Combined Inhibitory Effect of Canada Goldenrod Invasion and Soil Microplastics on Rice Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Pot Experiment Design and Plant Sampling
2.3. Plant Growth and Biomass Parameters
2.4. Chlorophyll Contents and Gaseous Exchange Parameters
2.5. Antioxidants Enzyme Activities
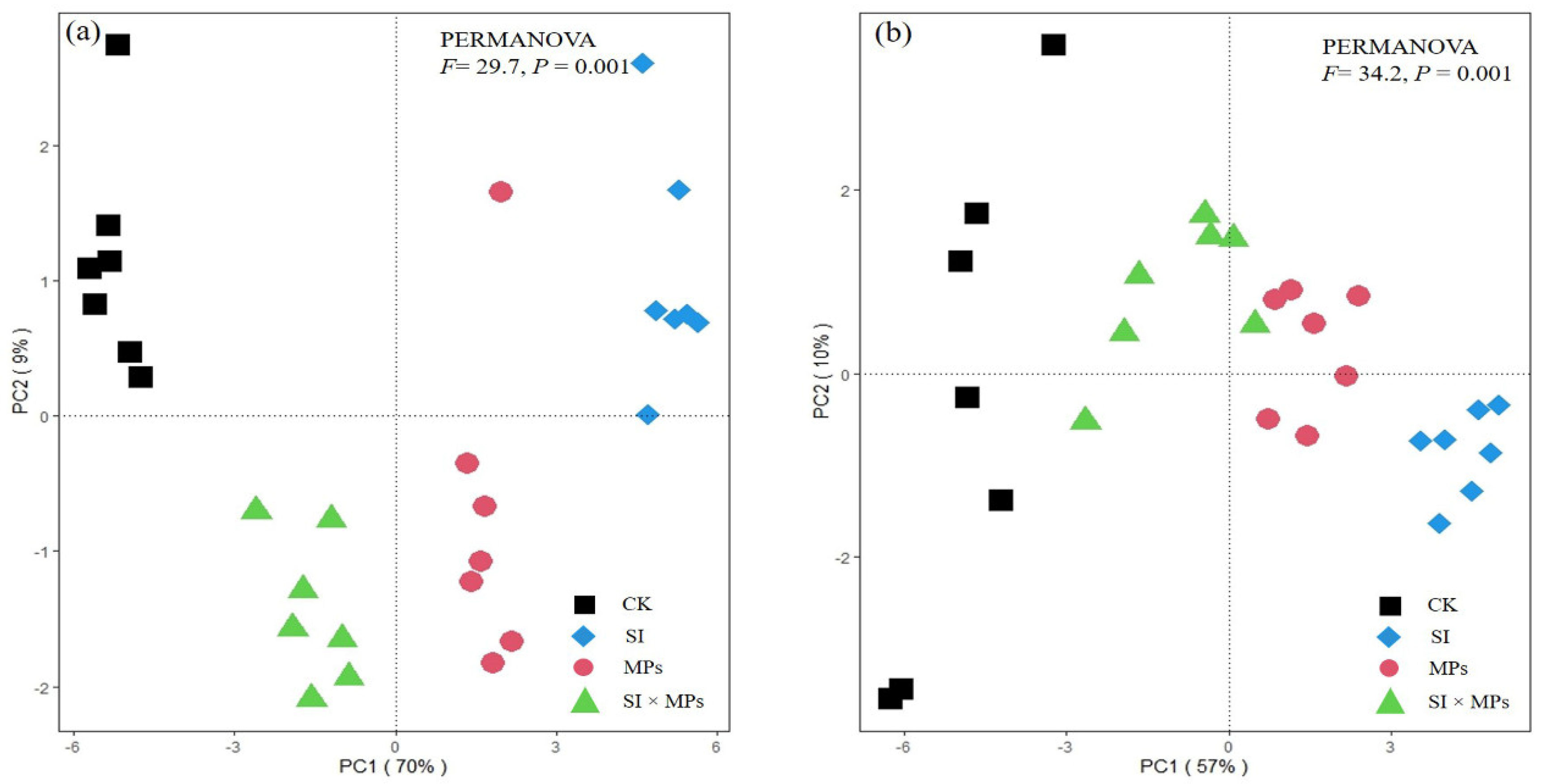
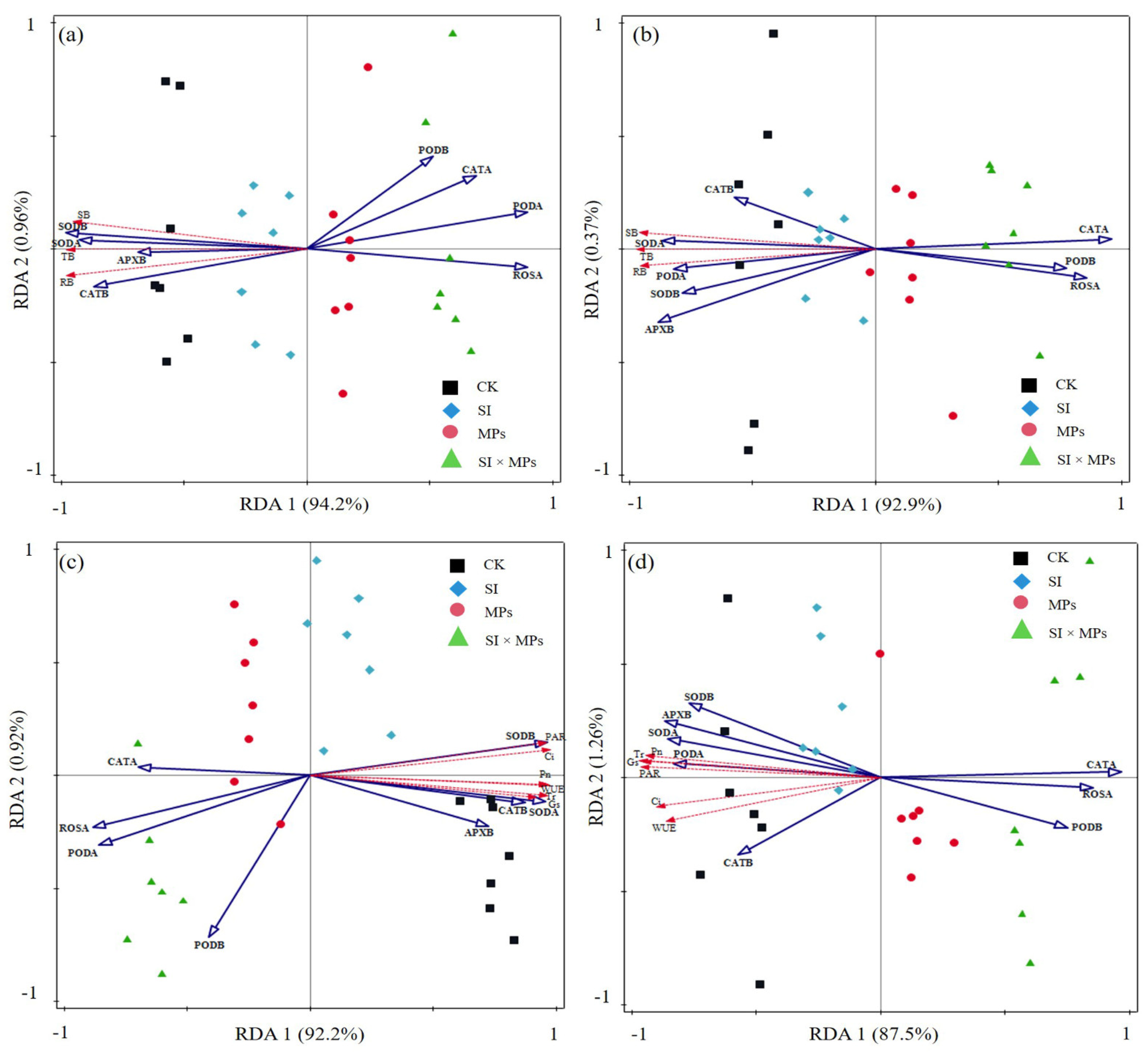
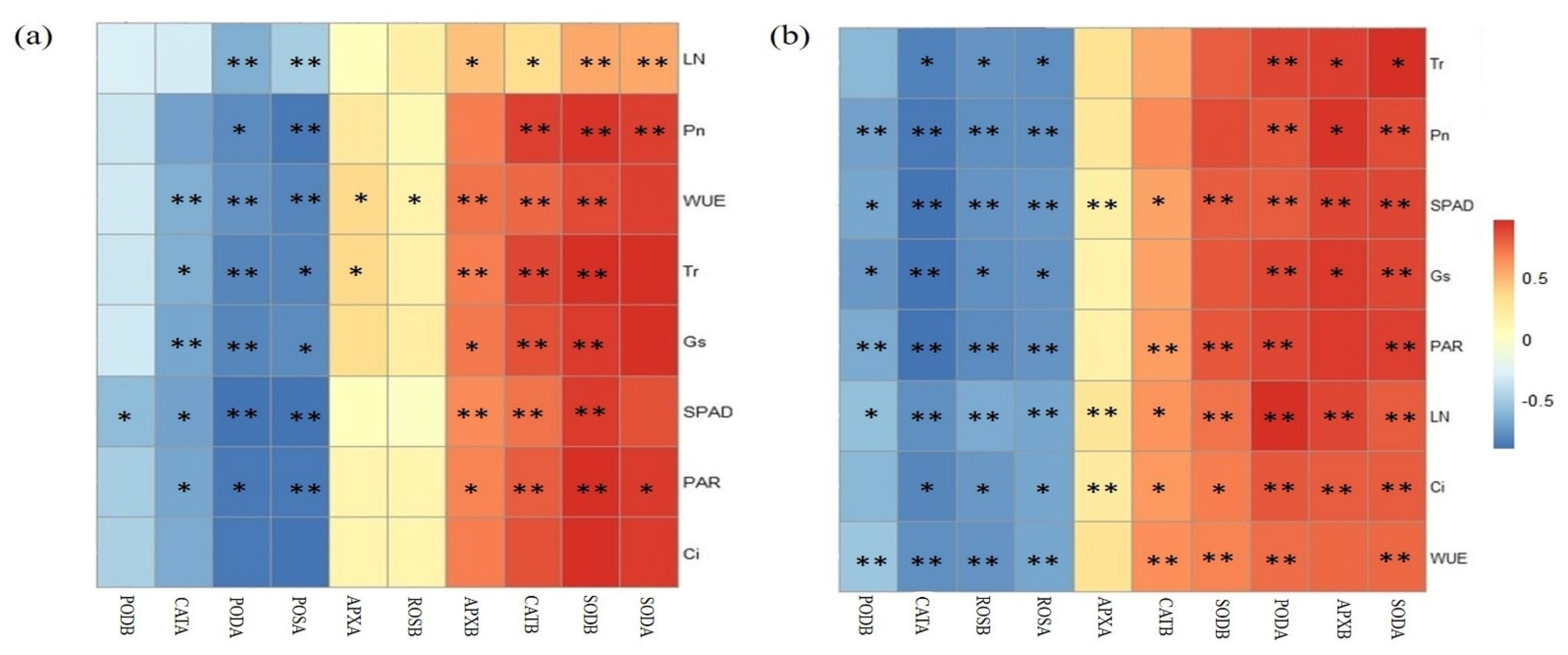
2.6. Statistical Analysis
3. Results
3.1. The Response of Rice Biomass and Phenological Indices to Treatments
3.2. The Response of Photosynthetic Parameters to Treatments
3.3. The Responses of Rice Antioxidants Enzymes Activities to Treatments
4. Discussion
4.1. Effects of S. canadensis Invasion and Residual Soil MPs on Rice Biomass and Phenology
4.2. Effects of S. canadensis Invasion and Residual Soil MPs on Photosynthesis
4.3. Effects of S. canadensis Invasion and Residual Soil MPs on the Antioxidant Enzyme Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 103, 689–710. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science 2013, 339, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Si, C.C.; Liu, X.Y.; Wang, C.Y.; Wang, L.; Dai, Z.C.; Qi, S.S.; Du, D.L. Different degrees of plant invasion significantly affect the richness of the soil fungal community. PLoS ONE 2013, 8, e85490. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.G.; Wang, B.; Zhang, S.S.; Tang, J.J.; Tu, C.; Hu, S.J.; Yong, J.W.H.; Chen, X. Enhanced allelopathy and competitive ability of invasive plant Solidago canadensis in its introduced range. J. Plant Ecol. 2013, 6, 253–263. [Google Scholar] [CrossRef]
- Pratt, C.F.; Constantine, K.L.; Murphy, S.T. Economic impacts of invasive alien species on African smallholder livelihoods. Glob. Food Secur. 2017, 14, 31–37. [Google Scholar] [CrossRef]
- Fried, G.; Chauvel, B.; Reynaud, P.; Sache, I. Decreases in crop production by non-native weeds, pests, and pathogens. In Impact of Biological Invasions on Ecosystem Services; Vilà, M., Hulme, P., Eds.; Invading Nature—Springer Series in Invasion Ecology; Springer: Cham, Switzerland, 2017; Volume 12. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Farooq, M.; Nawaz, A.; Yadav, L.; Chauhan, B.S.; Adkins, S. Impact of invasive plant species on the livelihoods of farming households: Evidence from Parthenium hysterophorus invasion in rural Punjab, Pakistan. Biol. Invasions 2019, 21, 3285–3304. [Google Scholar] [CrossRef]
- Hrideek, T.K.; Amruth, S.M. Invasive Alien Plants: A potential source of unique metabolites. In Plant Metabolites: Methods, Applications and Prospects; Sukumaran, S.T., Sugathan, S., Abdulhameed, S., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Wu, B.; Wang, S.; Wei, M.; Du, D.; Wang, C. Allelopathy of three compositae invasive alien species on indigenous Lactuca sativa L. enhanced under Cu and Pb pollution. Sci. Hortic. 2020, 267, 10932. [Google Scholar] [CrossRef]
- Dong, L.J.; Yu, H.W.; He, W.M. What determines positive, neutral, and negative impacts of Solidago canadensis invasion on native plant species richness? Sci. Rep. 2015, 5, 16804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.Z.; Weng, E.S.; Wu, X.W.; Weber, E.; Zhao, B.; Li, B. Potential distribution of Solidago canadensis in China. Acta Phytotaxon. Sin. 2007, 45, 670–674. [Google Scholar] [CrossRef]
- Dong, L.J.; Sun, Z.K.; Gao, Y.; He, W.M. Two-year interactions between invasive Solidago canadensis and soil decrease its subsequent growth and competitive ability. J. Plant Ecol. 2015, 8, 617–622. [Google Scholar] [CrossRef]
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423, 12723. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of microplastics in soil ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Plastic and plants. Nat. Sustain. 2020, 3, 887–888. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Qi, Y.L.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum L.) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.S.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Khalid, N.; Aqueel, M.; Noman, A. Microplastics could be a threat to plants in terrestrial systems directly or indirectly. Environ. Pollut. 2020, 267, 115653. [Google Scholar] [CrossRef]
- Brown, R.W.; Chadwick, D.R.; Thornton, H.; Marshall, M.R.; Bei, S.; Distaso, M.A.; Bargiela, R.; Marsden, K.A.; Clode, P.L.; Murphy, S.V.; et al. Field application of pure polyethylene microplastic has no significant short-term effect on soil biological quality and function. Soil Biol. Biochem. 2021, 165, 108496. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Liu, S.; Junaid, M.; Wang, J. Effects of micro(nano)plastics on higher plants and the rhizosphere environment. Sci. Total Environ. 2022, 807, 150841. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.L.; Lwanga, E.H.; Eldridge, S.M.; Johnston, P.; Hu, H.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Zhou, J.; Marshall, M.; Chadwick, D.; Wen, Y.; Jones, D. Microplastics in the agroecosystem: Are they an emerging threat to the plant-soil system? Soil Biol. Biochem. 2020, 148, 107926. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Li, B.; Huang, S.; Wang, H.; Liu, M.; Xue, S.; Tang, D.; Cheng, W.; Fan, T.; Yang, X. Effects of plastic particles on germination and growth of soybean (Glycine max): A pot experiment under field condition. Environ. Pollut. 2021, 272, 116418. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Guo, J.; Dong, Y.; Wang, Z.; Gong, L.; Li, X. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J. Hazard. Mater. 2021, 415, 125614. [Google Scholar] [CrossRef]
- Wu, X.; Hou, H.; Liu, Y.; Yin, S.; Bian, S.; Liang, S.; Wan, C.; Yuan, S.; Xiao, K.; Liu, B.; et al. Microplastics affect rice (Oryza sativa L.) quality by interfering metabolite accumulation and energy expenditure pathways: A field study. J. Hazard. Mater. 2022, 422, 126834. [Google Scholar] [CrossRef]
- Dong, Y.M.; Gao, M.L.; Song, Z.G.; Qiu, W.W. Microplastic particles increase arsenic toxicity to rice seedlings. Environ. Pollut. 2020, 259, 113892. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and translocation of nano/microplastics by rice seedlings: Evidence from a hydroponic experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef]
- Queval, G.; Foyer, C.H. Redox regulation of photosynthetic gene expression. Philos. Trans. R. Soc. B 2012, 367, 3475–3485. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Liu, C.Q.; Li, P.P.; Wang, J.Z.; Xing, D.; Wang, B.L. Photosynthetic characteristics involved in adaptability to Karst soil and alien invasion of paper mulberry (Broussonetia papyrifera (L.) Vent.) in comparison with mulberry (Morus alba L.). Photosynthetica 2009, 47, 155–160. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef] [PubMed]
- Pignattelli, S.; Broccoli, A.; Piccardo, M.; Felline, S.; Terlizzi, A.; Renzi, M. Short-term physiological and biometrical responses of Lepidium sativum seedlings exposed to PET-made microplastics and acid rain. Ecotoxicol. Environ. Saf. 2021, 208, 111718. [Google Scholar] [CrossRef] [PubMed]
- Pignattelli, S.; Broccoli, A.; Piccardo, M.; Terlizzi, A.; Renzi, M. Effects of polyethylene terephthalate (PET) microplastics and acid rain on physiology and growth of Lepidium sativum. Environ. Pollut. 2021, 282, 116997. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, Y.; Song, Z. Effects of polyethylene microplastic on the phytotoxicity of di-n-butyl phthalate in lettuce (Lactuca sativa L. var. ramosa Hort). Chemosphere 2019, 237, 124482. [Google Scholar] [CrossRef]
- Li, Z.; Li, R.; Li, Q.; Zhou, J.; Wang, G. Physiological response of cucumber (Cucumis sativus L.) leaves to polystyrene nanoplastics pollution. Chemosphere 2020, 255, 127041. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Luo, Z.; Lai, J.; Li, C.; Luo, X. Effects of polystyrene nanoplastics (PSNPs) on the physiology and molecular metabolism of corn (Zea mays L.) seedlings. Sci. Total Environ. 2022, 806, 150895. [Google Scholar] [CrossRef]
- Banta, J.A.; Stark, S.C.; Mhh, S.; Thi, P.; Baumert, A.; Carson, W.P. Light reduction predicts widespread patterns of dominance between asters and goldenrods. Plant Ecol. 2008, 199, 65–76. [Google Scholar] [CrossRef]
- Yang, R.Y.; Yu, G.D.; Tang, J.J.; Chen, X. Effects of metal lead on growth and mycorrhizae of an invasive plant species (Solidago canadensis L.). J. Environ. Sci. China 2008, 20, 739–744. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Sun, S.G.; Dai, C.; Gituru, R.W.; Chen, J.M.; Wang, Q.F. Genetic variation and structure in native and invasive Solidago canadensis populations. Weed Res. 2015, 55, 163–172. [Google Scholar] [CrossRef]
- Liu, M.T.; Lu, S.B.; Song, Y.; Lei, L.L.; Hu, J.N.; Lv, W.W.; Zhou, W.Z.; Cao, C.J.; Shi, H.H.; Yang, X.F. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Lozano, Y.M.; Rillig, M.C. Microplastics increase soil ph and decrease microbial activities as a function of microplastic shape, polymer type, and exposure time. Front. Environ. Sci. 2021, 9, 675803. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.D.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Veljovic-Jovanovic, S.; Noctor, G.; Foyer, C.H. Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol. Biochem. 2002, 40, 501–507. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, 4.1.1. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 4 September 2022).
- Zhang, Q.; Yang, R.; Tang, J.; Chen, X. Competitive interaction between the invasive Solidago canadensis and native Kummerowia striata in lead contaminated soil. Bot. Stud. 2008, 49, 385–391. [Google Scholar]
- Yan, X.; Wang, H.; Wang, Q.; Rudstam, L.G. Risk spreading, habitat selection and division of biomass in a submerged clonal plant: Responses to heterogeneous copper pollution. Environ. Pollut. 2013, 174, 114–120. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, H.; Zhao, L.; Liu, J.; Wang, L.; Zhang, F.; Shi, Y.; Du, D. The allelopathic effects of invasive plant Solidago canadensis on seed germination and growth of Lactuca sativa enhanced by different types of acid deposition. Ecotoxicology 2016, 25, 555–562. [Google Scholar] [CrossRef]
- Zhang, C.B.; Wang, J.; Qian, B.Y.; Li, W.H. Effects of the invader Solidago canadensis on soil properties. Appl. Soil Ecol. 2009, 43, 163–169. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobucar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Kong, F.; Ullah, I.; Ali, S.; Li, H.; Wang, J.; Khattak, W.A.; Zhou, Z. Phosphorus application improves the cotton yield by enhancing reproductive organ biomass and nutrient accumulation in two cotton cultivars with different phosphorus sensitivity. Agronomy 2020, 10, 153. [Google Scholar] [CrossRef]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological responses of garden cress (L. sativum) to different types of microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef] [PubMed]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef] [PubMed]
- Zubek, S.; Majewska, M.L.; Kapusta, P.; Stefanowicz, A.M.; Błaszkowski, J.; Rożek, K.; Stanek, M.; Karpowicz, F.; Gałosz, J.Z. Solidago canadensis invasion in abandoned arable fields induces minor changes in soil properties and does not affect the performance of subsequent crops. Land Degrad. Dev. 2019, 31, 334–345. [Google Scholar] [CrossRef]
- Zeb, A.; Liu, W.; Meng, L.; Lian, J.; Wang, Q.; Lian, Y.; Chen, C.; Wu, J. Effects of polyester microfibers (PMFs) and cadmium on lettuce (Lactuca sativa) and the rhizospheric microbial communities: A study involving physio-biochemical properties and metabolomic profiles. J. Hazard. Mater. 2022, 424, 127405. [Google Scholar] [CrossRef]
- Wu, J.; Peng, S.; Zhao, H.; Xiao, H. Allelopathic effects of Wedelia trilobata residues on lettuce germination and seedling growth. Allelopath. J. 2008, 22, 197–204. [Google Scholar]
- Meng, F.; Yang, X.; Riksen, M.; Xu, M.; Geissen, V. Response of common bean (Phaseolus vulgaris L.) growth to soil contaminated with microplastics. Sci. Total Environ. 2021, 755, 142516. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and bark extracts of Solidago canadensis L. Ind. Crops Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Gao, Y.B.; Wang, C.L.; Wu, J.Y.; Zhou, H.S.; Jiang, X.T.; Wu, J.; Zhang, S.L. Low temperature inhibits pollen tube growth by disruption of both tip-localized reactive oxygen species and endocytosis in Pyrus bretschneideri Rehd. Plant Physio. Biochem. 2014, 74, 255–262. [Google Scholar] [CrossRef]
| Treatments | Belowground Biomass | Aboveground Biomass | Total Biomass | |
|---|---|---|---|---|
| (×10−1 g) | (×10−1 g) | (g) | ||
| 50 DAT | CK | 4.59 ± 0.10 a | 5.26 ± 0.20 a | 1.56 ± 0.02 a |
| SI | 3.72 ± 0.61 b | 4.19 ± 0.06 b | 1.23 ± 0.00 b | |
| MPs | 2.62 ± 0.08 c | 2.50 ± 0.12 c | 0.78 ± 0.02 c | |
| SI × MPs | 1.60 ± 0.08 d | 1.40 ± 0.11 d | 0.48 ± 0.01 c | |
| 80 DAT | CK | 8.59 ± 0.07 a | 24.94 ± 1.32 a | 5.05 ± 0.08 a |
| SI | 6.78 ± 0.05 b | 17.45 ± 0.95 b | 3.52 ± 0.08 b | |
| MPs | 5.27 ± 0.04 c | 14.08 ± 0.51 c | 2.78 ± 0.08 bc | |
| SI × MPs | 4.45 ± 0.07 d | 8.16 ± 0.23 d | 1.74 ± 0.04 c | |
| Treatments | Stem Height | Total Height | Diameter | No. of Leaves | No. of Tillers | No. of Nodes | |
|---|---|---|---|---|---|---|---|
| (×10 cm) | (×10 cm) | (mm) | |||||
| 50 DAT | CK | 2.30 ± 0.10 a | 6.10 ± 0.23 a | 4.85 ± 0.34 a | 10.86 ± 0.40 | 2.70 ± 0.30 a | 8.40 ± 1.10 |
| SI | 2.08 ± 0.08 ab | 5.82 ± 0.12 ab | 3.85 ± 0.24 b | 9.29 ± 0.42 | 2.10 ± 0.30 ab | 8.10 ± 0.40 | |
| MPs | 1.92 ± 0.05 bc | 5.49 ± 0.19 b | 3.65 ± 0.13 b | 9.43 ± 0.48 | 1.70 ± 0.20 b | 7.60 ± 0.30 | |
| SI × MPs | 1.70 ± 0.12 c | 4.66 ± 0.17 c | 3.36 ± 0.11 b | 9.86 ± 0.34 | 1.60 ± 0.20 b | 7.10 ± 0.30 | |
| 80 DAT | CK | 3.00 ± 0.06 a | 6.49 ± 0.25 a | 6.33 ± 0.26 a | 14.00 ± 2.00 | 4.00 ± 0.30 a | 10.70 ± 2.00 a |
| SI | 2.54 ± 0.10 b | 5.99 ± 0.26 ab | 4.97 ± 0.13 b | 14.00 ± 1.00 | 3.00 ± 0.20 b | 6.60 ± 0.40 b | |
| MPs | 2.40 ± 0.09 b | 5.57 ± 0.10 b | 4.41 ± 0.12 c | 12.00 ± 1.00 | 2.30 ± 0.30 bc | 5.90 ± 0.60 b | |
| SI × MPs | 1.99 ± 0.05 c | 4.91 ± 0.17 c | 3.84 ± 0.21 d | 12.00 ± 1.00 | 1.90 ± 0.30 c | 4.40 ± 0.50 b | |
| Treatments | Pn | Tr | Gs | PAR | Ci | WUE | SPAD | Leaf N | |
|---|---|---|---|---|---|---|---|---|---|
| (×10 μmol m−2 s−1) | (μmol m−2 s−1) | (×10−1 μmol m−2 s−1) | (×103 μmol m−2 s−1) | (×102 ppm) | (%) | (×10) | |||
| 50 DAT | CK | 2.68 ± 0.11 a | 6.23 ± 0.08 a | 6.54 ± 0.16 a | 1.55 ± 0.02 a | 5.26 ± 0.06 a | 9.96 ± 0.35 a | 3.31 ± 0.08 a | 2.87 ± 0.06 a |
| SI | 2.03 ± 0.05 b | 3.62 ± 0.14 b | 4.05 ± 0.16 b | 1.32 ± 0.02 b | 4.21 ± 0.02 b | 5.91 ± 0.66 b | 2.93 ± 0.03 b | 2.70 ± 0.06 b | |
| MPs | 1.43 ± 0.07 c | 2.62 ± 0.07 c | 2.41 ± 0.17 c | 1.01 ± 0.02 c | 3.18 ± 0.04 c | 4.53 ± 0.06 c | 2.63 ± 0.03 c | 2.68 ± 0.07 c | |
| SI × MPs | 1.10 ± 0.04 d | 1.44 ± 0.13 d | 1.62 ± 0.08 d | 0.80 ± 0.02 d | 2.13 ± 0.09 d | 2.63 ± 0.17 d | 1.89 ± 0.05 d | 2.57 ± 0.08 d | |
| 80 DAT | CK | 2.22 ± 0.04 a | 7.22 ± 0.18 a | 6.52 ± 0.12 a | 1.40 ± 0.06 a | 5.61 ± 0.16 a | 7.80 ± 0.35 a | 3.57 ± 0.06 a | 3.35 ± 0.15 a |
| SI | 1.84 ± 0.01 b | 5.48 ± 0.12 b | 5.47 ± 0.10 b | 1.08 ± 0.03 b | 4.55 ± 0.14 b | 5.77 ± 0.27 b | 3.05 ± 0.06 b | 2.80 ± 0.06 b | |
| MPs | 1.21 ± 0.01 c | 3.93 ± 0.19 c | 3.52 ± 0.11 c | 0.80 ± 0.02 c | 3.33 ± 0.14 c | 4.29 ± 0.30 c | 2.48 ± 0.06 c | 2.45 ± 0.03 c | |
| SI × MPs | 0.81 ± 0.02 d | 2.78 ± 0.05 d | 1.49 ± 0.10 d | 0.31 ± 0.05 d | 2.64 ± 0.11 d | 2.51 ± 0.23 d | 1.63 ± 0.10 d | 2.10 ± 0.05 d | |
| Parts | DAT | Treatments | APX | CAT | POD | SOD | ROS |
|---|---|---|---|---|---|---|---|
| (×102 U min−1 g−1 FW) | (×102 U min−1 g−1 FW) | (×103 U min−1 g−1 FW) | (×102 U g−1 FW) | (×10 U min−1 g−1 FW) | |||
| Belowground | 50 | CK | 1.88± 0.19 a | 5.00 ± 0.41 a | 1.86 ± 0.14 b | 4.67 ± 0.11 a | 0.94 ± 0.04 a |
| SI | 1.24 ± 0.27 b | 3.58 ± 0.12 b | 1.23 ± 0.07 c | 3.65 ± 0.10 b | 0.71 ± 0.07 b | ||
| MPs | 1.11 ± 0.08 bc | 2.72 ± 0.17 c | 1.63 ± 0.21 bc | 2.74 ± 0.11 c | 0.65 ± 0.01 b | ||
| SI × MPs | 0.64 ± 0.10 c | 2.16 ± 0.14 c | 2.67 ± 0.10 a | 1.61 ± 0.11 d | 0.51 ± 0.02 c | ||
| 80 | CK | 2.86 ± 0.25 a | 4.39 ± 0.95 a | 3.26 ± 0.05 c | 2.48 ± 0.20 a | 1.40 ± 0.08 c | |
| SI | 2.27 ± 0.15 b | 3.26 ± 0.20 ab | 2.33 ± 0.33 d | 2.33 ± 0.04 a | 1.78 ± 0.04 b | ||
| MPs | 1.49 ± 0.10 c | 2.51 ± 0.35 b | 4.30 ± 0.13 b | 1.97 ± 0.05 b | 2.08 ± 0.16 b | ||
| SI × MPs | 1.10 ± 0.07 c | 1.83 ± 0.36 b | 5.21 ± 0.18 a | 1.48 ± 0.12 c | 2.70 ± 0.13 a | ||
| Aboveground | 50 | CK | 2.28 ± 0.21 a | 9.05 ± 1.01 c | 1.89 ± 0.08 c | 8.14 ± 0.18 a | 1.25 ± 0.04 b |
| SI | 1.03 ± 0.04 d | 13.70 ± 1.44 bc | 2.16 ± 0.13 c | 5.43 ± 0.26 b | 1.45 ± 0.08 b | ||
| MPs | 1.40 ± 0.13 c | 19.97 ± 3.06 ab | 2.55 ± 0.10 b | 4.30 ± 0.21 c | 2.02 ± 0.10 a | ||
| SI × MPs | 1.85 ± 0.10 b | 26.90 ± 4.44 a | 3.27 ± 0.74 a | 3.59 ± 0.14 d | 2.87 ± 0.16 a | ||
| 80 | CK | 3.95 ± 0.35 a | 2.50 ± 0.10 d | 4.36 ± 0.44 a | 4.29 ± 0.17 a | 1.40 ± 0.10 d | |
| SI | 1.44 ± 0.46 c | 3.44 ± 0.14 c | 3.25 ± 0.23 b | 3.31 ± 0.13 b | 1.84 ± 0.60 c | ||
| MPs | 1.83 ± 0.08 c | 5.32 ± 0.14 b | 2.55 ± 0.11 bc | 2.95 ± 0.27 b | 2.25 ± 0.23 b | ||
| SI × MPs | 3.06 ± 0.17 b | 9.26 ± 0.34 a | 1.96 ± 0.08 c | 2.36 ± 0.07 c | 2.85 ± 0.08 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Xie, H.; Zhao, X.; Zhang, J.; Li, Z.; Yin, W.; Yuan, A.; Zhou, H.; Manan, S.; Nazar, M.; et al. Combined Inhibitory Effect of Canada Goldenrod Invasion and Soil Microplastics on Rice Growth. Int. J. Environ. Res. Public Health 2022, 19, 11947. https://doi.org/10.3390/ijerph191911947
Zhao X, Xie H, Zhao X, Zhang J, Li Z, Yin W, Yuan A, Zhou H, Manan S, Nazar M, et al. Combined Inhibitory Effect of Canada Goldenrod Invasion and Soil Microplastics on Rice Growth. International Journal of Environmental Research and Public Health. 2022; 19(19):11947. https://doi.org/10.3390/ijerph191911947
Chicago/Turabian StyleZhao, Xiaoxun, Hongliang Xie, Xin Zhao, Jiaqi Zhang, Zhiliang Li, Weiqing Yin, Aiguo Yuan, Huan Zhou, Sehrish Manan, Mudasir Nazar, and et al. 2022. "Combined Inhibitory Effect of Canada Goldenrod Invasion and Soil Microplastics on Rice Growth" International Journal of Environmental Research and Public Health 19, no. 19: 11947. https://doi.org/10.3390/ijerph191911947
APA StyleZhao, X., Xie, H., Zhao, X., Zhang, J., Li, Z., Yin, W., Yuan, A., Zhou, H., Manan, S., Nazar, M., Iqbal, B., Li, G., & Du, D. (2022). Combined Inhibitory Effect of Canada Goldenrod Invasion and Soil Microplastics on Rice Growth. International Journal of Environmental Research and Public Health, 19(19), 11947. https://doi.org/10.3390/ijerph191911947










