Enzyme Activities in Reduction of Heavy Metal Pollution from Alice Landfill Site in Eastern Cape, South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.1.1. Collection and Preparation of Soil Sample
2.1.2. Enzyme Assay
2.1.3. The Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analysis
2.1.4. Statistical Analysis
3. Results
3.1. ICP-MS Elemental Analysis
| Soil Heavy Metal Concentration (mg/kg) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Sites | Al | Ba | Ca | Fe | K | Mg | Mn | Na | Ni | P | Sr | Zn | Co | Cr | Cu |
| Site 1A | 21,943 | 265 | 8131 | 15,339 | 5835 | 1986 | 623 | 676 | 11 | 403 | 46 | 107 | 12 | 35 | 31 |
| Site 1B | 36,265 | 336 | 6348 | 22,608 | 9513 | 3139 | 656 | 523 | 15 | 420 | 50 | 160 | 11 | 45 | 82 |
| Site 1C | 25,198 | 228 | 8880 | 15,463 | 7162 | 2279 | 437 | 374 | 11 | 470 | 63 | 112 | 9 | 43 | 75 |
| Site 1D | 24,100 | 145 | 3149 | 17,267 | 5105 | 2125 | 417 | 496 | 18 | 296 | 25 | 30 | 12 | 57 | 12 |
| Site 2A | 29,435 | 402 | 33,584 | 25,098 | 9179 | 3704 | 938 | 1541 | 21 | 1344 | 87 | 1031 | 17 | 68 | 179 |
| Site 2B | 40,654 | 302 | 3431 | 23,227 | 9717 | 3006 | 527 | 363 | 15 | 284 | 36 | 65 | 11 | 47 | 24 |
| Site 2C | 24,435 | 213 | 5536 | 15,322 | 6989 | 2055 | 404 | 288 | 10 | 328 | 52 | 58 | 8 | 40 | 18 |
| Site 2D | 25,589 | 176 | 3651 | 18,707 | 4773 | 2419 | 489 | 587 | 19 | 192 | 33 | 30 | 13 | 63 | 12 |
| Site 3A | 25,893 | 356 | 7815 | 19,079 | 6913 | 2403 | 776 | 643 | 15 | 412 | 56 | 105 | 13 | 43 | 28 |
| Site 3B | 31,172 | 298 | 6335 | 19,629 | 8185 | 2670 | 633 | 476 | 15 | 346 | 47 | 129 | 11 | 44 | 33 |
| Site 3C | 26,855 | 232 | 7808 | 16,678 | 7066 | 2336 | 433 | 396 | 13 | 403 | 56 | 98 | 9 | 45 | 39 |
| Site 3D | 26,646 | 169 | 5286 | 20,163 | 5499 | 2759 | 499 | 1029 | 19 | 235 | 35 | 37 | 13 | 58 | 17 |
| WHO permissible limits (mg/kg) [15] | n. a | n. a | n. a | 1000 | n. a | n. a | 500 | n. a | 75 | n. a | n. a | 50 | 50 | 63 | 30 |
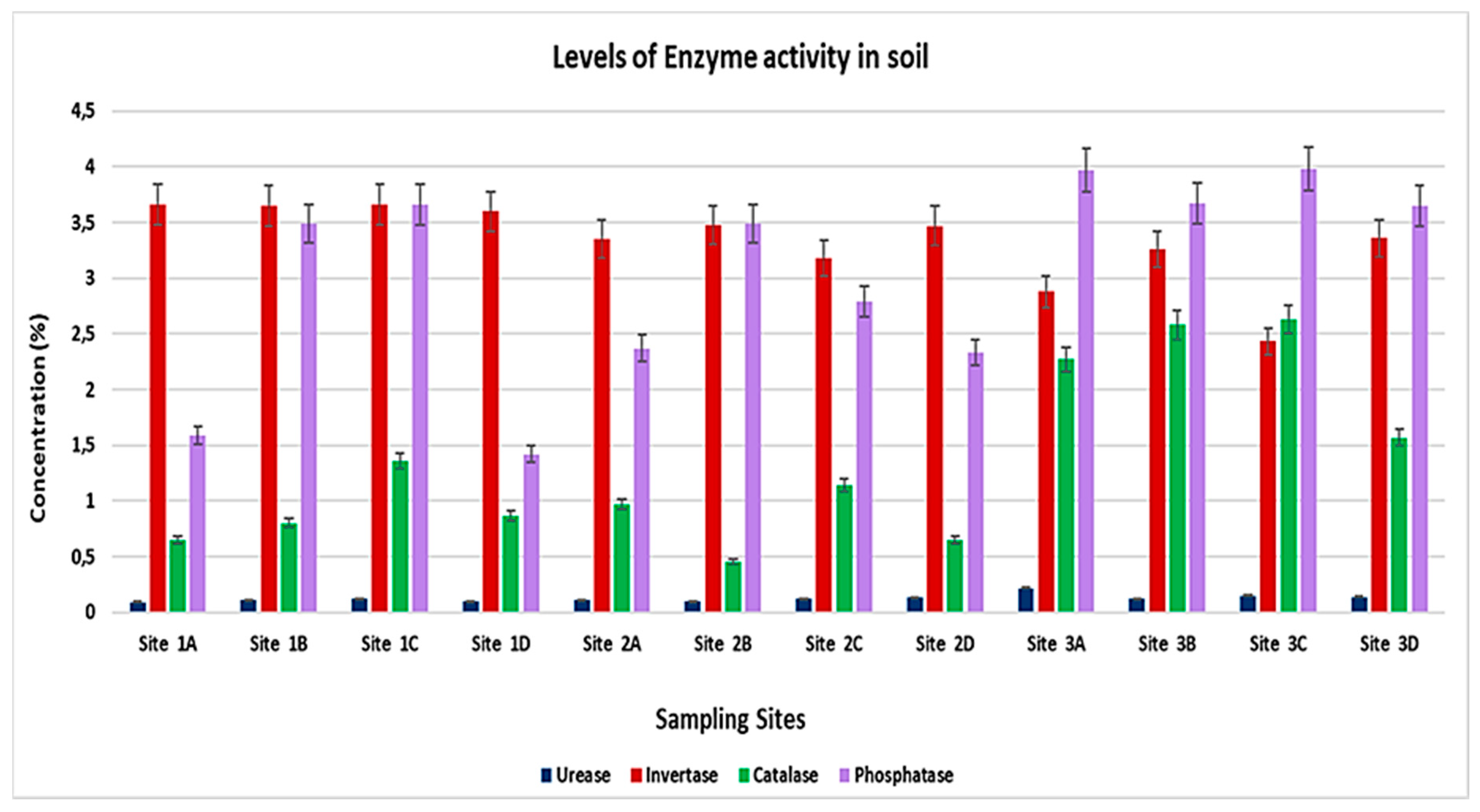
3.2. Concentration of Selected Soil Enzymes Activity in the Soil
3.3. The One-Way ANOVA Results of Soil Heavy Metals
3.4. Correlation between Enzyme Activity and Metal Content in the Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Titilawo, Y.; Adeniji, A.; Adeniyi, M.; Okoh, A. Determination of levels of some metal contaminants in the freshwater environments of Osun State, Southwest Nigeria: A risk assessment approach to predict health threat. Chemosphere 2018, 211, 834–843. [Google Scholar] [CrossRef]
- Agoro, M.A.; Adeniji, A.O.; Adefisoye, M.A.; Okoh, O.O. Heavy metals in wastewater and sewage sludge from selected municipal treatment plants in Eastern Cape province, South Africa. Water 2020, 12, 2746. [Google Scholar] [CrossRef]
- Nyika, J.M.; Onyari, E.K.; Dinka, M.O.; Mishra, S.B. Heavy metal pollution and mobility in soils within a landfill vicinity: A south African case study. Orient. J. Chem. 2019, 35, 1286. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.S.; Kim, J.G.; Kim, S.O. Use of Soil Enzymes as Indicators for Contaminated Soil Monitoring and Sustainable Management. Sustainability 2020, 12, 8209. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Liu, T.; Xu, C.; Duan, D.; Peng, C.; Zhu, S.; Shi, J. The variations in the soil enzyme activity, protein expression, microbial biomass, and community structure of soil contaminated by heavy metals. Int. Sch. Res. Not. 2013, 2013. [Google Scholar] [CrossRef]
- Mangizvo, R.V.; Wiseman, M. The management, practice, and environmental health implications of the municipal solid waste dumpsite in Alice, South Africa. Online J. Soc. Sci. Res. 2012, 1, 125–131. [Google Scholar]
- Ansari, T.M.; Marr, I.L.; Tariq, N. Heavy Metals in Marine Pollution Perspective—A Mini-Review. J. Appl. Sci. 2004, 4, 1–20. [Google Scholar] [CrossRef]
- Parajuli, P.B.; Duffy, S. Evaluation of soil organic carbon and soil moisture content from agricultural fields in Mississippi. Open J. Soil Sci. 2013, 3, 81. [Google Scholar] [CrossRef]
- Li, T.; Meng, L.; Herman, U.; Lu, Z.; Crittenden, J. A survey of soil enzyme activities along major roads in Beijing: The implications for traffic corridor green space management. Int. J. Environ. Res. Public Health 2015, 12, 12475–12488. [Google Scholar] [CrossRef]
- Xian, Y.; Wang, M.; Chen, W. Quantitative assessment of soil enzyme activities of heavy metal contaminated soils with various soil properties. Chemosphere 2015, 139, 604–608. [Google Scholar] [CrossRef]
- Durowoju, O.S.; Odiyo, J.O.; Ekosse, G.I.E. Variations of heavy metals from geothermal spring to surrounding soil and Mangifera indica–Siloam village, Limpopo province. Sustainability 2016, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Shadman, S.M.; Daneshi, M.; Shafiei, F.; Azimimehr, M.; Khorasgani, M.R.; Sadeghian, M.; Motaghi, H.; Mehrgardi, M.A. Aptamer-based electrochemical biosensors. In Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–251. [Google Scholar]
- Gebeyehu, H.R.; Bayissa, L.D. Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PLoS ONE 2020, 15, 0227883. [Google Scholar] [CrossRef]
- Abdulhamid, Z.; Agbaji, E.B.; Gimba, C.E.; Agbaji, A.S. Physicochemical parameters and heavy metals content of Soil samples from farms in Minna. Int. Lett. Chem. Phys. Astron. 2015, 58, 154–163. [Google Scholar] [CrossRef]
- Sarthou, G.; Baker, A.R.; Blain, S.; Achterberg, E.P.; Boye, M.; Bowie, A.R.; Croot, P.; Laan, P.; de Baar, H.J.; Jickells, T.D.; et al. Atmospheric iron deposition and sea-surface dissolved iron concentrations in the eastern Atlantic Ocean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2003, 50, 1339–1352. [Google Scholar] [CrossRef]
- Karaca, A.; Cetin, S.C.; Turgay, O.C.; Kizilkaya, R. Effects of heavy metals on soil enzyme activities. In Soil Heavy Metals, Soil Biology; Sherameti, I., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 19, pp. 237–265. [Google Scholar]
- Bartkowiak, A.; Breza-Boruta, B.; Lemanowicz, J. Assessment of the content of heavy metals and potential pathogenic microorganisms in soil under illegal dumping sites. Environ. Earth Sci. 2016, 75, 1401. [Google Scholar] [CrossRef]
- Aziza, K.; Naïma, E.G.; Naoual, R.; Khalid, D.; Mustapha, I.; Wifak, B. Leaching of Heavy Metals and Enzymatic Activities in Un-inoculated and Inoculated Soils with Yeast Strains. Soil Sediment Contam. Int. J. 2020, 29, 860–879. [Google Scholar] [CrossRef]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total. Environ. 2020, 737, 139744. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A.S. Effects of heavy metals on soil, plants, human health, and aquatic life. Int. J. Res. Chem. Environ. 2011, 1, 15–21. [Google Scholar]
- Angelovičová, L.; Lodenius, M.; Tulisalo, E.; Fazekašová, D. Effect of heavy metals on soil enzyme activity at different field conditions in Middle Spis mining area (Slovakia). Bull. Environ. Contam. Toxicol. 2014, 93, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Al-Temimi, A.; Al-Ghrairi, S.; Al-Ghrairi, F.; Razaq, I. Effect of potassium and micronutrient fertilization on the activity of catalase and yield of wheat grown in saline conditions. DYSO-NA-Appl. Sci. 2020, 1, 81–87. [Google Scholar]
- Rao, S.; Praveen, E. Quantitative Determination of Heavy Metals in the Soil along Musi River of Hyderabad. IOSR J. Appl. Chem. (IOSR-JAC) 2014, 7, 38–39. [Google Scholar] [CrossRef]
- Sardar, K.H.A.N.; Qing, C.A.O.; Hesham, A.E.L.; Yue, X.; He, J.Z. Soil enzymatic activities and microbial community structure with different application rates of Cd and Pb. J. Environ. Sci. 2007, 19, 834–840. [Google Scholar]



| Enzyme | Substrate | Incubation Hours | Metabolite |
|---|---|---|---|
| Invertase | 3,5-Dinitrosalicylic acid | 12 | Glucose |
| Urease | Urea (CH4N2O) | 3 | NH4–N (Ammonium) |
| Catalase | 3% H2O2 (Hydrogen peroxide) | 3 | KMnO4 (Potassium manganate) |
| Phosphatase | C6H5PO4Na2·2H2O (Phenyl phosphate disodium salt dihydrate) | 1 | Phenol (C6H6O) |
| Urease (μg NH4- N⋅g−1 Soil⋅h−1) | Invertase (μg Glucose⋅g−1 Soil⋅h−1) | Catalase (mL KmnO4⋅g−1 Soil⋅h−1) | Phosphatase (μg Phenol⋅g−1 Soil⋅h−1) | ||
|---|---|---|---|---|---|
| Week 1 | Site 1A | 0.09 ± 0.05 c | 3.66 ± 2.11 d | 0.65 ± 0.37 b | 1.59 ± 0.92 d |
| Site 1B | 0.11 ± 0.06 c | 3.65 ± 2.10 d | 0.80 ± 0.46 cd | 3.49 ± 2.01 ab | |
| Site 1C | 0.12 ± 0.07 c | 3.66 ± 2.11 d | 1.36 ± 0.78 a | 3.66 ± 2.11 e | |
| Site 2 | 0.10 ± 0.06 c | 3.60 ± 2.08 d | 0.87 ± 0.50 cd | 1.42 ± 0.82 d | |
| Week 2 | Site 1A | 0.11 ± 0.06 c | 3.35 ± 1.93 d | 0.97 ± 0.56 cd | 2.37 ± 1.37 c |
| Site 1B | 0.10 ± 0.05 c | 3.48 ± 2.01 d | 0.46 ± 0.27 ab | 3.49 ± 2.01 ab | |
| Site 1C | 0.12 ± 0.07 c | 3.18 ± 1.83 d | 1.14 ± 0.66 a | 2.79 ± 1.61 a | |
| Site 2 | 0.13 ± 0.07 c | 3.47 ± 2.01 d | 0.65 ± 0.37 b | 2.33 ± 1.34 c | |
| Week 3 | Site 1A | 0.22 ± 0.13 cd | 2.88 ± 1.66 c | 2.27 ± 1.31 c | 3.97 ± 2.29 e |
| Site 1B | 0.12 ± 0.07 c | 3.26 ± 1.88 d | 2.58 ± 1.49 d | 3.67 ± 2.12 e | |
| Site 1C | 0.15 ± 0.09 c | 2.43 ± 1.52 c | 2.63 ± 1.52 d | 3.98 ± 2.30 e | |
| Site 2 | 0.14 ± 0.0 c | 3.36 ± 1.94 d | 1.57 ± 0.91 a | 3.65 ± 2.10 e | |
| ANOVA | |||||
|---|---|---|---|---|---|
| Metals | |||||
| Sum of Squares | df | Mean Square | F | Sig. | |
| Between Groups | 11,565,655,644.36 | 13 | 889,665,818.797 | 112.556 | 0.000 |
| Within Groups | 1,217,252,533.27 | 154 | 7,904,237.229 | ||
| Total | 12,782,908,177.63 | 167 | |||
| Correlations | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urease | Invertase | Catalase | Phosphatase | Al | Ba | Ca | Co | Cr | Cu | Fe | K | Mg | Mn | Na | Ni | Sr | Zn | |
| Urease | 1 | |||||||||||||||||
| Invertase | −0.013 | 1 | ||||||||||||||||
| Catalase | 0.202 | −0.516 | 1 | |||||||||||||||
| Phosphatase | 0.448 | −0.230 | 0.754 ** | 1 | ||||||||||||||
| Al | −0.052 | −0.067 | −0.318 | −0.315 | 1 | |||||||||||||
| Ba | −0.343 | −0.153 | 0.016 | −0.282 | 0.480 | 1 | ||||||||||||
| Ca | −0.265 | −0.323 | 0.136 | −0.218 | −0.003 | 0.622 * | 1 | |||||||||||
| Co | −0.139 | −0.286 | −0.140 | −0.424 | 0.045 | 0.399 | 0.610 * | 1 | ||||||||||
| Cr | 0.044 | −0.333 | −0.369 | −0.443 | 0.054 | −0.075 | 0.448 | 0.707 * | 1 | |||||||||
| Cu | −0.117 | −0.483 | 0.232 | −0.071 | 0.205 | 0.675 * | 0.921 ** | 0.478 | 0.369 | 1 | ||||||||
| Fe | −0.136 | −0.212 | −0.379 | −0.572 | 0.753 ** | 0.621 * | 0.482 | 0.659 * | 0.550 | 0.552 | 1 | |||||||
| K | −0.162 | −0.108 | −0.059 | −0.216 | 0.818 ** | 0.786 ** | 0.390 | 0.018 | −0.121 | 0.576 * | 0.659 * | 1 | ||||||
| Mg | −0.162 | −0.251 | −0.279 | −0.475 | 0.684 * | 0.654 * | 0.639* | 0.615 * | 0.546 | 0.731 ** | 0.957 ** | 0.709 ** | 1 | |||||
| Mn | −0.303 | −0.218 | 0.006 | −0.376 | 0.219 | 0.875 ** | 0.745 ** | 0.761 ** | 0.242 | 0.694 * | 0.653 * | 0.473 | 0.670 * | 1 | ||||
| Na | −0.208 | −0.208 | −0.033 | −0.343 | −0.068 | 0.385 | 0.806 ** | 0.893 ** | 0.664 * | 0.665 * | 0.571 | 0.073 | 0.642 * | 0.717 ** | 1 | |||
| Ni | 0.132 | −0.243 | −0.406 | −0.485 | 0.171 | 0.062 | 0.387 | 0.831 ** | 0.931 ** | 0.324 | 0.682 * | −0.047 | 0.626 * | 0.402 | 0.709 ** | 1 | ||
| Sr | −0.366 | −0.202 | 0.175 | −0.048 | −0.012 | 0.702 * | 0.854 ** | 0.230 | 0.038 | 0.853 ** | 0.245 | 0.506 | 0.453 | 0.631 * | 0.481 | −0.041 | 1 | |
| Zn | −0.224 | −0.351 | 0.081 | −0.302 | 0.117 | 0.648 * | 0.986 ** | 0.645 * | 0.510 | 0.928 ** | 0.592 * | 0.460 | 0.724 ** | 0.765 ** | 0.804 ** | 0.466 | 0.796 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maphuhla, N.G.; Lewu, F.B.; Oyedeji, O.O. Enzyme Activities in Reduction of Heavy Metal Pollution from Alice Landfill Site in Eastern Cape, South Africa. Int. J. Environ. Res. Public Health 2022, 19, 12054. https://doi.org/10.3390/ijerph191912054
Maphuhla NG, Lewu FB, Oyedeji OO. Enzyme Activities in Reduction of Heavy Metal Pollution from Alice Landfill Site in Eastern Cape, South Africa. International Journal of Environmental Research and Public Health. 2022; 19(19):12054. https://doi.org/10.3390/ijerph191912054
Chicago/Turabian StyleMaphuhla, Nontobeko Gloria, Francis Bayo Lewu, and Opeoluwa Oyehan Oyedeji. 2022. "Enzyme Activities in Reduction of Heavy Metal Pollution from Alice Landfill Site in Eastern Cape, South Africa" International Journal of Environmental Research and Public Health 19, no. 19: 12054. https://doi.org/10.3390/ijerph191912054
APA StyleMaphuhla, N. G., Lewu, F. B., & Oyedeji, O. O. (2022). Enzyme Activities in Reduction of Heavy Metal Pollution from Alice Landfill Site in Eastern Cape, South Africa. International Journal of Environmental Research and Public Health, 19(19), 12054. https://doi.org/10.3390/ijerph191912054







