Abstract
The objective of our study is to determine the thickness of the pubovisceral fasciculus of the levator ani muscle and the area of the genital hiatus by means of three-dimensional perineal ultrasound, in pregnant women in the 2nd trimester, and to analyze the related maternal, perinatal and postpartum clinical variables. Furthermore, to compare the results of our study with two similar series previously published. An observational, prospective study of pelvic floor ultrasound was carried out, performed at week 20, whose delivery was attended in the obstetrics service of the Hospital General Universitario Gregorio Marañón de Madrid (HGUGM), during the period of August from 2021 to June 2022. Maternal, ultrasound, perinatal and postpartum clinical variables were collected from each participant. During the study period, a total of 54 patients were included in it. The mean gestational age at which the ultrasound was performed was 19.81 ± 0.91 weeks. In relation to the ultrasound variables, the mean thickness of the pubovisceral muscle was 0.87 ± 0.13 cm (95% CI, 0.64–1.38 cm), while, in the plane of minimum dimension of the genital hiatus, the hiatal area at rest was 13.41 ± 3.22 (95% CI, 4.60–18.78) cm2. There is a significant correlation between the age of pregnant women (over 35 years of age) and the increase in the area of the genital hiatus (r = 0.295, p = 0.031). 3D ultrasound of the pelvic floor performed at week 20 of gestation can to be an effective, non-invasive, reproducible and cheap tool in the prognosis of the development of labor and of possible subsequent perineal dysfunctions.
1. Introduction
The relationship between the Levator ani muscle (LAM) and pelvic floor morbidity has been studied in different studies. In fact, De Lancey et al. have related LAM trauma or increased urogenital hiatus with loss of pelvic floor support [1,2]. Pelvic floor ultrasound can increasingly offer us a prognostic window of how the pelvic structures are, to relate them to the different pathologies of the pelvic floor. For their part, Dietz and Hoyte demonstrated, through the use of 3D ultrasound of the pelvic floor and MRI, that there was a relationship between LAM defects and pelvic organ prolapse (POP), producing an increase in the urogenital hiatus, even suffering from a unilateral or bilateral avulsion of the muscle [3,4,5,6]. In fact, there are several exams including manual examination, ultrasound examination, ultrasound elastography exam as well combined ultrasound and biochemical exams (collagen and elastin) to evaluate the soft tissue of pelvic floor.
During childbirth, the LAM plays a fundamental role since it is the soft tissue that defines the biomechanical properties of the birth canal [7]. In fact, in other studies, the biomechanical properties of LAM in childbirth are very important, having a direct relationship between distention capacity and the type of delivery and even a shorter second stage labor [8,9].
LAM avulsion and consequently an increase in the genital hiatus are related to different pelvic floor dysfunctions (PFD), such as pelvic organ prolapse (POP) [10,11,12,13], urinary incontinence [14,15,16,17], fecal incontinence [18,19,20] and sexual dysfunctions [7,21,22].
Pelvic floor ultrasound has an increasing clinical application, for the measurement and visualization of different structures, such as the LAM, urogenital hiatus, LAM avulsions, etc. and its performance shows cheap, simple and very precise results, compared to MRI, in the visualization of the different perineal structures [23,24,25,26,27,28,29,30,31,32].
Transperineal ultrasound is considered the gold standard for exploration through imaging of the pelvic floor, being a tool for detecting different dysfunctions of the anterior, central or posterior compartment of the female genital sphere [33].
The LAM can elongate in very different ways in patients as a result of biomechanical stress or perineal trauma during childbirth, as well as having different morphological characteristics, among different patients that facilitate or slow down the second labor in childbirth [17], being able to generate in turn, damage to the different perineal structures, which can lead postpartum and even years later, to different dysfunctions of the pelvic floor, such as urinary incontinence (UI), pelvic organ prolapse (POP), anal incontinence (AI) or sexual dysfunctions, because of the likelihood of soft tissue trauma.
The objective of our study is to determine the thickness of the pubovisceral fasciculus of the LAM and the area of the genital hiatus by means of three-dimensional perineal ultrasound in 2nd trimester pregnant women and to analyze the related maternal, perinatal and postpartum clinical variables. Furthermore, to compare the results of our study with two similar series previously published.
2. Materials and Methods
A descriptive, observational and cross-sectional study has been carried out on a sample of pregnant women who attended the consultation for the routine ultrasound of week 20 at the Hospital General Universitario Gregorio Marañón, during the period between August and October 2021.
Among the inclusion criteria were a pregnant woman over 18 years of age, a singleton pregnancy without serious gestational pathology, a visit to perform a morphological ultrasound at week 20, and acceptance of informed consent. Exclusion criteria were delivery in another center and fetal death during pregnancy. Patients who did not respond to the postpartum questionnaire were excluded from the comparative analysis.
During the ultrasound examination, in addition to the obstetric ultrasound, measurements of the thickness of the pubovisceral fasciculus of the LAM and the area of the genital hiatus were made by three-dimensional transperineal ultrasound.
Images were obtained by 3D translabial ultrasound in the supine position, using a SAMSUNG ultrasound machine, model WS80A, with a 3D-4D volumetric probe (4–7 MHz). The method used to obtain the anatomical dimensions of the thickness of the pubovisceral muscle and the genital hiatus is that described in the study published by Dietz et al. [34] after being considered reproducible in other studies [35,36,37]. In order to follow the methodology of the aforementioned studies and in relation to the anatomical structures used in these studies, the same cut-off points have been used for the genital hiatus area and the thickness of the pubovisceral muscle, so that it can be reproducible and comparable in the 3D study of the pelvic floor structure. The ultrasound probe was oriented in the midsagittal plane. The acquisition angle was set at the transducer maximum of 70°. The volumetric images were obtained at rest and with an empty bladder, after checking in the midsagittal plane in 2D. Figure 1 shows the location of the plane taken in 2D, to determine the diameter of the hiatus and the thickness of the pubovisceral fasciculus. The plane of minimum dimensions of the hiatus was identified in the midsagittal plane, as the minimum distance between the hyperechoic posterior aspect of the pubic symphysis and the hyperechoic anterior border of the levator ani muscle, just after the anorectal image. When a correct 2D ultrasound image was obtained, the 3D volume was automatically obtained using the system’s 3D function, which maximizes acquisition quality and is saved in system memory. On this image, 4 measurements of the thickness of the pubovisceral fasciculus were made, from the internal muscle edge to the external In to In method on both sides at positions 3 and 5 o’clock, calculating the mean between the measurements to obtain the average thickness of the pubovisceral fasciculus of each patient. In addition, the perineal hiatus area was measured on the same image by delimiting its contours (Figure 2).
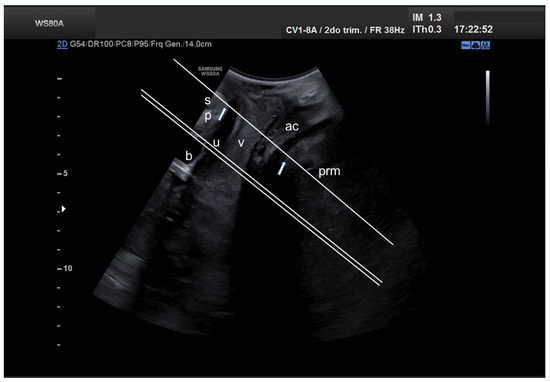
Figure 1.
Mid-sagittal translabial two-dimensional pelvic floor ultrasound, showing the location of planes used for determining hiatal diameters and areas (single line) as well as pubovisceral muscle thickness and area (double line). ac, anal canal; b, bladder; prm, puborectalis muscle; sp, pubic symphysis; u, urethra; v, vagina.
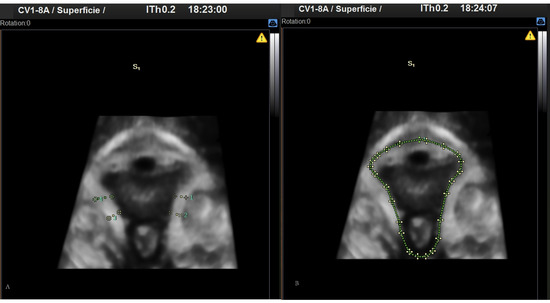
Figure 2.
3D images obtained by perineal ultrasound. (A) Pubovisceral muscle thickness. (B) Perineal hiatus area.
In Figure 3, an anatomical sketch of the coronal plane of the pelvic floor is shown in order to illustrate the exact plane and structures obtained by the 3D ultrasound examination.
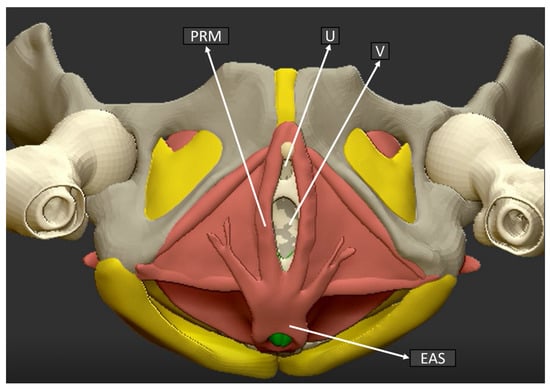
Figure 3.
Pelvic floor anatomical structure coronal plane. (U) Urethra, (V) Vagina, (PRM) Puborectalis muscle, (EAS) External anal sphincter.
In addition to the ultrasound examination, patients were offered to participate in a postpartum health questionnaire that assessed symptoms of perineal dysfunction. Those who accepted received a copy of the questionnaire to be completed so that they knew the questions beforehand. A non-standardized questionnaire was defined (Annex 1that included questions related to the possibility of suffering from some perineal dysfunction. Pelvic floor ultrasound measurement was performed by a single clinician during the study.
The surveys were conducted by telephone call three months after delivery, in the period between April and June 2022.
Maternal, ultrasound, perinatal and postpartum clinical variables were collected for each participant and included in a database created for this purpose.
Statistic Analysis
The data obtained from the study were included in a Microsoft Office Excel database, version 2019 (Microsoft, Redmond, WA, USA) and the statistical analysis was performed with SPSS version 25 programs (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY, USA) and Epidat 3.1 program for epidemiological data analysis. Version 3.1, Conselleria de Sanidade, Xunta de Galicia, Santiago, Spain; Pan American Health Organization (PAHO-WHO); CES University, Colombia. For each group, the descriptive parameters mean, standard deviation and 95% confidence interval were calculated for all the quantitative variables, and the percentage relative frequencies were calculated for the qualitative variables. A comparative analysis of the clinical variables of the patients was carried out based on the two groups of patients established according to presenting values of pubovisceral and hiatal thickness or equal to or less than the mean and above the mean. To calculate the effect correlation of each variable, a univariate logistic regression analysis was carried out to determine the value of the OR.
Subsequently, of the most representative variables in the results of the comparison of the groups, a calculation of the correlation between these ultrasound measurements and the most representative variables in the results of the comparison of the two groups was carried out.
To compare differences in anatomical measurements, statistical differences were calculated from the summary data (Mean, SD, and sample size) using Student’s t-test. A p < 0.05 has been defined for statistical significance.
The study protocol received the approval of the Ethics Committee for Medical Research of our center (Code: MSP-1, 13 July 2021).
3. Results
During the study period, 54 patients from our center who met the inclusion criteria were recruited. Maternal and ultrasound variables could be collected in all recruited patients.
No cases of LAM injury were detected in the ultrasounds performed, not even in patients with previous deliveries.
Nine (16.67%) patients were excluded due to delivery in another center and 1 (1.85%) patient due to fetal death, therefore 44 patients were included for the study of perinatal and postpartum variables.
Table 1 describes the maternal clinical and ultrasound variables. The mean age of the patients was 33.80 ± 4.59 years and they attended on average at 19.81 ± 0.91 weeks of gestation. It stands out that 44.4% of the patients were older than 35 years, as well as a multiparity percentage of 44.4%.

Table 1.
Characteristics of the study population. N values differ inside groups because of missing data of patients without childbirth variables and postpartum variables (N = 10).
In relation to the ultrasound variables, the mean thickness of the pubovisceral muscle was 0.87 ± 0.13 cm (95% CI, 0.64–1.38 cm), while, in the plane of minimum dimension of the genital hiatus, the hiatal area at rest was 13.41 ± 3.22 (95% CI, 4.60–18.78) cm2.
55.8% of deliveries were induced, with a frequency of 4.5% instrumental delivery and 9.1% cesarean section. 43.2% of perineal tears have been described in vaginal deliveries.
In order to analyze the results in the different variables, the patients have been classified into two groups based on the measurement of the pubovisceral muscle, classifying them into those with a measurement equal to or less than the mean (0.87 cm) and above the mean. In addition, they have been classified into two groups in relation to the size of the genital hiatus, also taking the mean of its area as the cut-off point (equal to or less than the mean of 13.41 cm2 and greater than the mean).
Table 1 describes the results of the clinical, delivery and postpartum variables in the two groups with the highest risk of possible association with pathology, which are the group with thickness of the pubovisceral muscle equal to or less than the average and the group with area of the pubovisceral muscle. higher than average hiatus.
Table 2 shows the analysis of the association of the different most relevant clinical variables with risk groups. There is no significant association between age over 35 years and the thickness of the pubovisceral fasciculus (r = 0.008, p = 0.952), however, this association has been established with the increase in the genital hiatus (r = 0.295, p = 0.031). None of the other variables studied have shown statistical significance, including parity, labor induction, or the presence of IU or POP postpartum. Only the frequency of labor induction is close to statistical significance, although its clinical etiological relationship is not clear.

Table 2.
Correlation between variables representative of the study population and the cutoff measure for pubovisceral muscle thickness and genital hiatus area.
In addition, it describes the degree of association through odds ratio. The variables with the greatest difference in cases, in the comparison between groups in Table 1 and that may have greater clinical relevance with the thickness of the pubovisceral muscle, are maternal age (OR = 1.03; p = 0.51), labor induction (OR = 3.34; p = 0.060) and urinary incontinence (OR = 1.97; p = 0.336). While the main variables with greater relevance to the genital hiatus area are maternal age (OR = 3.38; p = 0.033), multiparity (OR = 2.10; p = 0.183), urinary incontinence (OR = 1.33; p = 0.666) and pelvic organ prolapse (OR = 2.25; p = 0.261).
As previously mentioned, a comparison of the measurements obtained in our study with those published in the works of Dietz et al. and Yang et al. has been incorporated into the analysis. in non-pregnant women. The results of this comparison are shown in Table 3.

Table 3.
Patient sonographic and demographic data of the pelvic floor in tow series with different stage. Data are presented as mean ± SD (range). Comparison between these groups from the summary of data (mean, SD and sample size). * p value < 0.001 comparison with Yang et al. [35] study. ** p value < 0.001 comparison with Dietz et al. [34] study. *** p value < 0.001 comparison with Dietz et al. [34] study.
It can be noted that, in our study on pregnant women, the pubovisceral muscle is thicker than in the aforementioned studies (p < 0.001). However, these differences do exist in the area of the genital hiatus, which is higher in our study (p = 0.002). The age of the patients also differs between these studies, being higher in our sample, which could justify this second point.
4. Discussion
In this study we have performed a three-dimensional ultrasound evaluation of pregnant women in the second trimester of pregnancy in order to determine the measurements of the thickness of the LAM pubovisceral fascicle and the area of the genital hiatus.
The mean area of the genital hiatus appears to be related to age, being higher in the group of women older than 35 years (OR = 3.38; p = 0.033). It is widely described in other studies that age has an impact on the increase in different PFDs [37,38,39,40], so we believe that it is important to take this variable into account in pregnant women when it comes to anticipate the possibility of the appearance of any perineal dysfunction in the postpartum period. Multiple studies have linked increased genital hiatus area to POP [1,3,4,41,42]. The relationship shown in this study between age and the increase in the hiatal area corroborates these results and allows selecting those patients who present a high risk of suffering from UI or POP in the postpartum period.
POP occurs when there is a defect in the support mechanism of the different levels of suspension that Delancey et al. already described, in order to even be able to support normal intra-abdominal pressure [43,44]. In fact, Delancey et al. and other studies show that when the levator ani is damaged or weak, as can occur in an obstetric procedure, the area of the genital hiatus tends to increase [1,3,11,12,42]. In our study, we have not found any patient with unilateral or bilateral levator lesion, so we can think that this increase in the genital hiatus could be caused by the intra-abdominal pressure itself in relation to the fetus, as well as a possible weakness of the levator muscle. In non-pregnant women, POP occurs when the pelvic floor structures have compromised competence and/or integrity and must withstand sustained intra-abdominal pressure [3,7,17,44], so as we have observed in the results of the study, the pressure of the uterus itself during pregnancy and especially after week 20, on the entire suspension structure of the pelvic floor, can generate this increase in the area of the genital hiatus, and subsequently result in some type of POP. Although not statistically significant, in our study the risk of postpartum POP is doubled in pregnant women with an increase in the area of the genital hiatus at week 20 (OR = 2.25; p = 0.261).
In addition, we have been able to determine that in our study the measurement of the thickness of the pubovisceral muscle and the genital hiatus does not seem to be related to age or other clinical parameters such as parity. The only aspect related to thickness, in a significant way, has turned out to be the probability of labor induction, however, we do not know the underlying pathophysiological relationship if it exists. We have also not been able to find a significant association between variables related to childbirth and muscle thickness or hiatal area, despite the fact that they seem to be biologically related, such as the total duration of labor, the frequency of eutocic or instrumental vaginal delivery, or the of soft canal tears. This may be due to the low power due to the end of the study or to the fact that the examination during pregnancy around week 20 does not allow an association to be established with different events that may take place during childbirth.
In the evaluation of the risk factors for UI and POP, the presence of a lower-than-average thickness in the pubovisceral muscle shows an OR of 1.97 for postpartum IU, although without reaching statistical significance. In the case of increased genital hiatus, the OR for POP is 2.25, although with a p of 0.26. A higher n is likely to be needed to assess these outcomes, which are known risk factors for pelvic floor pathology. We must also take into account that the development of these pathologies can present years of latency, so that a single evaluation at 3 months postpartum may not adequately represent the evolution in the more distant future.
If we analyze our results by comparing them with the studies by Dietz et al. and Yang et al. the patients in our study present a larger area of the genital hiatus. This can be explained either by her gestational state in which the weight of the gravid uterus on this muscular diaphragm increases its diameter and is in turn related to the older average age of our population.
Another relevant aspect in the results of the study is the relationship that may exist between the decrease in thickness of the pubovisceral muscle and the performance of labor induction. In fact, labour induction has been studied as one of the important risk factors for pelvic floor tears [45,46,47,48]. In our study, patients who had a thickness of the pubovisceral fasciculus less than the average, have a higher probability of labour induction (OR = 3.34; CI 95% 0.94–11.85; p = 0.06). Therefore, an early detection of a thinner pubovisceral muscle (we can associate it with less muscle tone and strength), may indicate a higher risk of a perineal tear during childbirth, especially if it is an induced, long or instrumental delivery and its subsequent appearance of postpartum anal incontinence, due to the existing relationship already described in other studies [49,50,51,52,53,54].
Ultrasound is currently the fundamental tool in the study of the pelvic floor muscles, given its accessibility and low cost compared to other radiological techniques [55,56].
The possibility of obtaining these images has been very high, being able to capture volumes in all the patients recruited, so in practice it is a technique that is easy to acquire, minimally invasive and of reasonable cost compared to MRI. In addition, its performance during pregnancy can be framed within the obstetric ultrasound study and contribute to detecting and informing those patients with risk factors for pelvic floor pathology in the future and designing both primary prevention strategies (pelvic floor exercises, modify delivery care) as secondary if there are postpartum symptoms.
It is necessary to carry out more studies in pregnant patients, with more numerous samples that improve the statistical power and thus be able to determine which are the main risk factors associated with pelvic floor dysfunction, especially those antepartum that can be influenced in primary prevention. Interobserver studies are also advisable in order to compare the agreement between different sonographers. Despite other methods to assess the biomechanical parameters of the pelvic floor muscles have been described, such a biochemical markers like elastin and collagen, ultrasound assessment seems to be the first line of study given its availability, innocuity (no histology is required) and reproducibility [57,58].
A future research line could include the performance of a combination of imaging and biochemical studies in second or third trimester in order to obtain a more complete information of the status of the pelvic floor musculature and its possible behaviour under the stress of childbirth.
The timing of the 20-week examination was considered the optimal time for preventive action, in the case of high risk and as part of a complete examination that includes fetal morphological ultrasound, cervical measurement and second trimester analysis, although we can consider it a limitation of the study, not to have been able to perform measurements in the 32nd weeks to check for more significant associations. In fact, it would be interesting to be able to measure, biochemical parameters in the study population and combine them with the ultrasound study, to complete a pelvic floor examination.
5. Conclusions
3D ultrasound of the pelvic floor performed at week 20 of gestation can be an effective, non-invasive, reproducible and cheap tool in the prognosis of the development of labor and possible subsequent perineal dysfunction.
Author Contributions
Formal analysis, J.M.P.-M.; Investigation, J.A.B., C.B., S.G.T., R.A.-R., I.C.-H., M.P.P.-R., M.A.-M., M.A.O. and J.A.D.L.-L. All authors have read and agreed to the published version of the manuscript.
Funding
The study (FIS-PI21/01244) was supported by the Instituto de Salud Carlos III (grant no. Estatal de I + D + I 2020–2027) and co-financed by the European Development Regional Fund “A way to achieve Europe” and B2017/BMD-3804 MITIC-CM (Comunidad de Madrid), and Halekulani S.L. and MJR.
Institutional Review Board Statement
The study protocol received the approval of the Ethics Committee for Medical Research of our center (Code: MSP-1, 13 July 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data from this study are available at the obstetrics service of the Hospital General Universitario Gregorio Marañón in Madrid and will be provided upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| OR | Odds Ratio |
| C-Section | Cesarean birth |
| CC | Cephalic circumference |
| EFW | Estimated fetal weight |
| UI | Urinary incontinence |
| FI | Fecal incontinence |
| AI | Anal incontinence |
| POP | Pelvic organ prolapse |
| CI | Confidence interval |
| SD | standard deviation |
| BMI | Body mass index |
| LAM | Levator ani muscle |
| PFD | Pelvic floor diseases |
| HGUGM | Hospital General Universitario Gregorio Marañón de Madrid |
References
- DeLancey, J.O.L.; Hurd, W.W. Size of urogenital hiatus in the levator ani muscles in normal women and women with pelvic organ prolapse. Obstet. Gynecol. 1998, 91, 364–368. [Google Scholar] [CrossRef]
- DeLancey, J.O.L.; Morgan, D.M.; Fenner, D.E.; Kearney, R.; Guire, K.; Miller, J.M.; Hussain, H.; Umek, W.; Hsu, Y.; Ashton-Miller, J.A. Comparison of Levator Ani Muscle Defects and Function in Women With and Without Pelvic Organ Prolapse. Obstet. Gynecol. 2007, 109, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.; Simpson, J. Levator trauma is associated with pelvic organ prolapse. Br. J. Obstet. Gynaecol. 2008, 115, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Hoyte, L.; Schierlitz, L.; Zou, K.; Flesh, G.; Fielding, J.R. Two- and 3-dimensional MRI comparison of levator ani structure, volume, and integrity in women with stress incontinence and prolapse. Am. J. Obstet. Gynecol. 2001, 185, 11–19. [Google Scholar] [CrossRef]
- Ptaszkowski, K.; Małkiewicz, B.; Zdrojowy, R.; Paprocka-Borowicz, M.; Ptaszkowska, L. Assessment of the Elastographic and Electromyographic of Pelvic Floor Muscles in Postmenopausal Women with Stress Urinary Incontinence Symptoms. Diagnostics 2021, 11, 2051. [Google Scholar] [CrossRef]
- Gong, R.; Xia, Z. Collagen changes in pelvic support tissues in women with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 185–189. [Google Scholar] [CrossRef]
- Jung, S.-A.; Pretorius, D.H.; Padda, B.S.; Weinstein, M.M.; Nager, C.W.; Boer, D.J.D.; Mittal, R.K. Vaginal high-pressure zone assessed by dynamic 3-dimensional ultrasound images of the pelvic floor. Am. J. Obstet. Gynecol. 2007, 197, 52.e1–52.e7. [Google Scholar] [CrossRef]
- Lanzarone, V.; Dietz, H.P. Three-dimensional ultrasound imaging of the levator hiatus in late pregnancy and associations with delivery outcomes. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 176–180. [Google Scholar] [CrossRef]
- Balmforth, J.; Toosz-Hobson, P.; Cardozo, L. Ask not what childbirth can do to your pelvic floor but what your pelvic floor can do in childbirth. Neurourol. Urodyn. 2003, 22, 540–542. [Google Scholar]
- Dietz, H.P. Quantification of major morphological abnormalities of the levator ani. Ultrasound Obstet. Gynecol. 2007, 29, 329–334. [Google Scholar] [CrossRef]
- Dietz, H.; De Leon, J.; Shek, K. Ballooning of the levator hiatus. Ultrasound Obstet. Gynecol. 2008, 31, 676–680. [Google Scholar] [CrossRef]
- Dietz, H.P.; Franco, A.V.; Shek, K.L.; Kirby, A. Avulsion injury and levator hiatal ballooning: Two independent risk factors for prolapse? An observational study. Acta Obstet. Gynecol. Scand. 2012, 91, 211–214. [Google Scholar] [CrossRef]
- Rodrigo, N.; Shek, K.L.; Dietz, H.P. Rectal intussusception is associated with abnormal levator ani muscle structure and morphometry. Tech. Coloproctol. 2011, 15, 39–43. [Google Scholar] [CrossRef]
- Allen, R.; Hosker, G.L.; Smith, A.R.B.; Warrell, D.W. Pelvic floor damage and childbirth: A neurophysiological study. Br. J. Obstet. Gynaecol. 1990, 97, 770–779. [Google Scholar] [CrossRef]
- Wilson, P.D.; Berghmans, B.; Hagen, S.; Hay-Smith, J.; Moore, K.; Nygaard, I.; Sinclair, L.; Yamanishi, T.; Wyman, J. Adult conservative management. In Incontinence: Third International Consultation on Incontinence; Abrams, P., Cardozo, L., Khoury, S., Wein, A., Eds.; Health Publications Ltd.: Paris, France, 2005; Volume 2. [Google Scholar]
- Dietz, H.; Kirby, A.; Shek, K.; Bedwell, P. Does avulsion of the puborectalis muscle affect bladder function? Int. Urogynecol. J. 2009, 20, 967–972. [Google Scholar] [CrossRef]
- Morgan, D.M.; Cardoza, P.; Guire, K.; Fenner, D.E.; DeLancey, J.O.L. Levator ani defect status and lower urinary tract symptoms in women with pelvic organ prolapse. Int. Urogynecol. J. 2010, 21, 47–52. [Google Scholar] [CrossRef]
- Chantarasorn, V.; Shek, K.L.; Dietz, H.P. Sonographic detection of puborectalis muscle avulsion is not associated with anal incontinence. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 130–135. [Google Scholar] [CrossRef]
- van de Geest, L.; Steensma, A.B. Three-dimensional transperineal ultrasound imaging of anal sphincter injuries after surgical primary repair. Ultrasound Obstet. Gynecol. 2010, 36, 270. [Google Scholar] [CrossRef]
- Shek, K.; Guzman Rojas, R.; Dietz, H.P. Residual defects of the external anal sphincter are common after OASIS repair. Neurourol. Urodyn. 2012, 31, 913–914. [Google Scholar]
- Dietz, H.; Shek, K.; Chantarasorn, V.; Langer, S. Do women notice the effect of childbirth-related pelvic floor trauma? Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 277–281. [Google Scholar] [CrossRef]
- Thibault-Gagnon, S.; Yusuf, S.; Langer, S.; Wong, V.; Shek, K.L.; Martin, A.; Dietz, H.P. Do women notice the impact of childbirth-related tevator trauma on pelvic floor and sexual function? Int. Urogynecol. J. 2012, 23, S183–S185. [Google Scholar]
- Dietz, H.P. Ultrasound in the assessment of pelvic organ prolapse. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 54, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.P.; Campbell, S. Toward normal birthebut at what cost? Am. J. Obstet. Gynecol. 2016, 215, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Rahmanou, P.; Caudwell-Hall, J.; Atan, I.K.; Dietz, H.P. The association between maternal age at first delivery and risk of obstetric trauma. Am. J. Obstet. Gynecol. 2016, 215, 451.e1–451.e7. [Google Scholar] [CrossRef]
- Dietz, H.P. Virtual issue on urogynaecology. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 755–756. [Google Scholar] [CrossRef]
- Dietz, H.P. Ultrasound imaging of maternal birth trauma. Int. Urogynecol. J. 2021, 32, 1953–1962. [Google Scholar] [CrossRef]
- Dietz, H.P. Exoanal Imaging of the Anal Sphincters. J. Ultrasound Med. 2018, 37, 263–280. [Google Scholar] [CrossRef]
- Dietz, H.P. Pelvic Floor Ultrasound: A Review. Clin. Obstet. Gynecol. 2017, 60, 58–81. [Google Scholar] [CrossRef]
- Karjalainen, P.K.; Gillor, M.; Dietz, H.P. Predictors of occult stress urinary incontinence. Aust. N. Z. J. Obstet. Gynaecol. 2021, 61, 263–269. [Google Scholar] [CrossRef]
- Youssef, A.; Brunelli, E.; Pilu, G.; Dietz, H.P. The maternal pelvic floor and labor outcome. Am. J. Obstet. Gynecol. MFM 2021, 3, 100452. [Google Scholar] [CrossRef]
- Shek, K.L.; Dietz, H.P. Ultrasound imaging of slings and meshes in urogynecology. Ultrasound Obstet. Gynecol. 2021, 57, 526–538. [Google Scholar] [CrossRef]
- Dietz, H.P. Female pelvic floor dysfunction—an imaging perspective. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 113–121. [Google Scholar] [CrossRef]
- Dietz, H.P.; Shek, C.; Clarke, B. Biometry of the pubovisceral muscle and levator hiatus by three-dimensional pelvic floor ultrasound. Ultrasound Obstet. Gynecol. 2005, 25, 580–585. [Google Scholar] [CrossRef]
- Yang, J.-M.; Yang, S.-H.; Huang, W.-C. Biometry of the pubovisceral muscle and levator hiatus in nulliparous Chinese women. Ultrasound Obstet. Gynecol. 2006, 28, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Hoff Braekken, I.; Majida, M.; Ellstrom Engh, M.; Dietz, H.P.; Umek, W.; Bo, K. Test–retest and intra-observer repeatability of two-, three- and four-dimensional perineal ultrasound of pelvic floor muscle anatomy and function. Int. Urogynecol. J. 2007, 19, 227–235. [Google Scholar] [CrossRef]
- Wu, J.M.; Vaughan, C.P.; Goode, P.S.; Redden, D.T.; Burgio, K.L.; Richter, H.E.; Markland, A.D. Prevalence and Trends of Symptomatic Pelvic Floor Disorders in U.S. Women. Obstet. Gynecol. 2014, 123, 141–148. [Google Scholar] [CrossRef]
- FitzGerald, M.P.; Weber, A.M.; Howden, N.; Cundiff, G.W.; Brown, M.B. Risk Factors for Anal Sphincter Tear During Vaginal Delivery. Obstet. Gynecol. 2007, 109, 29–34. [Google Scholar] [CrossRef]
- Elvander, C.; Ahlberg, M.; Thies-Lagergren, L.; Cnattingius, S.; Stephansson, O. Birth position and obstetric anal sphincter injury: A population-based study of 113 000 spontaneous births. BMC Pregnancy Childbirth 2015, 15, 252. [Google Scholar] [CrossRef]
- Gurol-Urganci, I.; Cromwell, D.; Edozien, L.; Mahmood, T.; Adams, E.; Richmond, D.; Templeton, A.; van der Meulen, J. Third- and fourth-degree perineal tears among primiparous women in England between 2000 and 2012: Time trends and risk factors. Br. J. Obstet. Gynaecol. 2013, 120, 1516–1525. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Yang, J. Anatomical and functional significance of urogenital hiatus in primary urodynamic stress incontinence. Ultrasound Obstet. Gynecol. 2006, 27, 71–77. [Google Scholar] [CrossRef]
- Athanasiou, S.; Boos, K.; Khullar, V.; Anders, K.; Cardozo, L. Pathogenesis of genuine stress incontinence and urogenital prolapse. Neurourol. Urodynam. 1996, 15, 339–340. [Google Scholar]
- DeLancey, J.O. What’s new in the functional anatomy of pelvic organ prolapse? Curr. Opin. Obstet. Gynecol. 2016, 28, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.H.; Randall, C.L. Type of prolapse. In Vaginal Surgery, 4th ed.; Nichols, D.H., Randall, C.L., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1996; pp. 101–118. [Google Scholar]
- Abedzadeh-Kalahroudi, M.; Talebian, A.; Sadat, Z.; Mesdaghinia, E. Perineal trauma: Incidence and its risk factors. J. Obstet. Gynaecol. 2019, 39, 206–211. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Alliance (UK). Induction of Labour for Suspected Fetal Macrosomia: Inducing Labour: Evidence Review A; National Institute for Health and Care Excellence (NICE): London, UK, 2021. [Google Scholar]
- Risk Factors for Severe Perineal Lacerations during Childbirth: A Systematic Review and Meta-Analysis of Cohort Studies. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jocn.16438 (accessed on 19 July 2022). [CrossRef]
- Karaca, S.Y.; Adıyeke, M.; Ileri, A.; Vural, T.; Şenkaya, A.R.; Ileri, H.; Özeren, M. Obstetric Perineal Tears in Pregnant Adolescents and the Influencing Factors. J. Pediatr. Adolesc. Gynecol. 2022, 35, 323–328. [Google Scholar] [CrossRef]
- Laine, K.; Skjeldestad, F.E.; Sanda, B.; Horne, H.; Spydslaug, A.; Staff, A.C. Prevalence and risk factors for anal incontinence after obstetric anal sphincter rupture. Acta Obstet. Gynecol. Scand. 2011, 90, 319–324. [Google Scholar] [CrossRef]
- Mahony, R.; Behan, M.; Daly, L.; Kirwan, C.; O’Herlihy, C.; O’Connell, P.R. Internal anal sphincter defect influences continence outcome following obstetric anal sphincterinjury. Am. J. Obstet Gynecol. 2007, 196, 217.e1–217.e5. [Google Scholar] [CrossRef]
- Norderval, S.; Markskog, A.; Rossaak, K.; Vonen, B. Correlation between anal sphincter defects and anal incontinence following obstetric sphincter tears: Assessment using scoring systems for sonographic classification of defects. Ultrasound Obstet. Gynecol. 2008, 31, 78–84. [Google Scholar] [CrossRef]
- Nichols, C.M.; Nam, M.; Ramakrishnan, V.; Lamb, E.H.; Currie, N. Anal sphincter defects and bowel symptoms in women withand without recognized anal sphincter trauma. Am. J. Obstet. Gynecol. 2006, 194, 1450–1454. [Google Scholar] [CrossRef]
- Nichols, C.M.; Lamb, E.H.; Ramakrishnan, V. Differences in outcomes after third- versus fourth-degree perineal laceration repair: A prospective study. Am. J. Obstet. Gynecol. 2005, 193, 530–534. [Google Scholar] [CrossRef]
- Richter, H.E.; Fielding, J.R.; Bradley, C.S.; Handa, V.L.; Fine, P.; FitzGerald, M.P.; Visco, A.; Wald, A.; Hakim, C.; Wei, J.T.; et al. Endoanal ultrasound findings and fecal incontinence symptoms in women with and without recognized anal sphincter tears. Obstet. Gynecol. 2006, 108, 1394–1401. [Google Scholar] [CrossRef]
- Dietz, H.P. Ultrasound in the investigation of pelvic floor disorders. Curr. Opin. Obstet. Gynecol. 2020, 32, 431–440. [Google Scholar] [CrossRef]
- Falkert, A.; Willmann, A.; Endress, E.; Meint, P.; Seelbach-Göbel, B. Three-dimensional ultrasound of pelvic floor: Is there a correlation with delivery mode and persisting pelvic floor disorders 18–24 months after first delivery? Ultrasound Obstet. Gynecol. 2013, 41, 204–209. [Google Scholar] [CrossRef]
- Gardella, B.; Scatigno, A.L.; Belli, G.; Gritti, A.; Visoná, S.D.; Dominoni, M. Aging of Pelvic Floor in Animal Models: A Sistematic Review of Literature on the Role of the Extracellular Matrix in the Development of Pelvic Floor Prolapse. Front. Med. 2022, 9, 863945. [Google Scholar] [CrossRef]
- Lin, W.; Lin, L.; Dong, B.; Chen, L.; Lei, H.; Gao, Y.; Chen, Y.; Sun, P. The Role of Obstetric Factors, miRNA-30d and miRNA-181a in Postpartum Women with Pelvic Organ Prolapse. Risk Manag. Health Policy 2020, 13, 2309–2316. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).