed, J.H.; Alireza, S. Batch adsorptive removal of benzoic acid from aqueous solution onto modified natural vermiculite: Kinetic, isotherm and thermodynamic studies. J. Ind. Eng. Chem. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Caliskan, N.; Kul, A.R.; Alkan, S.; Sogut, E.G.; Alacabey, I. Adsorption of Zinc(II) on diatomite and manganese-oxide-modified diatomite: A kinetic and equilibrium study. J. Hazard. Mater. 2011, 193, 27–36. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Y.; Zhu, D.; Xia, T.; Qi, Y.; Yao, Y.; Guo, X.; Ji, R.; Chen, W. Polystyrene Nanoplastics-Enhanced Contaminant Transport: Role of Irreversible Adsorption in Glassy Polymeric Domain. Environ. Sci. Technol. 2018, 52, 2677–2685. [Google Scholar] [CrossRef]
- Barnes, A.J. Blue-shifting hydrogen bonds—are they improper or proper? J. Mol. Struct. 2004, 704, 3–9. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Schlegel, H.B. On the Physical Origin of Blue-Shifted Hydrogen Bonds. J. Am. Chem. Soc. 2002, 124, 9639–9647. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhao, Y.; Shan, S.; Yang, X.; Liu, D.; Cui, F.; Xing, B. Wrinkle- and Edge-Adsorption of Aromatic Compounds on Graphene Oxide as Revealed by Atomic Force Microscopy, Molecular Dynamics Simulation, and Density Functional Theory. Environ. Sci. Technol. 2018, 52, 7689–7697. [Google Scholar] [CrossRef] [PubMed]
- Nagayasu, T.; Yoshioka, C.; Imamura, K.; Nakanishi, K. Effects of carboxyl groups on the adsorption behavior of low-molecular-weight substances on a stainless steel surface. J. Colloid Interface Sci. 2004, 279, 296–306. [Google Scholar] [CrossRef]
- Effect of pH-Dependent Homo/Heteronuclear CAHB on Adsorption and Desorption Behaviors of Ionizable Organic Compounds on Carbonaceous Materials. Available online: https://chemicalize.com/ (accessed on 23 August 2022).
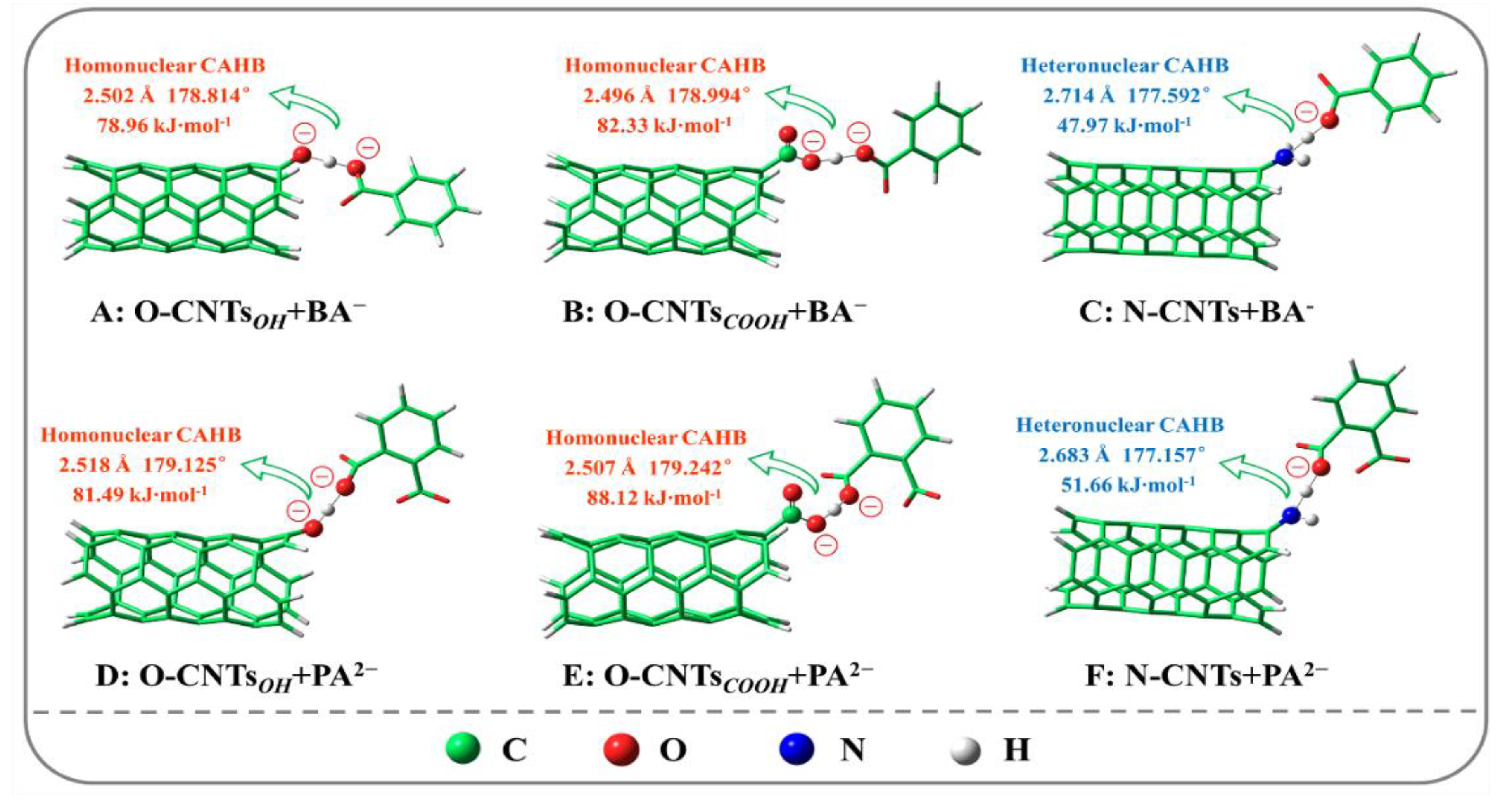
| Adsorption Configurations | Eads (kJ·mol−1) | Bond Length (Å) | Bond Angle (°) |
|---|---|---|---|
| O-CNTsOH···BA− | −78.96 | 2.502 | 178.814 |
| O-CNTsCOOH···BA− | −82.33 | 2.496 | 178.994 |
| N-CNTs···BA− | −47.97 | 2.714 | 177.592 |
| O-CNTsOH···PA2− | −81.49 | 2.518 | 179.125 |
| O-CNTsCOOH···PA2− | −88.12 | 2.507 | 179.242 |
| N-CNTs···PA2− | −51.66 | 2.683 | 177.157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, J.; Jin, Y.; Liu, Y.; Li, N.; Wang, Y.; Du, C.; Xue, Z.; Zhang, N.; Chen, Q. Effect of pH-Dependent Homo/Heteronuclear CAHB on Adsorption and Desorption Behaviors of Ionizable Organic Compounds on Carbonaceous Materials. Int. J. Environ. Res. Public Health 2022, 19, 12118. https://doi.org/10.3390/ijerph191912118
Li X, Zhang J, Jin Y, Liu Y, Li N, Wang Y, Du C, Xue Z, Zhang N, Chen Q. Effect of pH-Dependent Homo/Heteronuclear CAHB on Adsorption and Desorption Behaviors of Ionizable Organic Compounds on Carbonaceous Materials. International Journal of Environmental Research and Public Health. 2022; 19(19):12118. https://doi.org/10.3390/ijerph191912118
Chicago/Turabian StyleLi, Xiaoyun, Jinlong Zhang, Yaofeng Jin, Yifan Liu, Nana Li, Yue Wang, Cong Du, Zhijing Xue, Nan Zhang, and Qin Chen. 2022. "Effect of pH-Dependent Homo/Heteronuclear CAHB on Adsorption and Desorption Behaviors of Ionizable Organic Compounds on Carbonaceous Materials" International Journal of Environmental Research and Public Health 19, no. 19: 12118. https://doi.org/10.3390/ijerph191912118
APA StyleLi, X., Zhang, J., Jin, Y., Liu, Y., Li, N., Wang, Y., Du, C., Xue, Z., Zhang, N., & Chen, Q. (2022). Effect of pH-Dependent Homo/Heteronuclear CAHB on Adsorption and Desorption Behaviors of Ionizable Organic Compounds on Carbonaceous Materials. International Journal of Environmental Research and Public Health, 19(19), 12118. https://doi.org/10.3390/ijerph191912118







