Verbal Instruction for Pelvic Floor Muscle Contraction among Healthy Young Males
Abstract
:1. Introduction
2. Methods
2.1. Participants and Setting
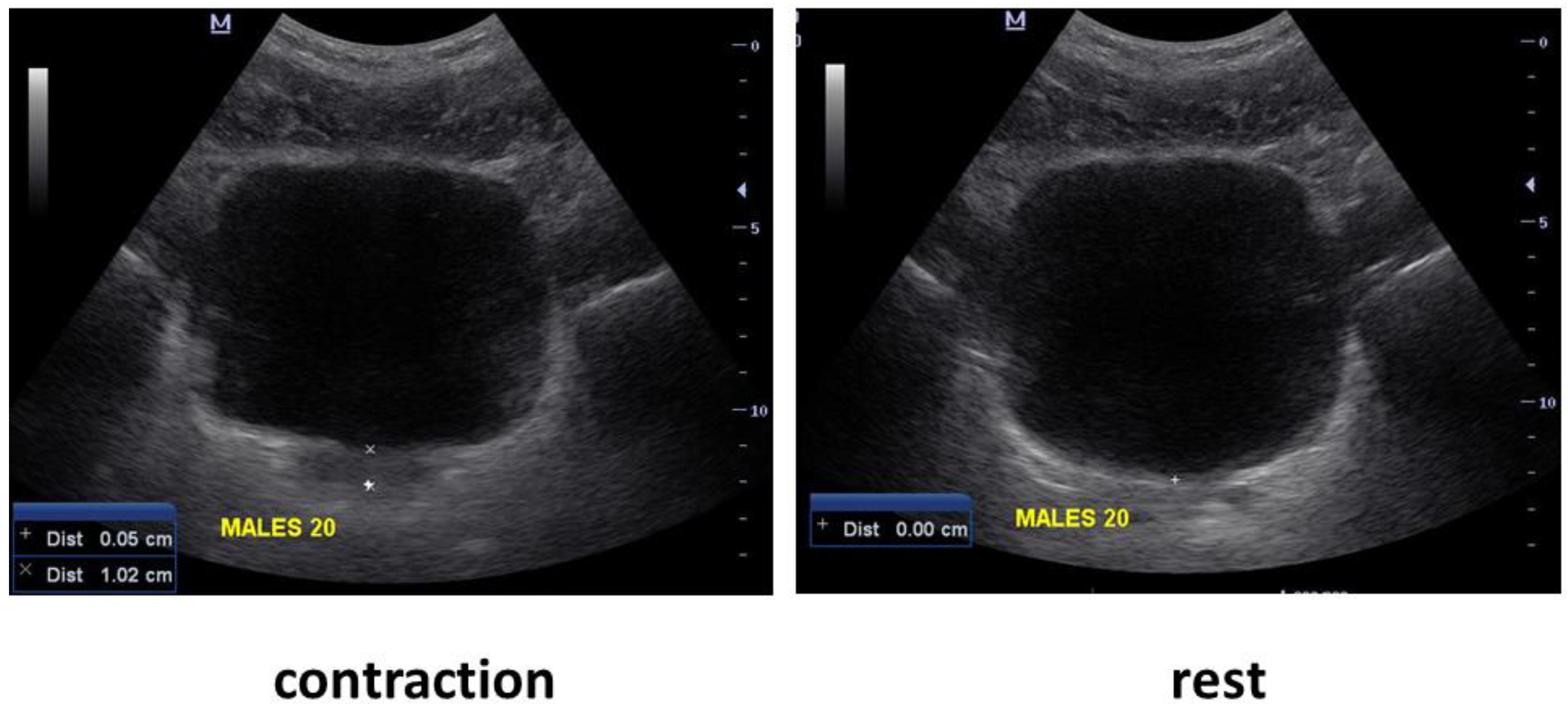
2.2. Protocol
- Squeeze your pelvic floor muscles (“general instruction”)
- Squeeze your anus
- Shorten the penis
- Elevate the scrotum
- Stop the flow of urine
- Take a moderate breath in, let the breath out, lift your pelvic floor muscles, and draw in your umbilicus. (“draw in”)
2.3. Statistical Analysis
3. Results
3.1. The Direction of Bladder Base Displacement
3.2. Bladder Base Displacement
3.3. Pelvic Floor Muscle Endurance
3.4. Urinary Incontinence
3.5. Participants Preferred Verbal Instructions
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, A.L. Pelvic Floor Muscle Training in Males: Practical Applications. Urology 2014, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Gonzalez, J.; Goldstein, I. The Role of Pelvic Floor Muscles in Male Sexual Dysfunction and Pelvic Pain. Sex. Med. Rev. 2016, 4, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska-Labon, E.; Bakuła, S.; Kucharzewski, M.; Śliwiński, Z. Efficacy of physiotherapy for urinary incontinence following prostate cancer surgery. Biomed Res. Int. 2014, 2014, 785263. [Google Scholar] [CrossRef] [PubMed]
- Dorey, G.; Speakman, M.J.; Feneley, R.C.L.; Swinkels, A.; Dunn, C.D.R. Pelvic floor exercises for erectile dysfunction. BJU Int. 2005, 96, 595–597. [Google Scholar] [CrossRef]
- Dorey|PDF|Urinary Incontinence|Pelvis. Available online: https://www.scribd.com/document/352273177/Dorey (accessed on 1 November 2021).
- Centemero, A.; Rigatti, L.; Giraudo, D.; Lazzeri, M.; Lughezzani, G.; Zugna, D.; Montorsi, F.; Rigatti, P.; Guazzoni, G. Preoperative pelvic floor muscle exercise for early continence after radical prostatectomy: A randomised controlled study. Eur. Urol. 2010, 57, 1039–1044. [Google Scholar] [CrossRef]
- Goonewardene, S.S.; Gillatt, D.; Persad, R. A systematic review of PFE pre-prostatectomy. J. Robot. Surg. 2018, 12, 397–400. [Google Scholar] [CrossRef]
- Overview|Lower Urinary Tract Symptoms in Men: Management|Guidance|NICE. 2010. Available online: https://www.nice.org.uk/guidance/cg97 (accessed on 1 November 2021).
- Sueppel, C.; Kreder, K.; See, W. Improved continence outcomes with preoperative pelvic floor muscle strengthening exercises—PubMed. Urol. Nurs. 2001, 21, 201–210. [Google Scholar]
- Anderson, C.A.; Omar, M.I.; Campbell, S.E.; Hunter, K.F.; Cody, J.D.; Glazener, C.M.A. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst. Rev. 2015, 2015, 17. [Google Scholar] [CrossRef]
- Nahon, I.; Waddington, G.; Adams, R.; Dorey, G. Assessing muscle function of the male pelvic floor using real time ultrasound. Neurourol. Urodyn. 2011, 30, 1329–1332. [Google Scholar] [CrossRef]
- Wyndaele, J.J.; Van Eetvelde, B. Reproducibility of digital testing of the pelvic floor muscles in men. Arch. Phys. Med. Rehabil. 1996, 77, 1179–1181. [Google Scholar] [CrossRef]
- Stafford, R.E.; Ashton-Miller, J.A.; Constantinou, C.E.; Hodges, P.W. Novel insight into the dynamics of male pelvic floor contractions through transperineal ultrasound imaging. J. Urol. 2012, 188, 1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, R.E.; Ashton-Miller, J.A.; Constantinou, C.E.; Hodges, P.W. A new method to quantify male pelvic floor displacement from 2D transperineal ultrasound images. Urology 2013, 81, 685. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.E.; Coughlin, G.; Lutton, N.J.; Hodges, P.W. Validity of estimation of pelvic floor muscle activity from transperineal ultrasound imaging in men. PLoS ONE 2015, 10, e0144342. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, B.; Arab, A.M.; Gilani, M.A.S.; Samadi, V.; Assadi, H. Transabdominal Ultrasound Measurement of Pelvic Floor Muscle Mobility in Men With and Without Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Urology 2012, 80, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.E.; Ashton-Miller, J.A.; Constantinou, C.; Coughlin, G.; Lutton, N.J.; Hodges, P.W. Pattern of activation of pelvic floor muscles in men differs with verbal instructions. Neurourol. Urodyn. 2016, 35, 457–463. [Google Scholar] [CrossRef]
- Milios, J.; Atkinson, C.L.; Naylor, L.; Millar, D.; Thijssen, D.; Ackland, T.; Green, D. Pelvic floor muscle assessment in men post prostatectomy: Comparing digital rectal examination and real-time ultrasound approaches. Aust. N. Zeal. Cont. J. 2018, 24, 105. [Google Scholar]
- Thompson, J.A.; O’Sullivan, P.B.; Briffa, K.; Neumann, P.; Court, S. Assessment of pelvic floor movement using transabdominal and transperineal ultrasound. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2005, 16, 285–292. [Google Scholar] [CrossRef]
- Crotty, K.; Bartram, C.I.; Pitkin, J.; Cairns, M.C.; Taylor, P.C.; Dorey, G.; Chatoor, D. Investigation of optimal cues to instruction for pelvic floor muscle contraction: A pilot study using 2D ultrasound imaging in pre-menopausal, nulliparous, continent women. Neurourol. Urodyn. 2011, 30, 1620–1626. [Google Scholar] [CrossRef]
- Kandadai, P.; O’Dell, K.; Saini, J. Correct performance of pelvic muscle exercises in women reporting prior knowledge. Female Pelvic Med. Reconstr. Surg. 2015, 21, 135–140. [Google Scholar] [CrossRef]
- Ami, N.B.; Dar, G. What is the most effective verbal instruction for correctly contracting the pelvic floor muscles? Neurourol. Urodyn. 2018, 37, 2904–2910. [Google Scholar] [CrossRef]
- Hajebrahimi, S.; Corcos, J.; Lemieux, M.C. International consultation on incontinence questionnaire short form: Comparison of physician versus patient completion and immediate and delayed self-administration. Urology 2004, 63, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Bø, K.; Sherburn, M.; Allen, T. Transabdominal ultrasound measurement of pelvic floor muscle activity when activated directly or via a transversus abdominis muscle contraction. Neurourol. Urodyn. 2003, 22, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; O’Sullivan, P.B.; Briffa, N.K.; Neumann, P. Differences in muscle activation patterns during pelvic floor muscle contraction and Valsalva maneuver. Neurourol. Urodyn. 2006, 25, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef]
- Sapsford, R.R.; Hodges, P.W. Contraction of the pelvic floor muscles during abdominal maneuvers. Arch. Phys. Med. Rehabil. 2001, 82, 1081–1088. [Google Scholar] [CrossRef]
- Sapsford, R.R.; Hodges, P.W.; Richardson, C.A.; Cooper, D.H.; Markwell, S.J.; Jull, G.A. Co-activation of the abdominal and pelvic floor muscles during voluntary exercises. Neurourol. Urodyn. 2001, 20, 31–42. [Google Scholar] [CrossRef]
- Neumann, P.; Gill, V. Pelvic floor and abdominal muscle interaction: EMG activity and intra-abdominal pressure. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2002, 13, 125–132. [Google Scholar] [CrossRef]
- Kelly, M.; Tan, B.K.; Thompson, J.; Carroll, S.; Follington, M.; Arndt, A.; Seet, M. Healthy adults can more easily elevate the pelvic floor in standing than in crook-lying: An experimental study. Aust. J. Physiother. 2007, 53, 187–191. [Google Scholar] [CrossRef] [Green Version]
| Variable * | Participants N = 35 |
|---|---|
| Age (years) | 25.97 ± 1.9 |
| Married, n (%) | 12 (34.2%) |
| Body mass index (BMI) † | 24.6 ± 3 |
| Smoke, n (%) | 1 (2.9%) |
| Participate in regular physical activity, n (%) | 32 (91.4%) |
| Perform pelvic floor muscle training regularly, n (%) | 1 (2.9%) |
| Reporting urinary leakage about once a week or less often, n (%) | 1 (2.9%) |
| Variable | Instruction 1: Squeeze the Pelvic Floor | Instruction 2: Squeeze Your Anus | Instruction 3: Shorten the Penis | Instruction 4: Elevate the Scrotum | Instruction 5: Stop the Flow of Urine | Instruction 6: Draw in * | p-Value ** (Effect Size) |
|---|---|---|---|---|---|---|---|
| Number of participants with cranial bladder base displacement N (%) | 24 (69%) | 33 (94.3%) | 33 (94.3%) | 32 (91.4%) | 31 (88.6%) | 9 (25.7%) | <0.0001 (0.20) |
| Cranial bladder base displacement (cm), mean ± SD | 0.91 ± 0.3 (N = 24) | 0.91 ± 0.4 (N = 33) | 0.79 ± 0.3 (N = 33) | 0.87 ± 0.3 (N = 32) | 0.90 ± 0.3 (N = 31) | 0.82 ± 0.4 (N = 9) | 0.015 (0.06) |
| Endurance (seconds) mean ± SD (N = number of participants who succeeded in holding the contraction | 14.43 ± 6.0 (N = 23) | 14.48 ± 6.7 (N = 31) | 14.24 ± 6.7 (N = 33) | 14.91 ± 6.1 (N = 32) | 15.57 ± 6.7 (N = 30) | 8.33 ± 8.4 (N = 7) | <0.0001 (0.14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ami, N.; Feldman, R.; Dar, G. Verbal Instruction for Pelvic Floor Muscle Contraction among Healthy Young Males. Int. J. Environ. Res. Public Health 2022, 19, 12031. https://doi.org/10.3390/ijerph191912031
Ben Ami N, Feldman R, Dar G. Verbal Instruction for Pelvic Floor Muscle Contraction among Healthy Young Males. International Journal of Environmental Research and Public Health. 2022; 19(19):12031. https://doi.org/10.3390/ijerph191912031
Chicago/Turabian StyleBen Ami, Noa, Ron Feldman, and Gali Dar. 2022. "Verbal Instruction for Pelvic Floor Muscle Contraction among Healthy Young Males" International Journal of Environmental Research and Public Health 19, no. 19: 12031. https://doi.org/10.3390/ijerph191912031
APA StyleBen Ami, N., Feldman, R., & Dar, G. (2022). Verbal Instruction for Pelvic Floor Muscle Contraction among Healthy Young Males. International Journal of Environmental Research and Public Health, 19(19), 12031. https://doi.org/10.3390/ijerph191912031







