Lycium barbarum Polysaccharide Regulates the Lipid Metabolism and Alters Gut Microbiota in High-Fat Diet Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Design
2.2. Serum and Liver Lipid-Related Index Analysis
2.3. RT-PCR Analysis
2.4. Total DNA Isolation and 16S rDNA Sequencing Analysis
2.5. Statistical Analysis
3. Results
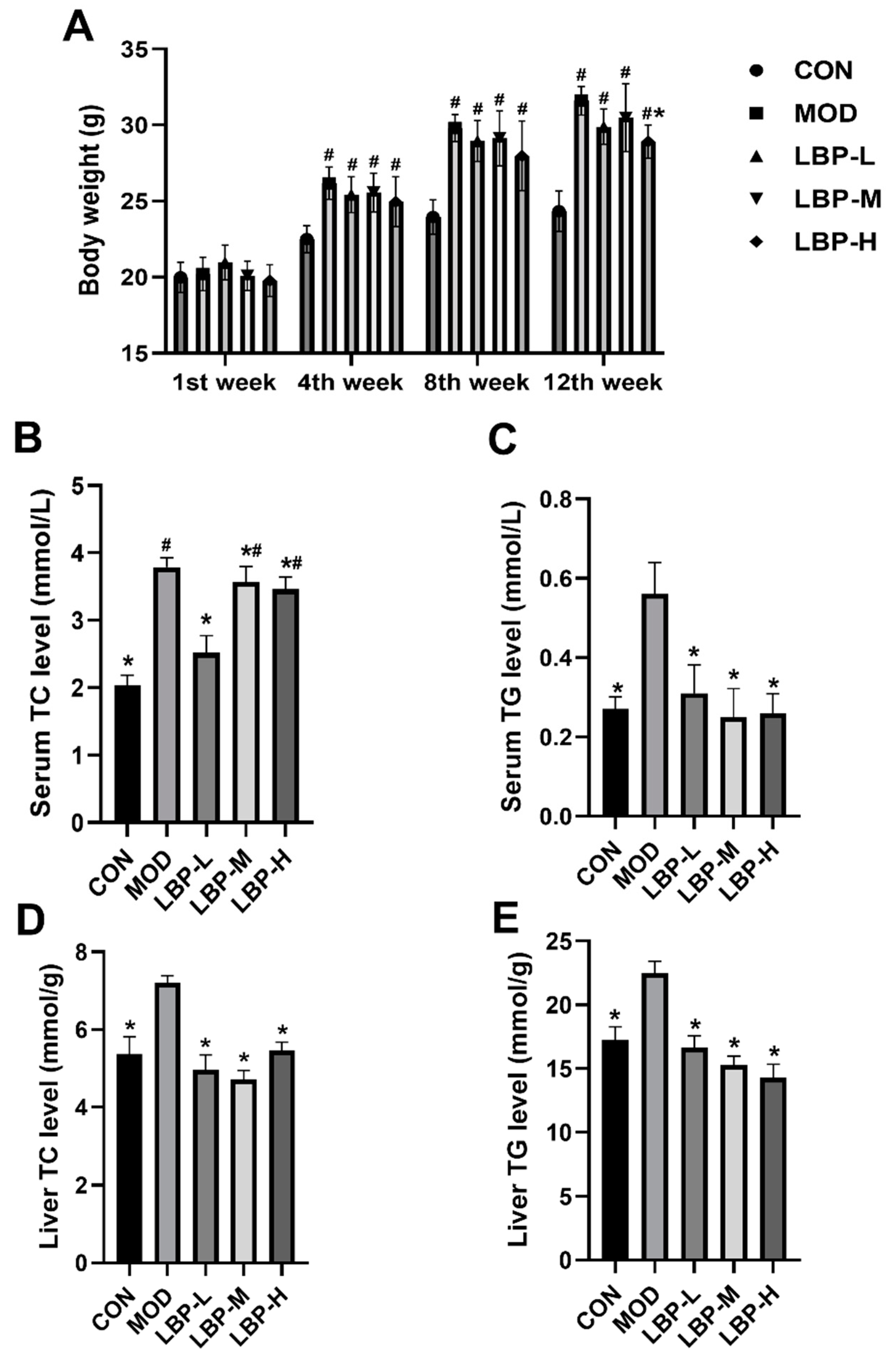
3.1. Effects of LBP Feeding on Lipid Metabolism in Preventing Obesity
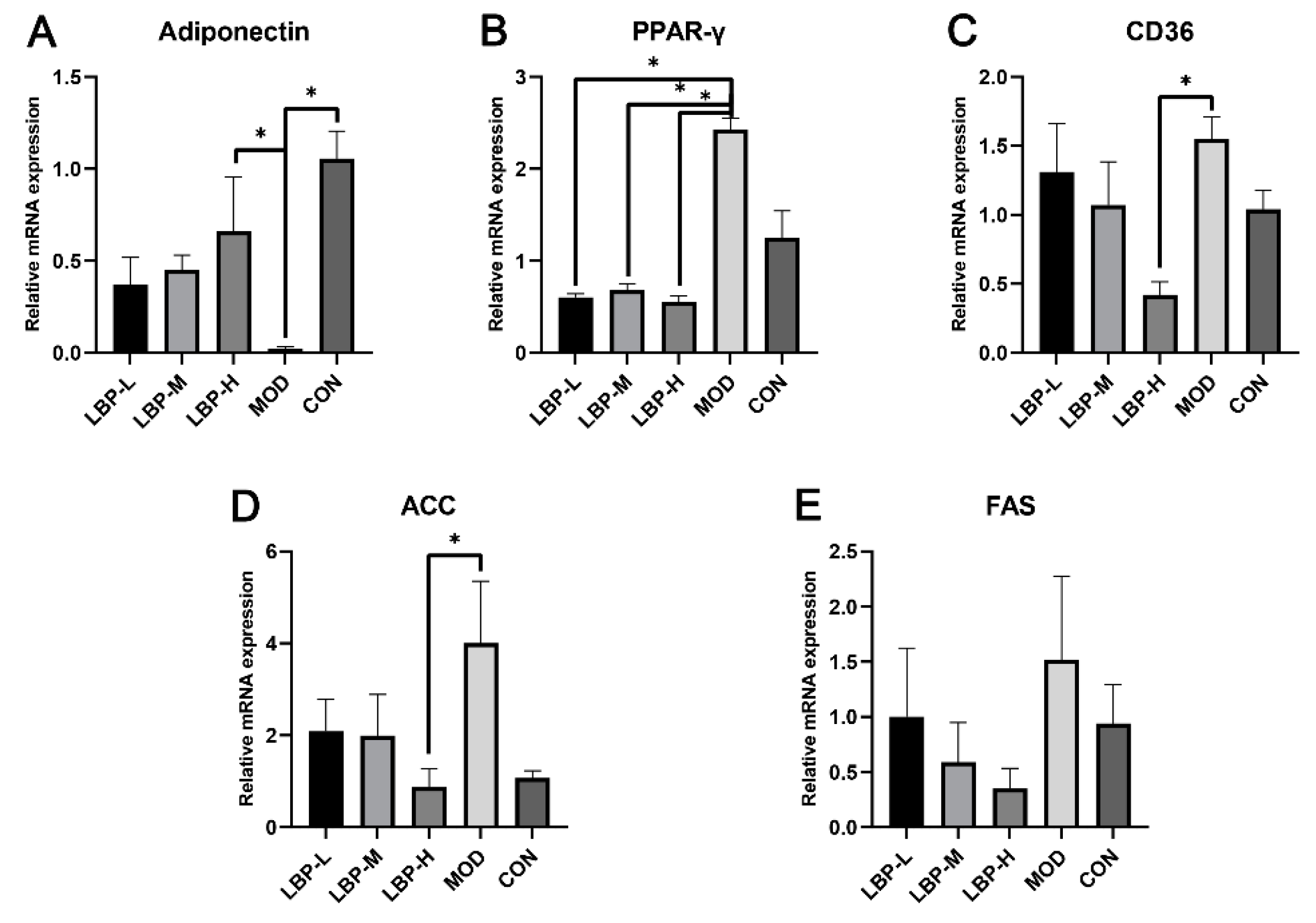
3.2. LBP Feeding Improved the Lipid-Related Gene Expression in High-Fat Diet Induced Mice
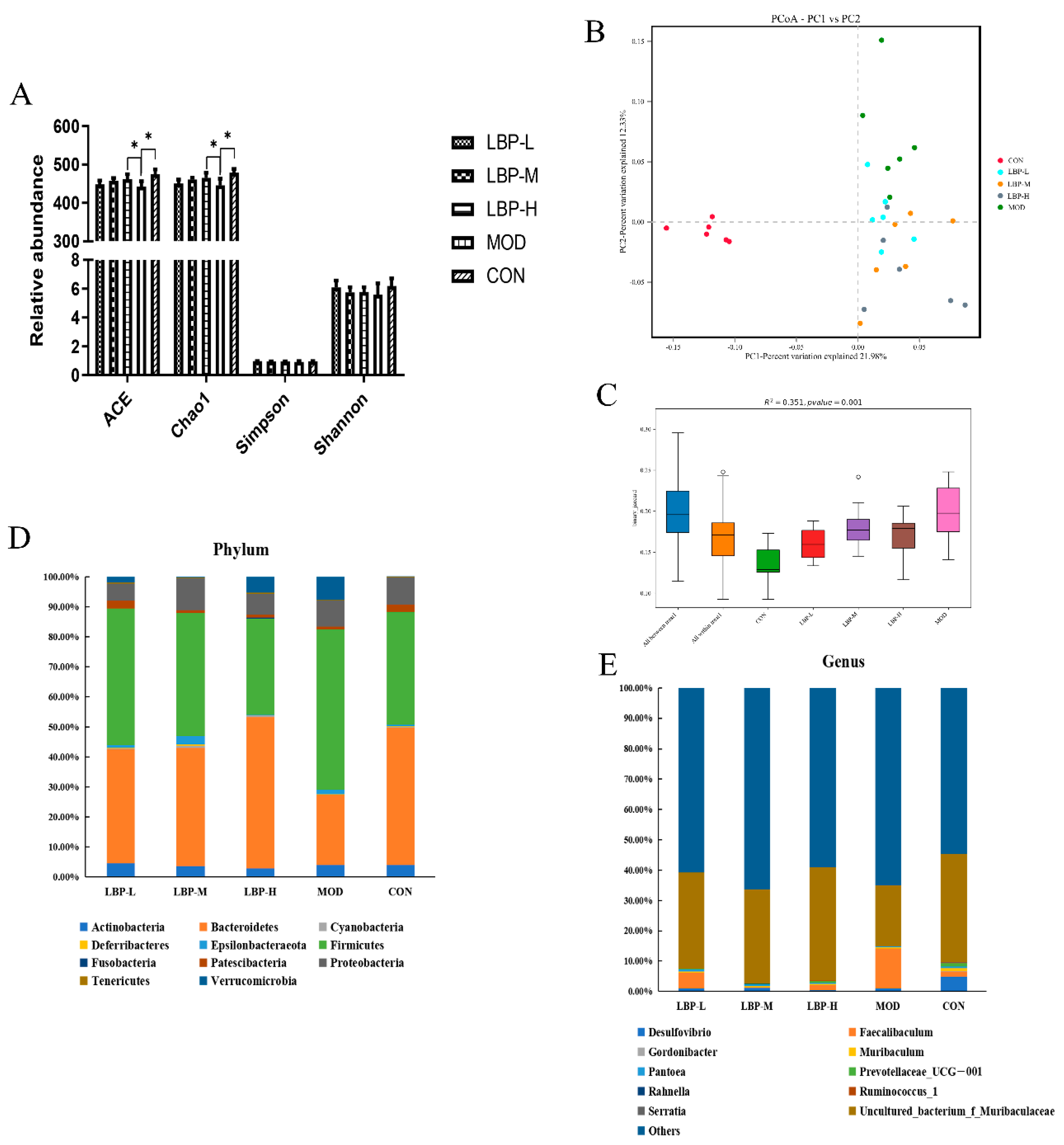
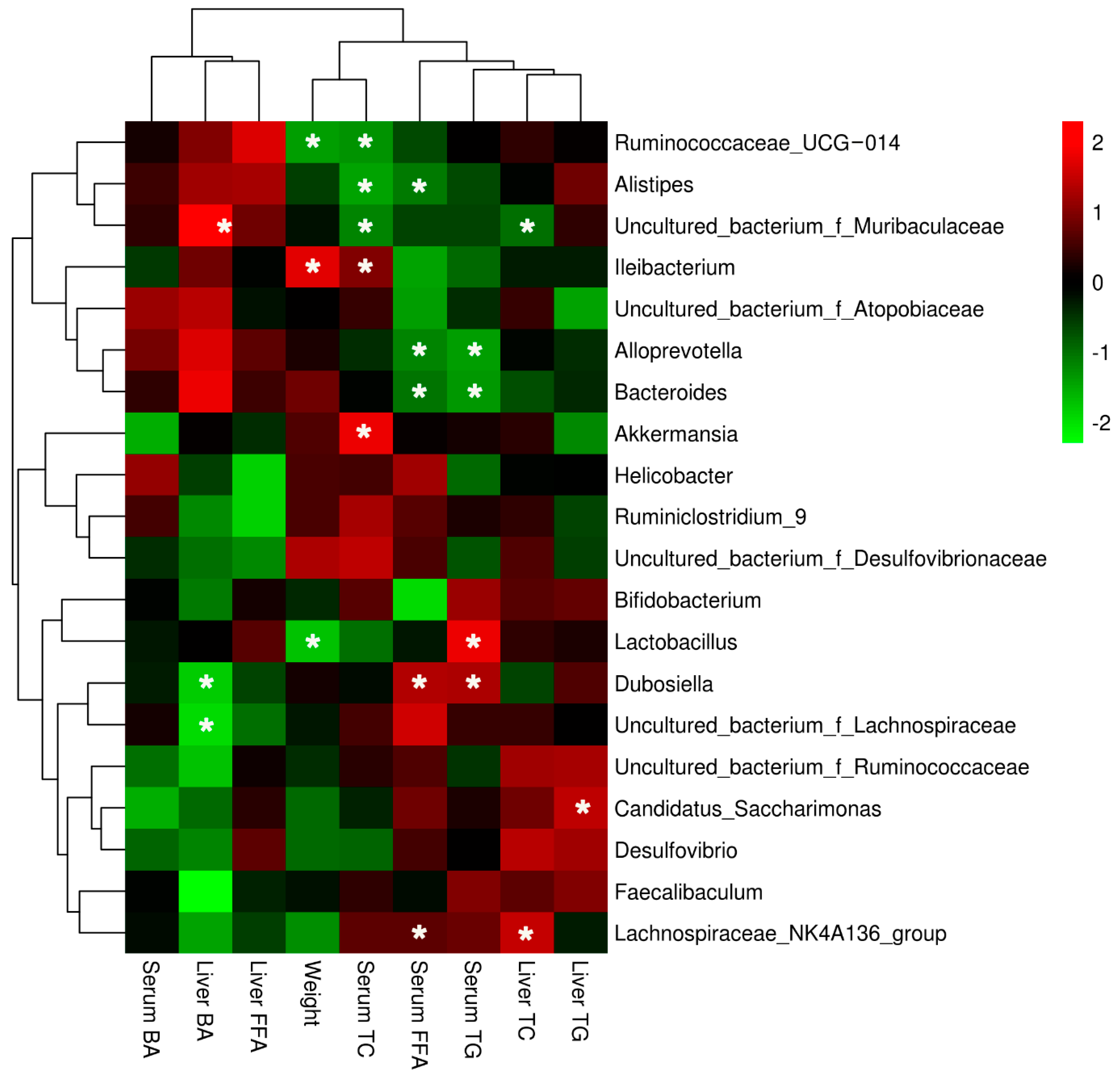
3.3. LBP Feeding Changed the Composition of Gut Microbiota in Obese Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- World Health Organization. Draft Recommendations for the Prevention and Management of Obesity over the Life Course, including Potential Targets. Available online: https://cdn.who.int/media/docs/default-source/obesity/who-discussion-paper-on-obesity---final190821.pdf?sfvrsn=4cd6710a_1&download=true (accessed on 19 August 2021).
- Stanhope, K.L.; Goran, M.I.; Bosy-Westphal, A.; King, J.C.; Schmidt, L.A.; Schwarz, J.M.; Stice, E.; Sylvetsky, A.C.; Turnbaugh, P.J.; Bray, G.A.; et al. Pathways and mechanisms linking dietary components to cardiometabolic disease: Thinking beyond calories. Obes. Rev. 2018, 19, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, V.; Sanz-Lamora, H.; Arias, G.; Marrero, P.F.; Haro, D.; Relat, J. Metabolic Impact of Flavonoids Consumption in Obesity: From Central to Peripheral. Nutrients 2020, 12, 2393. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M., Jr.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.O.; Lundgren, P.; Nath, K.; Thaiss, C.A. Metabolic control by the microbiome. Genome Med. 2022, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wang, M.; Zhong, R.; Yi, B.; Schroyen, M.; Zhang, H. Depletion of Gut Microbiota Inhibits Hepatic Lipid Accumulation in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2022, 23, 9350. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef]
- Zhou, B.; Xia, H.; Yang, L.; Wang, S.; Sun, G. The effect of Lycium barbarum polysaccharide on the glucose and lipid metabolism: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2021, 41, 618–626. [Google Scholar] [CrossRef]
- Xiao, J.; Xing, F.; Huo, J.; Fung, M.L.; Liong, E.C.; Ching, Y.P.; Xu, A.; Chang, R.C.C.; So, K.F.; Tipoe, G.L. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci. Rep. 2014, 4, 5587. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Wang, Q.; Yang, Y. Crude extracts from Lycium barbarum suppress SREBP-1c expression and prevent diet-induced fatty liver through AMPK activation. BioMed Res. Int. 2014, 2014, 196198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, M.; Jin, H.; Yang, J.; Kang, S.; Liu, Y.; Yang, S.; Ma, S.; Ni, J. Effects of Lycium barbarum Polysaccharides on Immunity and the Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice. Front. Microbiol. 2021, 12, 701566. [Google Scholar] [CrossRef]
- Hsieh, S.-Y.; Lian, Y.Z.; Lin, I.H.; Yang, Y.-C.; Tinkov, A.A.; Skalny, A.V.; Chao, J.C.J. Combined Lycium babarum polysaccharides and C-phycocyanin increase gastric Bifidobacterium relative abundance and protect against gastric ulcer caused by aspirin in rats. Nutr. Metab. 2021, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jiang, X.; Wang, T.; Zhang, B.; Zhao, H. Lyciumbarbarum Polysaccharide (LBP): A Novel Prebiotics Candidate for Bifidobacterium and Lactobacillus. Front. Microbiol. 2018, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Tang, H.; Wang, F.; Yang, X.; Wang, Z.; Liu, H.; Pan, D.; Wang, S.; Sun, G. Metabolic effects of dietary supplementation of Lycium barbarum polysaccharides on serum and urine metabolomics in a young healthy male population. J. Funct. Foods 2018, 46, 440–448. [Google Scholar] [CrossRef]
- Bustin, S.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.; Shipley, G.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Xia, H.; Tang, H.; Wang, F.; Yang, X.; Wang, Z.; Liu, H.; Pan, D.; Yang, C.; Wang, S.; Sun, G. An untargeted metabolomics approach reveals further insights of Lycium barbarum polysaccharides in high fat diet and streptozotocin-induced diabetic rats. Food Res. Int. 2019, 116, 20–29. [Google Scholar] [CrossRef]
- Domínguez-Reyes, T.; Astudillo-López, C.C.; Salgado-Goytia, L.; Muñoz-Valle, J.F.; Salgado-Bernabé, A.B.; Guzmán-Guzmán, I.P.; Castro-Alarcón, N.; Moreno-Godínez, M.E.; Parra-Rojas, I. Interaction of dietary fat intake with APOA2, APOA5 and LEPR polymorphisms and its relationship with obesity and dyslipidemia in young subjects. Lipids Health Dis. 2015, 14, 106. [Google Scholar] [CrossRef]
- Twisk, J.; Hoekman, M.F.; Mager, W.H.; Moorman, A.F.; de Boer, P.A.; Scheja, L.; Princen, H.M.; Gebhardt, R. Heterogeneous expression of cholesterol 7 α-hydroxylase and sterol 27-hydroxylase genes in the rat liver lobulus. J. Clin. Investig. 1995, 95, 1235–1243. [Google Scholar] [CrossRef]
- Koutsari, C.; Mundi, M.S.; Ali, A.H.; Patterson, B.W.; Jensen, M.D. Systemic Free Fatty Acid Disposal Into Very Low-Density Lipoprotein Triglycerides. Diabetes 2013, 62, 2386. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, W.; Zhou, M.; Tang, Y. MicroRNA-27a regulates hepatic lipid metabolism and alleviates NAFLD via repressing FAS and SCD1. Sci. Rep. 2017, 7, 14493. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Carlson, C.A.; Kim, K.H. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. Arch. Biochem. Biophys. 1974, 164, 478–489. [Google Scholar] [CrossRef]
- Skat-Rørdam, J.; Højland Ipsen, D.; Lykkesfeldt, J.; Tveden-Nyborg, P. A role of peroxisome proliferator-activated receptor γ in non-alcoholic fatty liver disease. Basic Clin. Pharmacol. Toxicol. 2019, 124, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Lim, S.; Chang, D.H.; Ahn, S.; Kim, B.C. Whole genome sequencing of "Faecalibaculum rodentium" ALO17, isolated from C57BL/6J laboratory mouse feces. Gut Pathog. 2016, 8, 3. [Google Scholar] [CrossRef]
- Mamo, G. Anaerobes as Sources of Bioactive Compounds and Health Promoting Tools. In Anaerobes in Biotechnology; HattiKaul, R., Mamo, G., Mattiasson, B., Eds.; Springer: Cham, Switzerland, 2016; Volume 156, pp. 433–464. [Google Scholar]
- Mu, H.; Zhou, Q.; Yang, R.; Zeng, J.; Li, X.; Zhang, R.; Tang, W.; Li, H.; Wang, S.; Shen, T.; et al. Naringin Attenuates High Fat Diet Induced Non-alcoholic Fatty Liver Disease and Gut Bacterial Dysbiosis in Mice. Front. Microbiol. 2020, 11, 585066. [Google Scholar] [CrossRef]
- Jang, S.E.; Hyam, S.R.; Han, M.J.; Kim, S.Y.; Lee, B.G.; Kim, D.H. Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages. J. Appl. Microbiol. 2013, 115, 888–896. [Google Scholar] [CrossRef]
- Ye, Y.; Jiang, R.; Guo, X.; Zhang, Y.; Xu, Q.; Li, M.; Teng, Y.; Tao, M. Correlations analysis of gut microbiota with blood glucose and lipids in the third trimester pregnancy. Microbiol. China 2021, 48, 2118–2130. [Google Scholar]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.-J.; Hu, R.-K.; Wu, L.-X.; Lv, X.-C.; Li, X.; Zhao, L.-N.; Liu, B. Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters. J. Food Biochem. 2020, 44, e13109. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Li, M.; Huang, Z.; Jiang, J.; Chen, Y.; Chen, J.; Jia, Y.; Zhang, L.; Zhou, F. Ferulic Acid Ameliorates Atherosclerotic Injury by Modulating Gut Microbiota and Lipid Metabolism. Front. Pharmacol. 2021, 12, 621339. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Song, G.; Zhang, M.; Shi, J.; Xu, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Wu, Z.; Miao, J.; Li, S.; Wang, S.; Yu, Q.; He, F. Exploration on the characteristics of intestinal microbiota in association with metabolism and immunity in the elderlys. Acta Nutr. Sin. 2017, 39, 228–233. [Google Scholar]
- Wang, Y.; Ye, L.; Jia, Y.; Pang, G.; Zhao, P.; Yan, Y.; Chen, Q. Lactobacillus helveticus TS206 Regulates Serum Lipid Levels and Fecal Bile Acid Levels in Mice. Food Sci. 2017, 38, 196–201. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Zhou, B.; Sui, J.; Ma, W.; Wang, S.; Yang, L.; Sun, G. Lycium barbarum Polysaccharide Regulates the Lipid Metabolism and Alters Gut Microbiota in High-Fat Diet Induced Obese Mice. Int. J. Environ. Res. Public Health 2022, 19, 12093. https://doi.org/10.3390/ijerph191912093
Xia H, Zhou B, Sui J, Ma W, Wang S, Yang L, Sun G. Lycium barbarum Polysaccharide Regulates the Lipid Metabolism and Alters Gut Microbiota in High-Fat Diet Induced Obese Mice. International Journal of Environmental Research and Public Health. 2022; 19(19):12093. https://doi.org/10.3390/ijerph191912093
Chicago/Turabian StyleXia, Hui, Beijia Zhou, Jing Sui, Wenqing Ma, Shaokang Wang, Ligang Yang, and Guiju Sun. 2022. "Lycium barbarum Polysaccharide Regulates the Lipid Metabolism and Alters Gut Microbiota in High-Fat Diet Induced Obese Mice" International Journal of Environmental Research and Public Health 19, no. 19: 12093. https://doi.org/10.3390/ijerph191912093
APA StyleXia, H., Zhou, B., Sui, J., Ma, W., Wang, S., Yang, L., & Sun, G. (2022). Lycium barbarum Polysaccharide Regulates the Lipid Metabolism and Alters Gut Microbiota in High-Fat Diet Induced Obese Mice. International Journal of Environmental Research and Public Health, 19(19), 12093. https://doi.org/10.3390/ijerph191912093








