Good Practices on Endoscope Reprocessing in Italy: Findings of a Nationwide Survey
Abstract
:1. Introduction
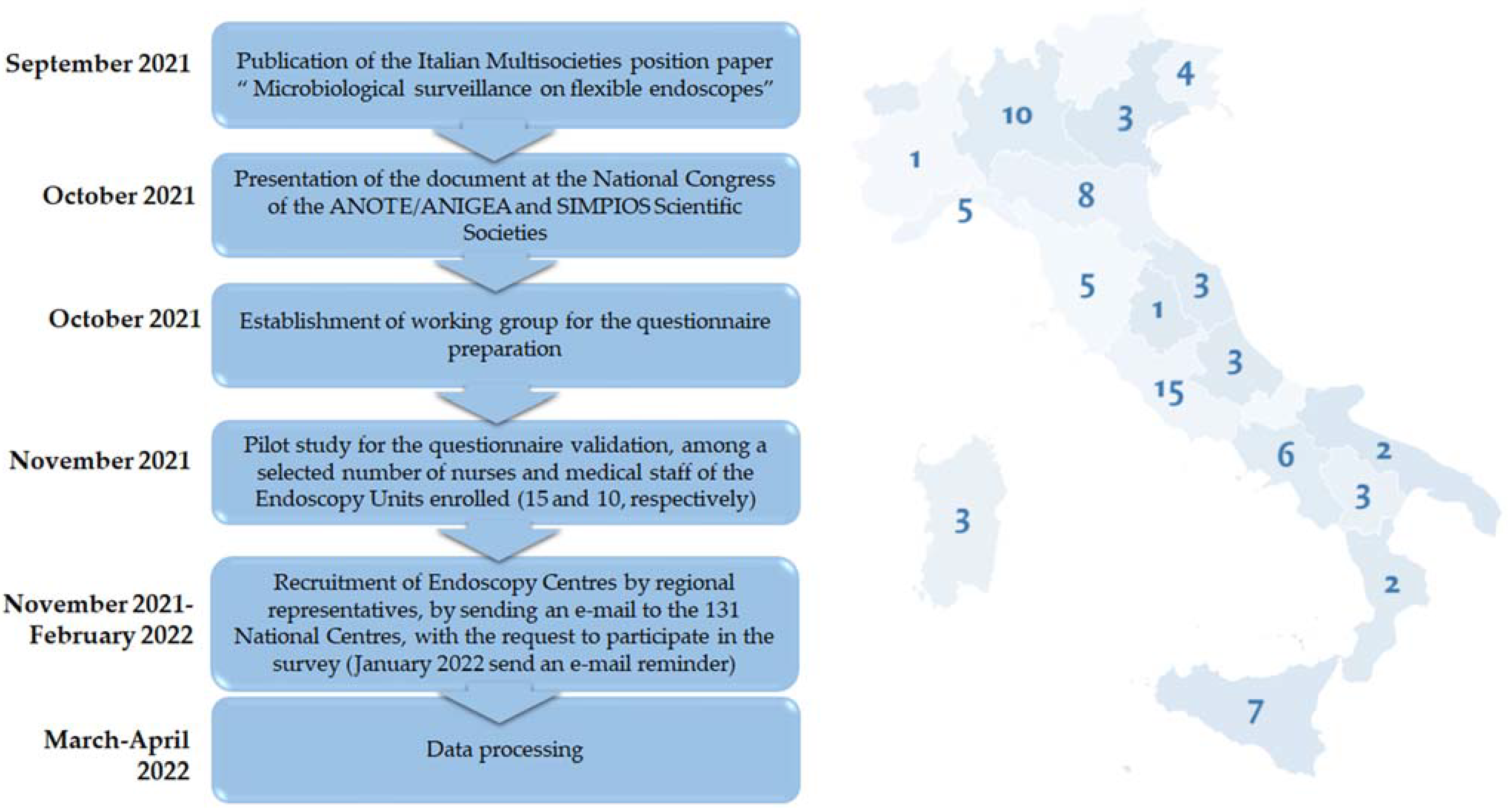
2. Methods
3. Results
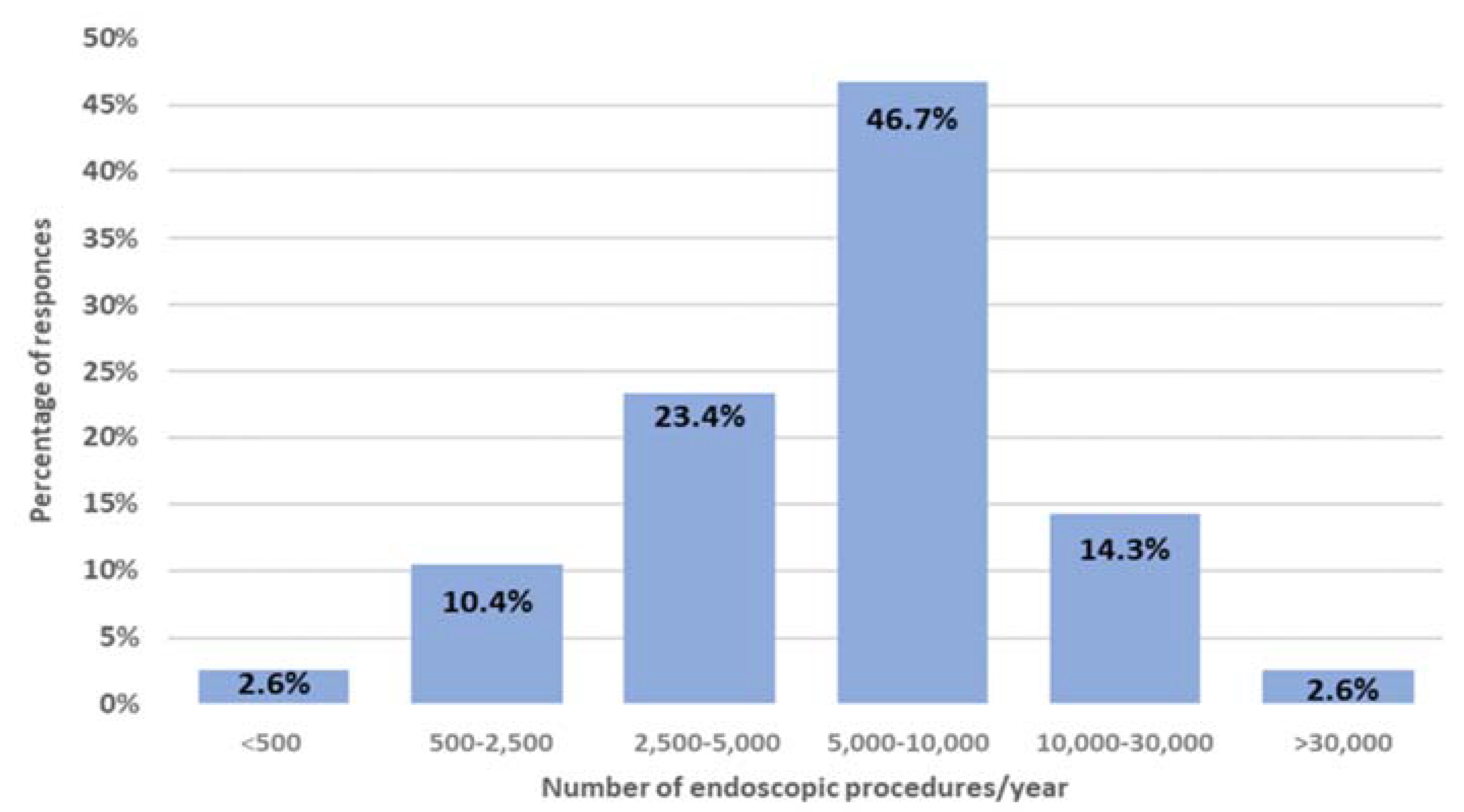
3.1. Characteristics of Centres
3.2. Endoscopes and the Devices Utilized
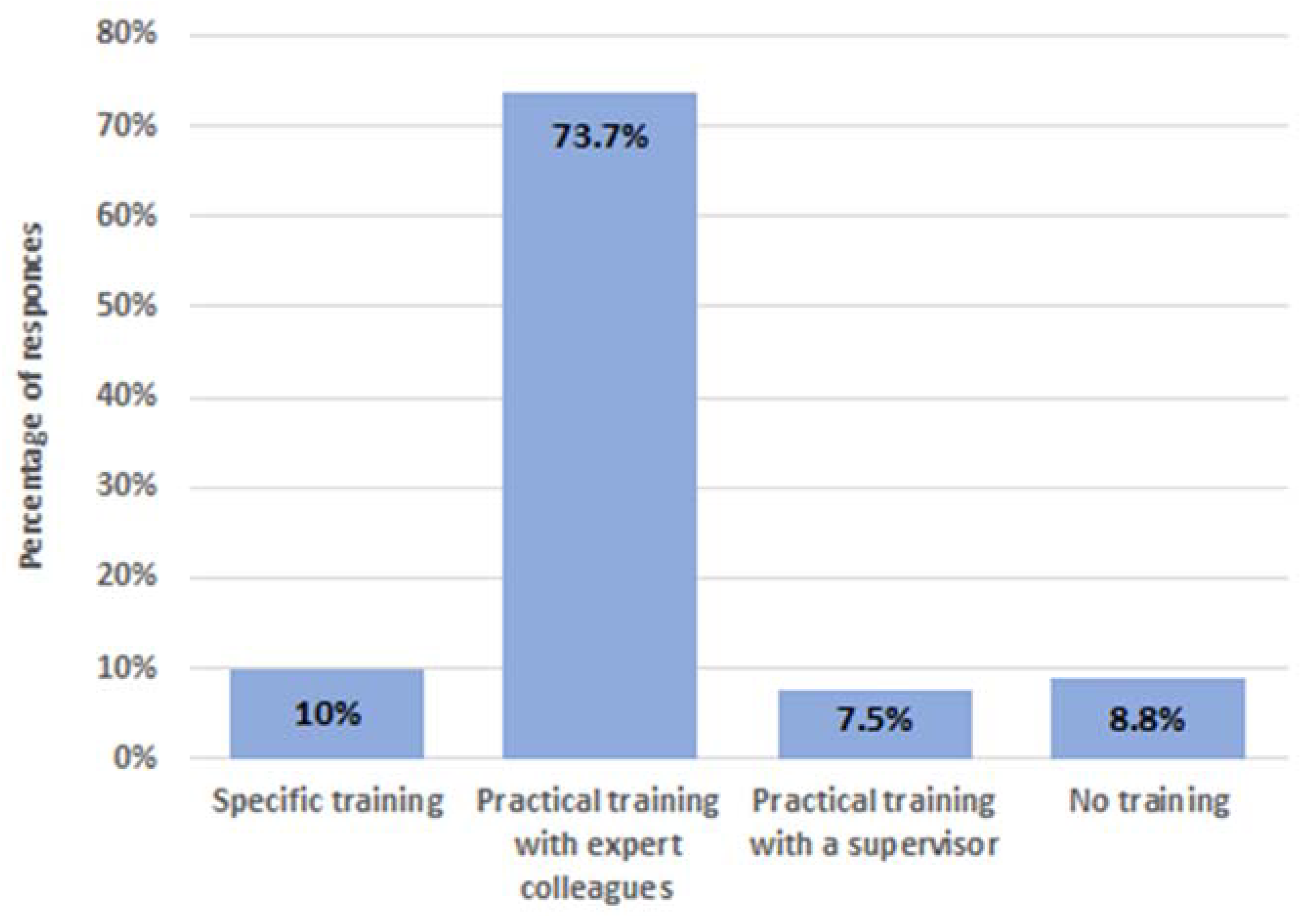
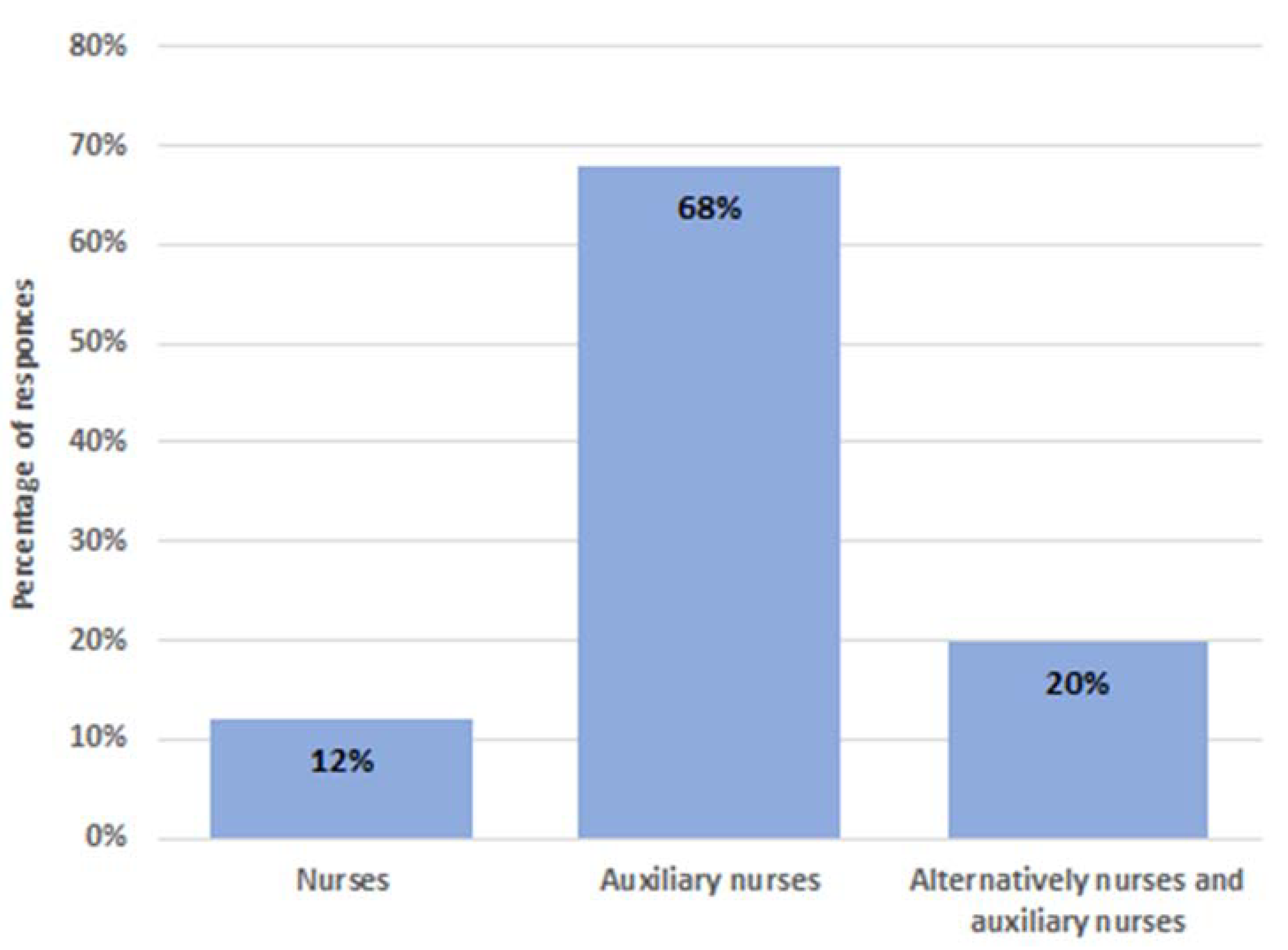
3.3. Training on Reprocessing
3.4. Reprocessing Procedures
3.5. Microbiological Surveillance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muscarella, L.F. Risk of transmission of carbapenem-resistant Enterobacteriaceae and related “superbugs” during gastrointestinal endoscopy. World J. Gastrointest. Endosc. 2014, 6, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Rubin, Z.A.; Kim, S.; Thaker, A.M.; Muthusamy, V.R. Safely reprocessing duodenoscopes: Current evidence and future directions. Lancet Gastroenterol. Hepatol. 2018, 3, 499–508. [Google Scholar] [CrossRef]
- Murray, P.; Washington, DC, USA; Senate Health, Education, Labor and Pensions Committee. Preventable Tragedies: Superbugs and How Ineffective Monitoring of Medical Device Safety Fails Patients; 2016. Available online: https://www.help.senate.gov/imo/media/doc/Duodenoscope%20Investigation%20FINAL%20Report.pdf (accessed on 17 August 2022).
- Larsen, S.; Russell, R.V.; Ockert, L.K.; Spanos, S.; Travis, H.S.; Ehlers, L.H.; Mærkedahl, A. Rate and impact of duodenoscope contamination: A systematic review and meta-analysis. EClinicalMedicine 2020, 25, 100451. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Reprocessing semicritical items: Outbreaks and current issues. Am. J. Infect. Control 2019, 47, A79–A89. [Google Scholar] [CrossRef] [PubMed]
- Interim Protocol for Healthcare Facilities Regarding Surveillance for Bacterial Contamination of Duodenoscopes after Reprocessing; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2015.
- Beilenhoff, U.; Biering, H.; Blum, R.; Brljak, J.; Cimbro, M.; Dumonceau, J.M.; Hassan, C.; Jung, M.; Kampf, B.; Neumann, C.; et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA)–Update 2018. Endoscopy 2018, 50, 1205–1234. [Google Scholar] [CrossRef] [PubMed]
- Gastroenterological Society of Australia; Gastroenterological Nurses College of Australia. Infection Control in Endoscopy, 3rd ed.; Gastroenterological Society of Australia: Sydney, Australia, 2010. [Google Scholar]
- Day, L.W.; Muthusamy, V.R.; Collins, J.; Kushnir, V.M.; Sawhney, M.S.; Thosani, N.C.; Wani, S. Multisociety guideline on reprocessing flexible GI endoscopes and accessories. Gastrointest. Endosc. 2021, 93, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Duodenoscope Surveillance Sampling & Culturing: Reducing the Risks of Infection; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2018.
- Beilenhoff, U.; Neumann, C.S.; Rey, J.F.; Biering, H.; Blum, R.; Schmidt, V. ESGE Guidelines Committee. ESGE-ESGENA guideline for quality assurance in reprocessing: Microbiological surveillance testing in endoscopy. Endoscopy 2007, 39, 175–181. [Google Scholar] [CrossRef]
- Casini, B.; Pan, A.; Guarini, A.; Rivara, C.; Zullo, A.; Monica, F.; Cimbro, M.; Casarano, S.; Inglese, A.; Vaghi, A.; et al. Working Team on Infections in Endoscopy. Multisocieties position paper: Microbiological surveillance on flexible endoscopes. Dig. Liver Dis. 2021, 53, 1105–1111. [Google Scholar] [CrossRef]
- Archibald, L.K.; Jarvis, W.R. Health care-associated infection outbreak investigations by the Centers for Disease Control and Prevention, 1946–2005. Am. J. Epidemiol. 2011, 174 (Suppl. 11), S47–S64. [Google Scholar] [CrossRef]
- Ramsey, A.H.; Oemig, T.V.; Davis, J.P.; Massey, J.P.; Toöroök, T.J. An outbreak of bronchoscopy-related Mycobacterium tuberculosis infections due to lack of bronchoscope leak testing. Chest 2002, 121, 976–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, K.; Jang, S. Single Use (disposable) Duodenoscope: Recent Development and Future. Clin. Endosc. 2022, 55, 191–196. [Google Scholar] [CrossRef]
- Trindade, A.J.; Copland, A.; Bhatt, A.; Bucobo, J.C.; Chandrasekhara, V.; Krishnan, K.; Parsi, M.A.; Kumta, N.; Law, R.; Pannala, R.; et al. Single-use duodenoscopes and duodenoscopes with disposable end caps. Gastrointest. Endosc. 2021, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Aumeran, C.; Robin, F.; Traore, O.; Forestier, C. Antibiotic resistance and plasmid transfer capacity in biofilm formed with a CTX-M-15-producing Klebsiella pneumoniae isolate. J. Antimicrob. Chemother. 2012, 67, 2123–2130. [Google Scholar] [CrossRef]
- Alfa, M.J.; Singh, H. Impact of wet storage and other factors on biofilm formation and contamination of patient-ready endoscopes: A narrative review. Gastrointest. Endosc. 2020, 91, 236–247. [Google Scholar] [CrossRef]
- Beilenhoff, U.; Biering, H.; Blum, R.; Brljak, J.; Cimbro, M.; Dumonceau, J.M.; Hassan, C.; Jung, M.; Neumann, C.; Pietsch, M.; et al. Prevention of multidrug-resistant infections from contaminated duodenoscopes: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA). Endoscopy 2017, 49, 1098–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpaci, M.; Cosci, T.; Tuvo, B.; Guarini, A.; Iannone, T.; Zullo, A.; Casini, B., on behalf of SIMPIOS and ANOTE-ANIGEA Study Group. Good Practices on Endoscope Reprocessing in Italy: Findings of a Nationwide Survey. Int. J. Environ. Res. Public Health 2022, 19, 12082. https://doi.org/10.3390/ijerph191912082
Scarpaci M, Cosci T, Tuvo B, Guarini A, Iannone T, Zullo A, Casini B on behalf of SIMPIOS and ANOTE-ANIGEA Study Group. Good Practices on Endoscope Reprocessing in Italy: Findings of a Nationwide Survey. International Journal of Environmental Research and Public Health. 2022; 19(19):12082. https://doi.org/10.3390/ijerph191912082
Chicago/Turabian StyleScarpaci, Michela, Tommaso Cosci, Benedetta Tuvo, Alessandra Guarini, Teresa Iannone, Angelo Zullo, and Beatrice Casini on behalf of SIMPIOS and ANOTE-ANIGEA Study Group. 2022. "Good Practices on Endoscope Reprocessing in Italy: Findings of a Nationwide Survey" International Journal of Environmental Research and Public Health 19, no. 19: 12082. https://doi.org/10.3390/ijerph191912082
APA StyleScarpaci, M., Cosci, T., Tuvo, B., Guarini, A., Iannone, T., Zullo, A., & Casini, B., on behalf of SIMPIOS and ANOTE-ANIGEA Study Group. (2022). Good Practices on Endoscope Reprocessing in Italy: Findings of a Nationwide Survey. International Journal of Environmental Research and Public Health, 19(19), 12082. https://doi.org/10.3390/ijerph191912082







