Analysis of the Flammability and the Mechanical and Electrostatic Discharge Properties of Selected Personal Protective Equipment Used in Oxygen-Enriched Atmosphere in a State of Epidemic Emergency
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Limiting Oxygen Index (LOI)
2.2.2. Cone Calorimeter Test
2.2.3. Differential Scanning Calorimeter Test
2.2.4. Oxygen Bomb Calorimeter Test
2.2.5. Tensile Test
2.2.6. Abrasion Resistance Test
2.2.7. Electrostatic Discharge Test
3. Results and Discussion
3.1. Burning Behavior
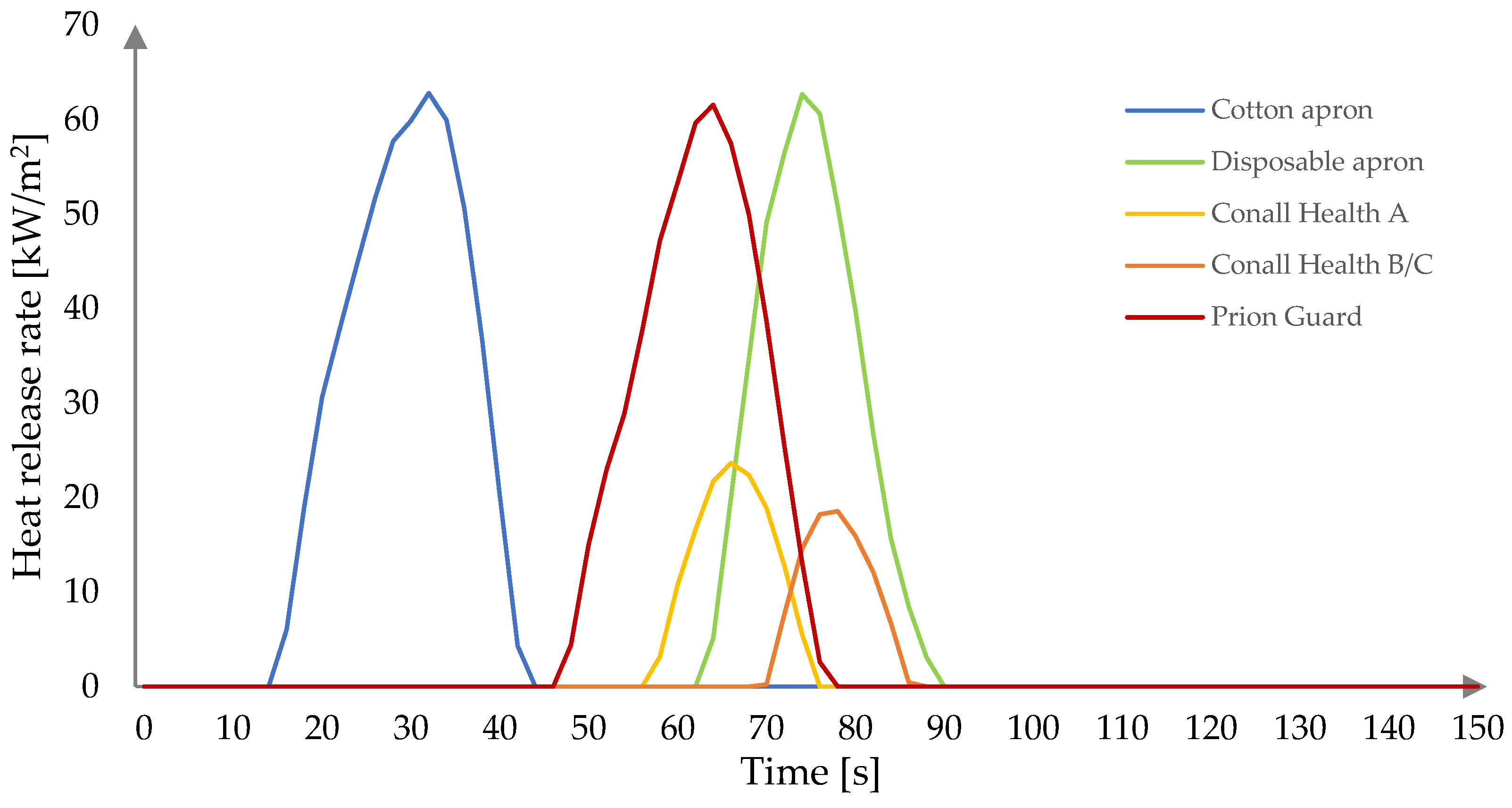
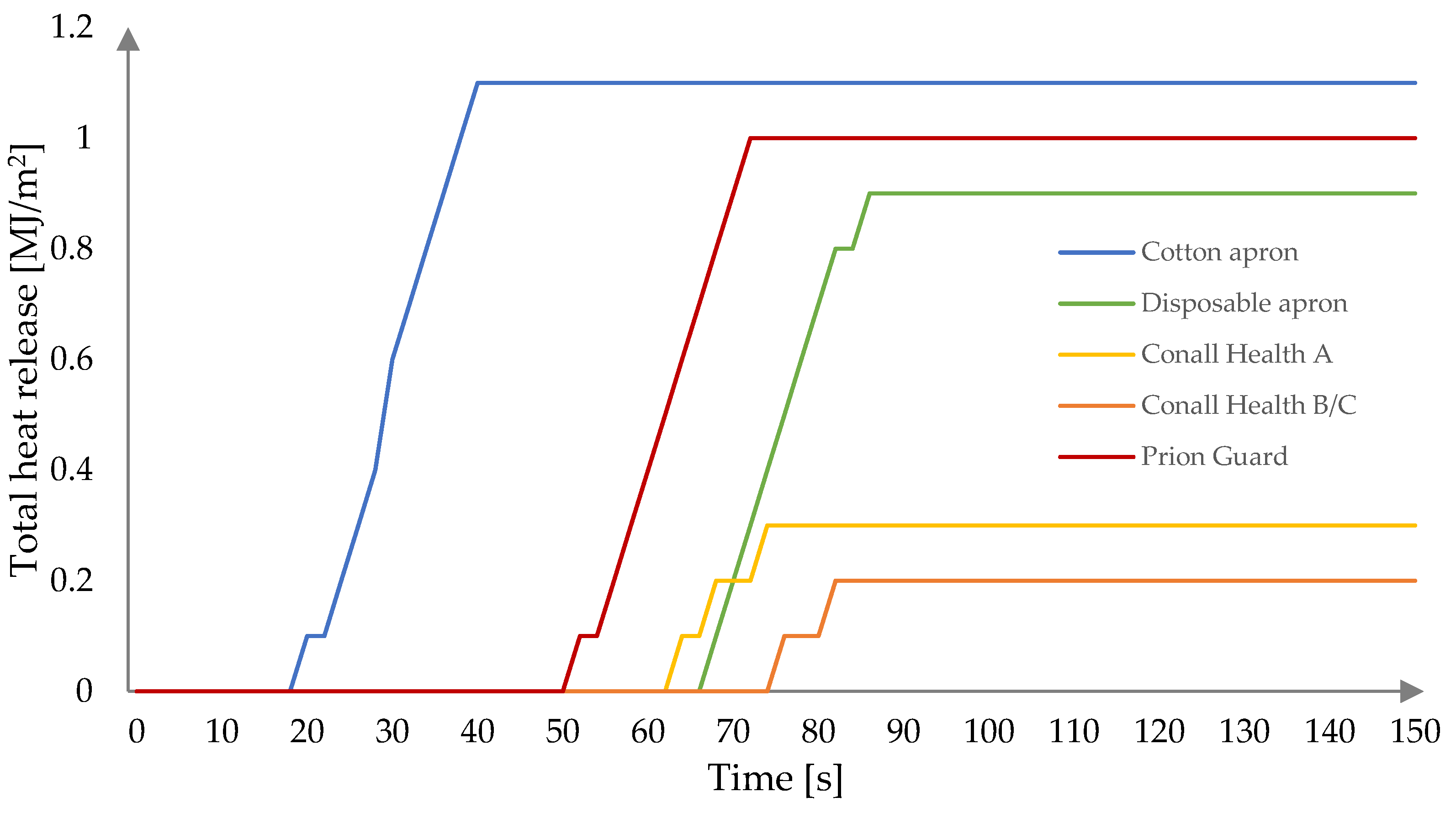
3.2. Fire Behavior
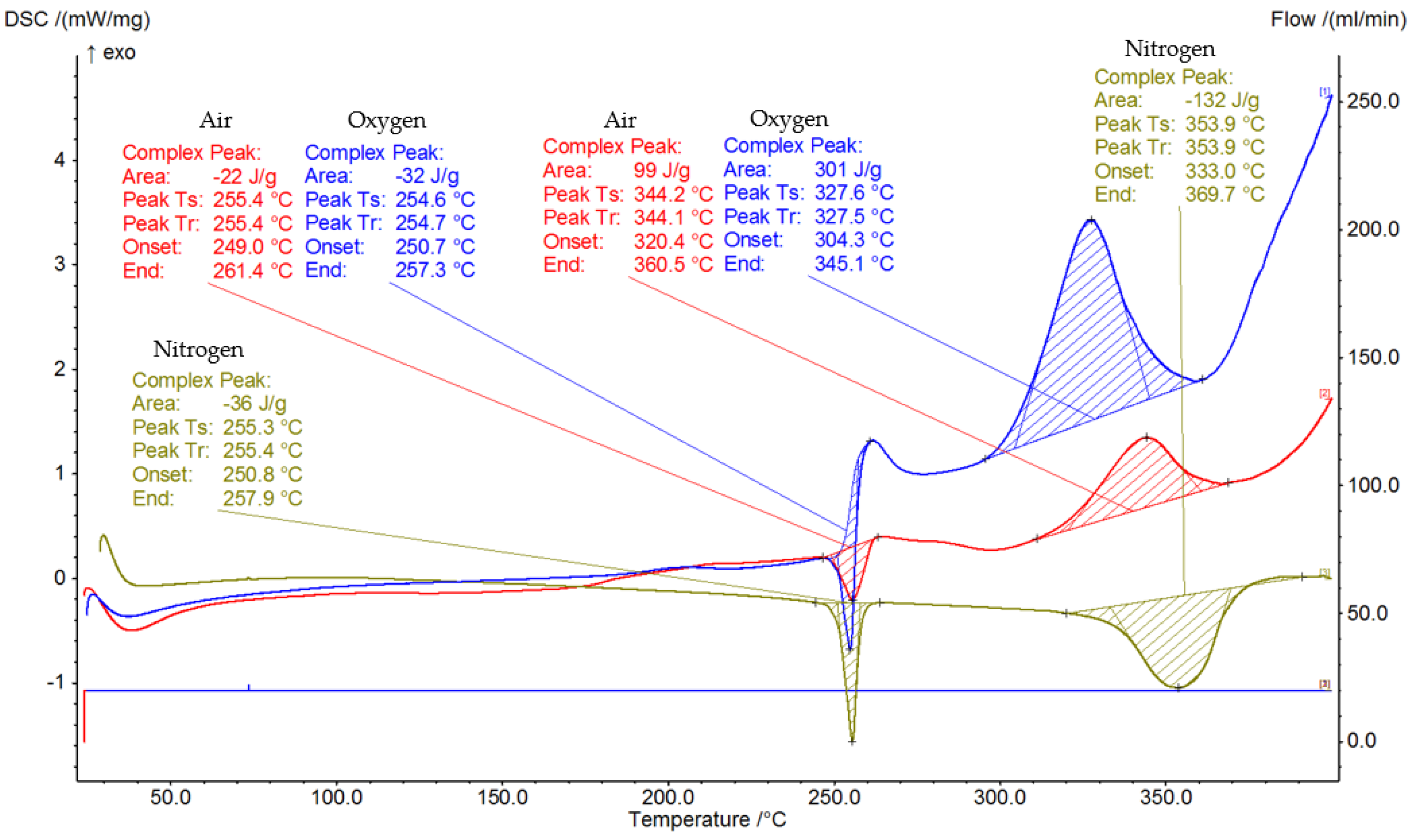
3.3. Thermal Analysis
3.4. Gross Heat of Combustion (Calorific Value)
3.5. Tensile Properties, Abrasion Resistance, and Electrostatic Discharge Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zangoue, M.; Safari, H.; Royce, S.G.; Zangooie, A.; Rezapour, H.; Zangouei, A.; Fereidouni, M. The high level of adherence to personal protective equipment in health care workers efficiently protects them from COVID-19 infection. Work 2021, 69, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, K.; Surbhi; Balaji, A.; Ganesh, R.; Daniel, R.A.; Aggarwal, R.; Soni, K.D.; Singh, A.K.; Khanna, P.; Gupta, V.; et al. Is it a challenging task to work with personal protective equipment in a COVID-19 ICU: Findings from a hospital-based cross-sectional study from north India. J. Fam. Med. Prim. Care 2022, 11, 1935–1942. [Google Scholar] [CrossRef]
- Noman, M.A.; Sun, J.; Hossain, M.B. COVID-19 Generated Personal Protective Equipment: Sources of Microplastics and Pathogen Vectors in Marine Environments? Front. Mar. Sci. 2021, 8, 798047. [Google Scholar] [CrossRef]
- Rault, F.; Giraud, S.; Salaün, F.; Almeras, X. Development of a Halogen Free Flame Retardant Masterbatch for Polypropylene Fibers. Polymers 2015, 7, 220–234. [Google Scholar] [CrossRef]
- Malviya, A.K.; Mulchandani, M.; Singh, J.; Singh, A.; Gupta, A. Increasing Hospital Fires During the COVID-19 Pandemic in India: Are the Current Policies and Infrastructure Adequate? J. Patient Saf. 2022, 18, e869–e870. [Google Scholar] [CrossRef]
- Wood, M.H.; Hailwood, M.; Koutelos, K. Reducing the risk of oxygen-related fires and explosions in hospitals treating Covid-19 patients. Process. Saf. Environ. Prot. 2021, 153, 278–288. [Google Scholar] [CrossRef]
- Risk of Oxygen-Related Fires in Hospitals Treating COVID-19 Patients. Available online: https://minerva.jrc.ec.europa.eu/en/shorturl/minerva/llb_on_risk_of_oxygen_related_fires_in_hospitals_treating_covid_19_patients (accessed on 31 August 2022).
- Thomson, L.; Paton, J. Oxygen toxicity. Paediatr. Respir. Rev. 2014, 15, 120–123. [Google Scholar] [CrossRef]
- Matrich, R.L.; Slusser, J.W.; Lievre, K.A. Oxygen enrichment: Recognizing and addressing the hazards. In Proceedings of the 68th Conference on Glass Problems, Columbus, OH, USA, 16–17 October 2008. [Google Scholar]
- Al-Dahhan, W.H.; Yousif, E. Fire and Explosion Hazards Due to Medical Oxygen Handling During Coronavirus Pandemic. J. Clin. Med. Case Rep. 2021, 2, 2–4. [Google Scholar] [CrossRef]
- Wicki, R. Liquid Oxygen—Fire Hazards of Oxygen and Oxygen-Enriched Atmospheres. Chimia 2003, 57, 781–783. [Google Scholar] [CrossRef][Green Version]
- Wróblewski, W.; Tuśnio, N.; Wolny, P.; Siuta, D.; Trzebicki, J.; Bączkowska, T.; Dzikowska-Diduch, O.; Pruszczyk, P. Fire Safety of Healthcare Units in Conditions of Oxygen Therapy in COVID-19: Empirical Establishing of Effects of Elevated Oxygen Concentrations. Sustainability 2022, 14, 4315. [Google Scholar] [CrossRef]
- ISO 4589-2; Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. ISO: Geneva, Switzerland, 2017; 27p.
- Kukfisz, B.; Półka, M. Analysis of Oxygen Index to Support Candle-like Combustion of Polyurethane. Procedia Eng. 2017, 172, 604–611. [Google Scholar] [CrossRef]
- Dowbysz, A.M.; Samsonowicz, M. Smoke Generation Parameters from the Cone Calorimeter Method and Single-Chamber Test. Environ. Sci. Proc. 2021, 9, 22. [Google Scholar] [CrossRef]
- ISO 5660-1; Reaction to Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015; 55p.
- Lee, J.S. Clarifying the Terms of Heating Values; University of British Columbia: Vancouver, Canada, 2017. [Google Scholar] [CrossRef]
- ISO 1716; Reaction to Fire Tests for Products—Determination of the Gross Heat of Combustion (Calorific Value). ISO: Geneva, Switzerland, 2018; 30p.
- Asadi, H.; Uhlemann, J.; Stranghöner, N. Uniaxial Strip and Grab Test Methods for Tensile Testing of Architectural Fabrics. In Proceedings of the TensiNet Symposium: Softening the Habitats: Sustainable Innovations in Minimal Mass Structures and Lighweight Architectures, Milan, Italy, 3–5 June 2019. [Google Scholar]
- ISO 13934-1; Textiles—Tensile Properties of Fabrics—Part 1: Determination of Maximum Force and Elongation at Maximum Force Using the Strip Method. ISO: Geneva, Switzerland, 2013; 12p.
- ISO 12947-2; Textiles—Determination of Abrasion Resistance of Fabrics by the Martindale Method—Part 2: Determination of Specimen Breakdown. ISO: Geneva, Switzerland, 2017; 15p.
- Somogyi Skoc, M.; Pezelj, E. Abrasion resistance of High Performance Fabrics. In Abrasion Resistance of Materials; Adamiak, M., Ed.; InTech: London, UK, 2012; pp. 35–52. [Google Scholar]
- EN 1149:3; Protective Clothing—Electrostatic Properties—Part 3: Test. Methods for Measurement of Charge Decay. EN: Pilsen, Czech Republic, 2004; 20p.
- Koncar, V. Smart textiles for monitoring and measurement applications. In Smart Textiles for In Situ Monitoring of Composites; Koncar, V., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 1–151. [Google Scholar]
- Rengasamy, S.; Niezgoda, G.; Shaffer, R. Flammability of Respirators and other Head and Facial Personal Protective Equipment. J. Int. Soc. Respir. Prot. 2018, 35, 1–13. [Google Scholar] [PubMed]
- Silva-Santos, M.C.; Peixoto, J.J.; Fangueiro, R.; Gasi, F.; Baruque-Ramos, J. The influence of textile materials on flame resistance ratings of professional uniforms. SN Appl. Sci. 2019, 1, 1650. [Google Scholar] [CrossRef]
- Mouritz, A.P. Durability of composites exposed to elevated temperature and fire. In Durability of Composites for Civil Structural Applications; Karbhari, V.M., Ed.; Woodhead Publishing: Sawston, UK, 2007; pp. 98–125. [Google Scholar]
- Sachdev, V.S.; Kotresh, T.M.; Vyawahare, M.K.; Agrawal, G.P. Heat Release Characteristics of the basic materials used for flying clothing. Indian J. Aerosp. Med. 2004, 48, 53–58. [Google Scholar]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.-D.; Novoselov, K.S. Sustainable Personal Protective Clothing for Healthcare Applications: A Review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef]
- Marquis, D.; Guillaume, E.; Lesenechal, D. Accuracy (Trueness and Precision) of Cone Calorimeter Tests with and Without a Vitiated Air Enclosure. Proc. Eng. 2013, 62, 103–119. [Google Scholar] [CrossRef]
- Makovicka Osvaldova, L.; Kadlicova, P.; Rychly, J. Fire Characteristics of Selected Tropical Woods without and with Fire Retardant. Coatings 2020, 10, 527. [Google Scholar] [CrossRef]
- Majder-Łopatka, M.; Węsierski, T.; Ankowski, A.; Ratajczak, K.; Duralski, D.; Piechota-Polanczyk, A.; Polanczyk, A. Thermal Analysis of Plastics Used in the Food Industry. Materials 2022, 15, 248. [Google Scholar] [CrossRef]
- Marlair, G.; Cwiklinski, C.; Tewarson, A. An analysis of some practical methods for estimating heats of combustion in fire safety studies. In Proceedings of the INTERFLAM ′99: Fire Science and Engineering Conference, Edinburgh, UK, 1 July 1999. [Google Scholar]
- Walters, R.N. Development of Instrumental and Computational Tool for Investigation of Polymer Flammability. Ph.D. Thesis, University of Central Lancashire, Preston, UK, 2013. [Google Scholar]
- Martinka, J.; Rantuch, P.; Hladová, M.; Sulová, J.; Nečas, A.; Benko, D.; Balog, K. Heat of Combustion as the Key Fire Characteristics of Electrical Cables. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2019, 27, 29–39. [Google Scholar] [CrossRef]
- EN 1149-5; Protective clothing—Electrostatic properties—Part 5: Material performance and design requirements. EN: Pilsen, Czech Republic, 2018; 20p.
- Zhang, S.; Horrocks, A.R. A review of flame retardant polypropylene fibres. Prog. Polym. Sci. 2003, 28, 1517–1538. [Google Scholar] [CrossRef]
- Brzeziński, S.; Kowalczyk, D.; Borak, B.; Jasiorski, M.; Tracz, A. Applying the sol–gel method to the deposition of nanocoats on textiles to improve their abrasion resistance. J. Appl. Polym. Sci. 2012, 125, 3058–3067. [Google Scholar] [CrossRef]

| Number | Name | Abbreviation | Material | Mass Per Unit Area [g/cm2] | Color |
|---|---|---|---|---|---|
| 1 | Cotton apron | CO | Cotton (100%) | 0.0206 | White |
| 2 | Disposable apron | DISP | Non-woven polypropylene fabric with a basis weight of 20 g/m2 | 0.0061 | White |
| 3 | Conall Health A brand apron | CHA | Non-woven polypropylene fabric with a basis weight of 35 g/m2 | 0.0037 | Blue |
| 4 | Conall Health B/C brand apron | CHB | Non-woven polypropylene fabric with a basis weight of 35 g/m2, with an additional protective inner layer against microbiological agents | 0.0036 | Blue outer layer and white internal layer |
| 5 | Prion Guard suit | PG | Non-woven polypropylene fabric | 0.0075 | Blue |
| Fabric | LOI (vol %) | Classification |
|---|---|---|
| CO | 17.17 | Combustible |
| DISP | 17.39 | Combustible |
| CH/A | 23.37 | Self-extinguishing |
| CH/B | 23.51 | Self-extinguishing |
| PG | 24.51 | Self-extinguishing |
| Sample | CO | DISP | CH/A | CH/B | PG |
|---|---|---|---|---|---|
| TTI (s) | 10 | 60 | 50 | 66 | 42 |
| pHRR (kW/m2) | 62.81 | 62.70 | 23.65 | 18.56 | 61.57 |
| tpHRR (s) | 32 | 74 | 66 | 78 | 64 |
| THR (MJ/m2) | 1.1 | 0.9 | 0.3 | 0.2 | 1.0 |
| MARHE (kW/m2) | 26.40 | 9.90 | 3.59 | 2.19 | 13.75 |
| Mean CO yield (kg/kg) | 132.82 | 562.46 | 687.51 | 700.43 | 332.65 |
| Mean CO2 yield (kg/kg) | 40.08 | 253.80 | 175.32 | 173.25 | 88.47 |
| Residue (%) | 32.24 | 54.82 | 37.29 | 50.69 | 22.68 |
| Fabric | Series 1 (MJ/kg) | Series 2 (MJ/kg) | Average Gross Heat of Combustion (MJ/kg) |
|---|---|---|---|
| CO | 19.343 | 19.460 | 19.402 |
| DISP | 35.227 | 35.523 | 35.375 |
| CH/A | 43.648 | 44.313 | 43.981 |
| CH/B | 44.496 | 44.258 | 44.377 |
| PG | 45.919 | 44.004 | 44.962 |
| Tensile Properties | Abrasion Resistance | Electrostatic Discharge Properties | |||
|---|---|---|---|---|---|
| Fabric | Maximum Force (N) | Elongation at Maximum Force (%) | Elongation at Rupture (%) | Number of Cycles | Half Decay Time of Charges t50 (s) |
| CO | 592.1 ± 8.8 | 7.9 ± 0.4 | 17.3 | 38,000 | 1 |
| DISP | 52.0 ± 10.2 | 39.0 ± 1.2 | 1.5 | 2000 | 20 |
| CH/A | 87.2 ± 9.2 | 59.0 ± 1.5 | 2.4 | 8000 | 16 |
| CH/B | 91.0 ± 10.3 | 65.0 ± 1.0 | 1.7 | 10,000 | 15 |
| PG | 274.5 ± 6.1 | 19.1 ± 1.1 | 5.3 | 40,000 1 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dowbysz, A.; Kukfisz, B.; Siuta, D.; Samsonowicz, M.; Maranda, A.; Kiciński, W.; Wróblewski, W. Analysis of the Flammability and the Mechanical and Electrostatic Discharge Properties of Selected Personal Protective Equipment Used in Oxygen-Enriched Atmosphere in a State of Epidemic Emergency. Int. J. Environ. Res. Public Health 2022, 19, 11453. https://doi.org/10.3390/ijerph191811453
Dowbysz A, Kukfisz B, Siuta D, Samsonowicz M, Maranda A, Kiciński W, Wróblewski W. Analysis of the Flammability and the Mechanical and Electrostatic Discharge Properties of Selected Personal Protective Equipment Used in Oxygen-Enriched Atmosphere in a State of Epidemic Emergency. International Journal of Environmental Research and Public Health. 2022; 19(18):11453. https://doi.org/10.3390/ijerph191811453
Chicago/Turabian StyleDowbysz, Adriana, Bożena Kukfisz, Dorota Siuta, Mariola Samsonowicz, Andrzej Maranda, Wojciech Kiciński, and Wojciech Wróblewski. 2022. "Analysis of the Flammability and the Mechanical and Electrostatic Discharge Properties of Selected Personal Protective Equipment Used in Oxygen-Enriched Atmosphere in a State of Epidemic Emergency" International Journal of Environmental Research and Public Health 19, no. 18: 11453. https://doi.org/10.3390/ijerph191811453
APA StyleDowbysz, A., Kukfisz, B., Siuta, D., Samsonowicz, M., Maranda, A., Kiciński, W., & Wróblewski, W. (2022). Analysis of the Flammability and the Mechanical and Electrostatic Discharge Properties of Selected Personal Protective Equipment Used in Oxygen-Enriched Atmosphere in a State of Epidemic Emergency. International Journal of Environmental Research and Public Health, 19(18), 11453. https://doi.org/10.3390/ijerph191811453







