How Internet Websites Portray Herbal Vitality Products Containing Eurycoma longifolia Jack: An Evaluation of the Quality and Risks of Online Information
Abstract
:1. Introduction
2. Methods
2.1. Website Identification and Selection
2.2. Assessment of the Quality of Websites Containing Information about TA Supplements
2.3. Assessment of the Risks of Websites Containing Information about TA Supplements
2.4. Identification of Health Claims for TA in the Websites
2.5. Statistical Analysis
3. Results
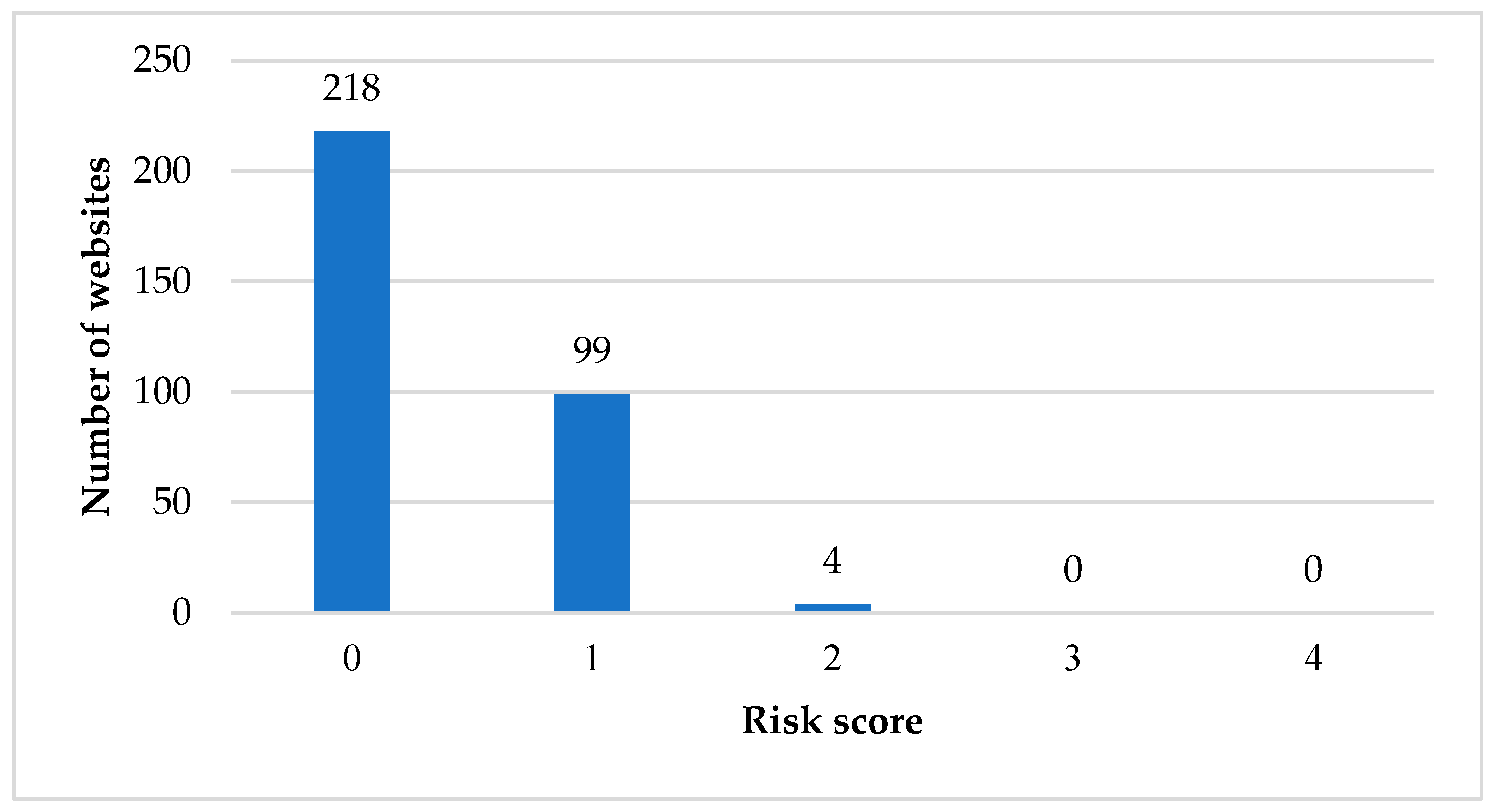
3.1. Risks of Websites Containing Information about TA Supplements
3.2. Health Claims for TA on the Websites
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassim, A.R.; Niiyama, K.; Ripin, A.; Appanah, S.; Iida, S. Species assembly and site preference of tree species in a primary seraya-ridge forest of Peninsular Malaysia. J. Trop. For. Sci. 2002, 14, 287–303. [Google Scholar]
- Remli, R.; Chan, S. Use of complementary medicine amongst diabetic patients in a public primary care clinic in Ipoh. Med. J. Malays. 2003, 58, 688–693. [Google Scholar]
- Ab Rahman, A.A.; Al-Sadat, N.; Yun Low, W. Help seeking behaviour among men with erectile dysfunction in primary care setting. J. Men’s Health 2011, 8, S94–S96. [Google Scholar] [CrossRef]
- Ulbricht, C.; Conquer, J.; Flanagan, K.; Isaac, R.; Rusie, E.; Windsor, R.C. An evidence-based systematic review of tongkat ali (Eurycoma longifolia) by the natural standard research collaboration. J. Diet. Suppl. 2013, 10, 54–83. [Google Scholar] [CrossRef]
- Wernsdorfer, W.H.; Ismail, S.; Chan, K.L.; Congpuong, K.; Wernsdorfer, G. Activity of Eurycoma longifolia root extract against Plasmodium falciparum in vitro. Wien. Klin. Wochenschr. 2009, 121, 23–26. [Google Scholar] [CrossRef]
- Chan, K.; Lee, S.; Sam, T.; Tan, S.; Noguchi, H.; Sankawa, U. 13β, 18-dihydroeurycomanol, a quassinoid from Eurycoma longifolia. Phytochemistry 1991, 30, 3138–3141. [Google Scholar] [CrossRef]
- Farouk, A.-E.; Benafri, A. Antibacterial activity of Eurycoma longifolia Jack. Saudi Med. J. 2007, 28, 1422–1424. [Google Scholar]
- Sriwilaijaroen, N.; Kondo, S.; Nanthasri, P.; Auparakkitanon, S.; Suzuki, Y.; Wilairat, P. Antiplasmodial effects of Brucea javanica (L.) Merr. and Eurycoma longifolia Jack extracts and their combination with chloroquine and quinine on Plasmodium falciparum in culture. Trop. Med. Health 2010, 38, 61–68. [Google Scholar] [CrossRef]
- Ang, H.H.; Cheang, H.S. Studies on the anxiolytic activity of Eurycoma longifolia Jack roots in mice. Jpn. J. Pharmacol. 1999, 79, 497–500. [Google Scholar]
- Sobri, H.; Rusli, I.; Kiong, L. A summary of reported chemical constituents and medicinal uses of Eurycoma longifolia. J. Trop. Med. Plants 2007, 8, 103–110. [Google Scholar]
- Toyama, H. Randomized Controlled Trial of the Effects of Tongkat Ali Intake on Stress Markers and Sleep Quality in Healthy Japanese Adults. Jpn. Pharmacol. Ther. 2022, 50, 871–876. [Google Scholar]
- Ismail, S.B.; Wan Mohammad, W.M.Z.; George, A.; Nik Hussain, N.H.; Musthapa Kamal, Z.M.; Liske, E. Randomized clinical trial on the use of PHYSTA freeze-dried water extract of Eurycoma longifolia for the improvement of quality of life and sexual well-being in men. Evid.-Based Complement. Altern. Med. 2012, 2012, 429268. [Google Scholar] [CrossRef] [PubMed]
- Leitão, A.E.; de Souza Vieira, M.C.; Pelegrini, A.; da Silva, E.L.; de Azevedo Guimarães, A.C. A 6-month, double-blind, placebo-controlled, randomized trial to evaluate the effect of Eurycoma longifolia (Tongkat Ali) and concurrent training on erectile function and testosterone levels in androgen deficiency of aging males (ADAM). Maturitas 2021, 145, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Udani, J.K.; George, A.A.; Musthapa, M.; Pakdaman, M.N.; Abas, A. Effects of a proprietary freeze-dried water extract of Eurycoma longifolia (Physta) and Polygonum minus on sexual performance and well-being in men: A randomized, double-Blind, placebo-controlled study. Evid.-Based Complement. Altern. Med. 2014, 2014, 179529. [Google Scholar] [CrossRef]
- Kotirum, S.; Ismail, S.B.; Chaiyakunapruk, N. Efficacy of Tongkat Ali (Eurycoma longifolia) on erectile function improvement: Systematic review and meta-analysis of randomized controlled trials. Complementary Ther. Med. 2015, 23, 693–698. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Thirumavalavan, N.; Srivatsav, A.; Yu, J.; Hotaling, J.M.; Lipshultz, L.I.; Pastuszak, A.W. An analysis of popular online erectile dysfunction supplements. J. Sex. Med. 2019, 16, 843–852. [Google Scholar] [CrossRef]
- Sulaiman, N.A.; Ahmed, A.; Tan, C.L.; Jatau, A.I.; Lua, P.L.; Jofrry, S.M.; Abdullah, A.H.; Wong, T.W. Consumption of herbal products: A study of urban community survey. Australas. Med. J. 2017, 10, 124–131. [Google Scholar] [CrossRef]
- Wahab, M.S.A.; Zaini, M.H.; Ali, A.A.; Sahudin, S.; Mehat, M.Z.; Hamid, H.A.; Mustaffa, M.F.; Othman, N.; Maniam, S. The use of herbal and dietary supplement among community-dwelling elderly in a suburban town of Malaysia. BMC Complement. Med. Ther. 2021, 21, 110. [Google Scholar] [CrossRef]
- Wardah, W.; Setiawan, M. An Ethnobotanical Study of Tongkat Ali (Eurycoma longifolia Jack) on Malay Ethnic Group in Tanjung Balai, Karimun, Riau Islands. J. Trop. Ethnobiol. 2021, 4, 58–65. [Google Scholar] [CrossRef]
- Wahab, M.S.A.; Jalani, M.M.; Goh, K.W.; Ming, L.C.; Faller, E.M. Why Did I Consult My Pharmacist about Herbal and Dietary Supplements? An Online Survey Amid the COVID-19 Pandemic in Malaysia. Int. J. Environ. Res. Public Health 2022, 19, 10994. [Google Scholar] [CrossRef]
- Ting, C.Y.; Abd Wahab, M.S.; Lee, K.S.; Tan, R.T.-H.; Ming, L.C. A cross-sectional study on the use of, preference for, and perceived reliability of mass media for drug-related information among the general public in Sarawak. Ther. Innov. Regul. Sci. 2017, 51, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Thakor, V.; Leach, M.J.; Gillham, D.; Esterman, A. The quality of information on websites selling St. John’s wort. Complement. Ther. Med. 2011, 19, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Baudischova, L.; Straznicka, J.; Pokladnikova, J.; Jahodar, L. The quality of information on the internet relating to top-selling dietary supplements in the Czech Republic. Int. J. Clin. Pharm. 2018, 40, 183–189. [Google Scholar] [CrossRef]
- Charnock, D.; Shepperd, S.; Needham, G.; Gann, R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J. Epidemiol. Community Health 1999, 53, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Ernst, E. Assessing websites on complementary and alternative medicine for cancer. Ann. Oncol. 2004, 15, 733–742. [Google Scholar] [CrossRef]
- Charnock, D.; Shepperd, S. Learning to DISCERN online: Applying an appraisal tool to health websites in a workshop setting. Health Educ. Res. 2004, 19, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Borgmann, H.; Wölm, J.-H.; Vallo, S.; Mager, R.; Huber, J.; Breyer, J.; Salem, J.; Loeb, S.; Haferkamp, A.; Tsaur, I. Prostate Cancer on the Web—Expedient Tool for Patients’ Decision-Making? J. Cancer Educ. 2017, 32, 135–140. [Google Scholar] [CrossRef]
- Nguyen, S.K.A.; Ingledew, P.-A. Tangled in the breast cancer web: An evaluation of the usage of web-based information resources by breast cancer patients. J. Cancer Educ. 2013, 28, 662–668. [Google Scholar] [CrossRef]
- Batchelor, J.M.; Ohya, Y. Use of the DISCERN instrument by patients and health professionals to assess information resources on treatments for asthma and atopic dermatitis. Allergol. Int. 2009, 58, 141–145. [Google Scholar] [CrossRef]
- Azer, S.A. Evaluation of gastroenterology and hepatology articles on Wikipedia: Are they suitable as learning resources for medical students? Eur. J. Gastroenterol. Hepatol. 2014, 26, 155–163. [Google Scholar] [CrossRef]
- Munsour, E.E.; Awaisu, A.; Hassali, M.A.A.; Ali, H.; Dabbous, Z. A comparative evaluation of written medicine information of antidiabetic medicines from Qatar, Australia and Europe. Cogent Med. 2019, 6, 1620904. [Google Scholar] [CrossRef]
- Prusti, M.; Lehtineva, S.; Pohjanoksa-Mäntylä, M.; Bell, J.S. The quality of online antidepressant drug information: An evaluation of English and Finnish language Web sites. Res. Soc. Adm. Pharm. 2012, 8, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Nakhal, S.A.; Domiati, S.A.; Amin, M.E.; El-Lakany, A.M. Assessment of pharmacy students’ knowledge, attitude, and practice toward herbal dietary supplements. J. Am. Coll. Health 2020, 70, 1826–1830. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.; Toone, T.; Steed-Ivie, M. A survey of dietary supplement knowledge, attitudes, and use in a rural population. J. Nutr. Food Sci. 2014, 4, 304. [Google Scholar] [CrossRef]
- Abd Wahab, M.S.; Othman, N.; Othman, N.H.I.; Jamari, A.A.; Ali, A.A. Exploring the use of and perceptions about honey as complementary and alternative medicine among the general public in the state of Selangor, Malaysia. J. Appl. Pharm. Sci. 2017, 7, 144–150. [Google Scholar]
- Azizah, N.; Halimah, E.; Puspitasari, I.M.; Hasanah, A.N. Simultaneous use of herbal medicines and antihypertensive drugs among hypertensive patients in the community: A review. J. Multidiscip. Healthc. 2021, 14, 259. [Google Scholar] [CrossRef]
- Agbabiaka, T.B.; Wider, B.; Watson, L.K.; Goodman, C. Concurrent use of prescription drugs and herbal medicinal products in older adults: A systematic review. Drugs Aging 2017, 34, 891–905. [Google Scholar] [CrossRef] [Green Version]
- Salman, S.; Amrah, S.; Wahab, M.; Ismail, Z.; Ismail, R.; Yuen, K.; Gan, S. Modification of propranolol’s bioavailability by Eurycoma longifolia water-based extract. J. Clin. Pharm. Ther. 2010, 35, 691–696. [Google Scholar] [CrossRef]
- Reddy, K.; Nurdijati, S.; Salleh, B. Efficacy of aqueous medicinal plant extracts on growth and citrinin production by Penicillium citrinum isolated from rice grains. Afr. J. Microbiol. Res. 2010, 4, 2562–2565. [Google Scholar]
- Husen, R.; Pihie, A.H.L.; Nallappan, M. Screening for antihyperglycaemic activity in several local herbs of Malaysia. J. Ethnopharmacol. 2004, 95, 205–208. [Google Scholar] [CrossRef]
- Moncada, I.; Martinez-Salamanca, J.; Ruiz-Castañe, E.; Romero, J. Combination therapy for erectile dysfunction involving a PDE5 inhibitor and alprostadil. Int. J. Impot. Res. 2018, 30, 203–208. [Google Scholar] [CrossRef]
- Abd Wahab, M.S.; Abd Malik, N.A.; Sahudin, S.; Affandi, M.M.R.M.M.; Othman, N.; Ali, A.A. Exploring the factors associated with the intention to assess customers’ herbal and dietary supplement use by community pharmacists in Kuala Lumpur, Malaysia. J. Appl. Pharm. Sci. 2019, 9, 108–116. [Google Scholar]
- Wahab, M.S.A.; Sakthong, P.; Winit-Watjana, W. Qualitative exploration of pharmacist care for herbal and dietary supplement users in Thai community pharmacies. J. Pharm. Health Serv. Res. 2019, 10, 57–66. [Google Scholar] [CrossRef]
- Rapata, M.E.; Meyer, J.J. Evaluation of Online Information on Complementary and Alternative Treatments for Dry Eye Disease. Optom. Vis. Sci. 2021, 98, 355–361. [Google Scholar] [CrossRef]
- Gunasekera, V.; Ernst, E.; Ezra, D.G. Systematic internet-based review of complementary and alternative medicine for glaucoma. Ophthalmology 2008, 115, 435–439.e2. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.Y.; Ahmed, S.; Zhang, C.J. Dietary and herbal supplements for weight loss: Assessing the quality of patient information online. Nutr. J. 2021, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, A.; Bezuglov, E. Testosterone Boosters Intake in Athletes: Current Evidence and Further Directions. Endocrines 2021, 2, 109–120. [Google Scholar] [CrossRef]
- Mar, M.R.; Noor Rain, A.; Zhari, I.; Zakiah, I. Effect of Eurycoma longifolia extract on the Glutathione level in Plasmodium falciparum infected erythrocytes in vitro. Trop. Biomed. 2005, 22, 155–163. [Google Scholar]
- Chan, K.-L.; Choo, C.-Y.; Abdullah, N.R. Semisynthetic 15-O-acyl-and 1, 15-di-O-acyleurycomanones from Eurycoma longifolia as potential antimalarials. Planta Med. 2005, 71, 967–969. [Google Scholar] [CrossRef]
- Mohd Ridzuan, M.; Sow, A.; Noor Rain, A.; Mohd Ilham, A.; Zakiah, I. Eurycoma longifolia extract-artemisinin combination: Parasitemia suppression of Plasmodium yoelii-infected mice. Trop. Biomed. 2007, 24, 111–118. [Google Scholar]
- Olkun, H.K.; Demirkaya, A.A. Evaluation of internet information about lingual orthodontics using DISCERN and JAMA tools. Turk. J. Orthod. 2018, 31, 50. [Google Scholar] [CrossRef] [PubMed]
| Item Number | Modified DISCERN Item | Mean ± SD Score a |
|---|---|---|
| 1 | Are the aims clear? | 1.14 ± 0.60 |
| 2 | Is it relevant? | 2.29 ± 0.85 |
| 3 | Is it clear what sources of information were used to compile the information on the website (other than the author or producer)? | 2.31 ± 1.43 |
| 4 | Is it clear when the information used or detailed on the website was produced? | 2.26 ± 1.20 |
| 5 | Is the information balanced and unbiased? | 2.69 ± 1.24 |
| 6 | Does the website provide details of additional sources of support and information? | 1.15 ± 0.58 |
| 7 | Does the website refer to areas of uncertainty? | 1.05 ± 0.23 |
| 8 | Does the website describe how TA works? | 2.79 ± 1.31 |
| 9 | Does the website describe the benefits of TA? | 2.90 ± 1.26 |
| 10 | Are drug-supplement interactions mentioned? | 1.17 ± 0.63 |
| 11 | Are contraindications of TA mentioned? | 1.22 ± 0.65 |
| 12 | Are potential adverse effects of TA mentioned? | 1.53 ± 1.00 |
| 13 | Is it clear from the website that there may be more than one possible treatment choice? | 1.02 ± 0.16 |
| 14 | Based on the answers to all of the above questions, rate the overall quality of the publication as a source of information about TA. | 1.07 ± 0.51 b |
| Claims of Benefits | n (%) |
|---|---|
| Enhance testosterone level | 121 (37.7) |
| Antimalaria | 112 (34.9) |
| Improve libido | 108 (33.6) |
| Treat infertility | 89 (27.7) |
| Reduce stress | 80 (24.9) |
| Enhance energy | 59 (18.4) |
| Anticancer | 57 (17.8) |
| Increase muscle gain | 56 (17.4) |
| Treat fever | 48 (15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahab, M.S.A.; Abd Hamid, N.N.; Yassen, A.O.; Naim, M.J.; Ahamad, J.; Zulkifli, N.W.; Ismail, F.F.; Zulkifli, M.H.; Goh, K.W.; Ming, L.C. How Internet Websites Portray Herbal Vitality Products Containing Eurycoma longifolia Jack: An Evaluation of the Quality and Risks of Online Information. Int. J. Environ. Res. Public Health 2022, 19, 11853. https://doi.org/10.3390/ijerph191911853
Wahab MSA, Abd Hamid NN, Yassen AO, Naim MJ, Ahamad J, Zulkifli NW, Ismail FF, Zulkifli MH, Goh KW, Ming LC. How Internet Websites Portray Herbal Vitality Products Containing Eurycoma longifolia Jack: An Evaluation of the Quality and Risks of Online Information. International Journal of Environmental Research and Public Health. 2022; 19(19):11853. https://doi.org/10.3390/ijerph191911853
Chicago/Turabian StyleWahab, Mohd Shahezwan Abd, Nurfarah Nadiah Abd Hamid, Ali Omar Yassen, Mohd Javed Naim, Javed Ahamad, Nur Wahida Zulkifli, Farhana Fakhira Ismail, Muhammad Harith Zulkifli, Khang Wen Goh, and Long Chiau Ming. 2022. "How Internet Websites Portray Herbal Vitality Products Containing Eurycoma longifolia Jack: An Evaluation of the Quality and Risks of Online Information" International Journal of Environmental Research and Public Health 19, no. 19: 11853. https://doi.org/10.3390/ijerph191911853








