Cardiopulmonary Exercise Testing Distinguishes between Post-COVID-19 as a Dysfunctional Syndrome and Organ Pathologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Enrollment
2.2. Cardiopulmonary Exercise Testing
2.3. Statistics
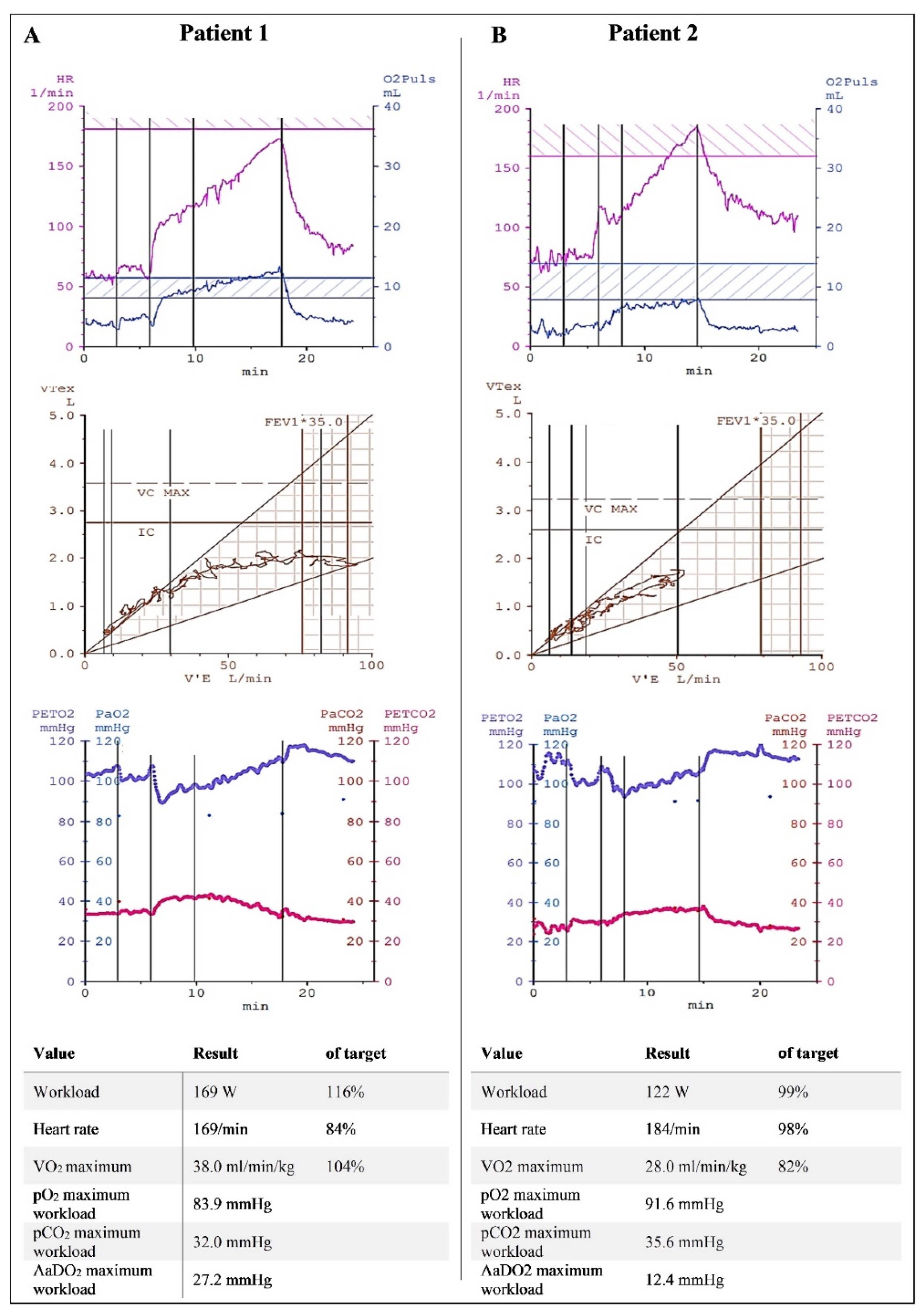
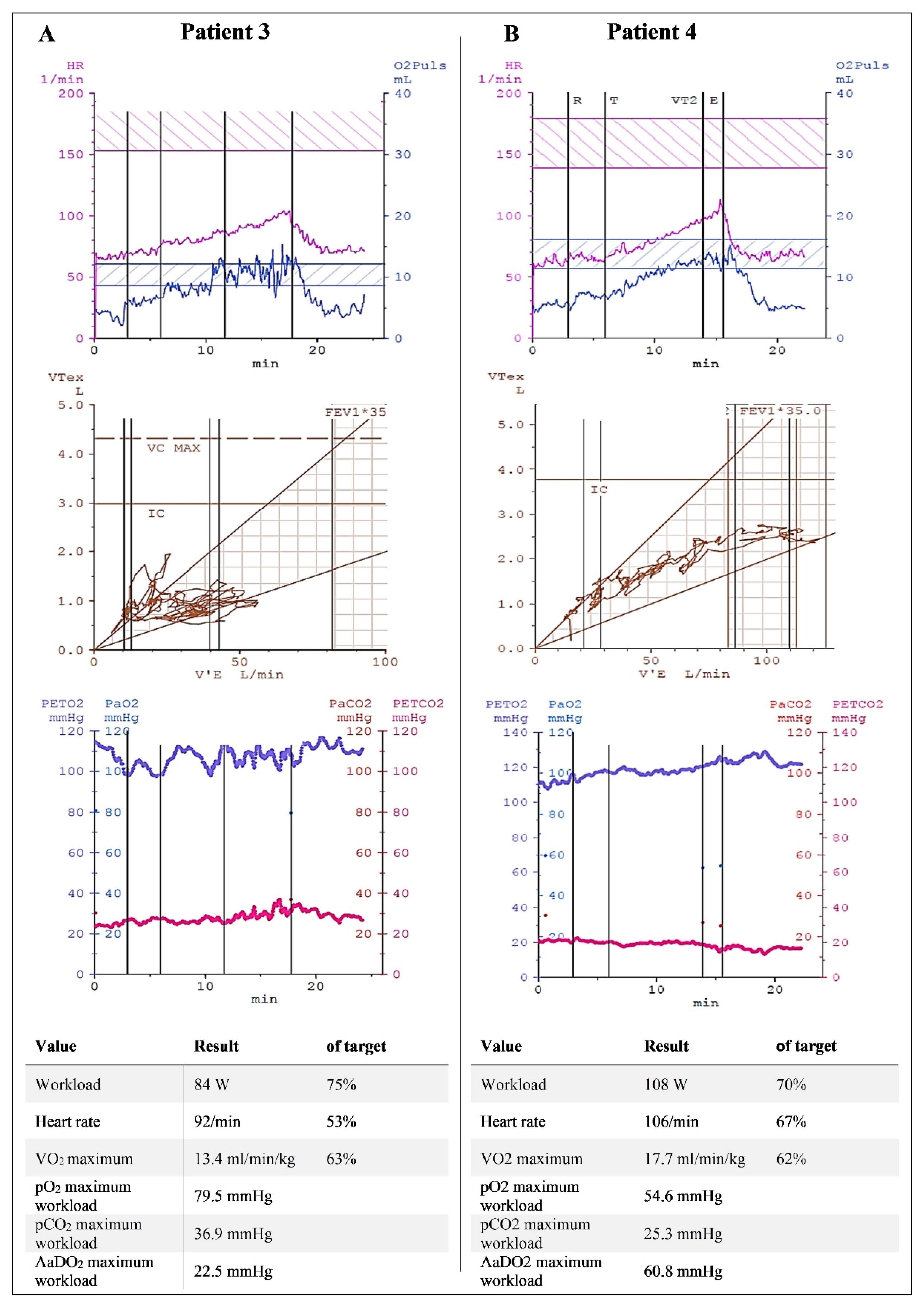
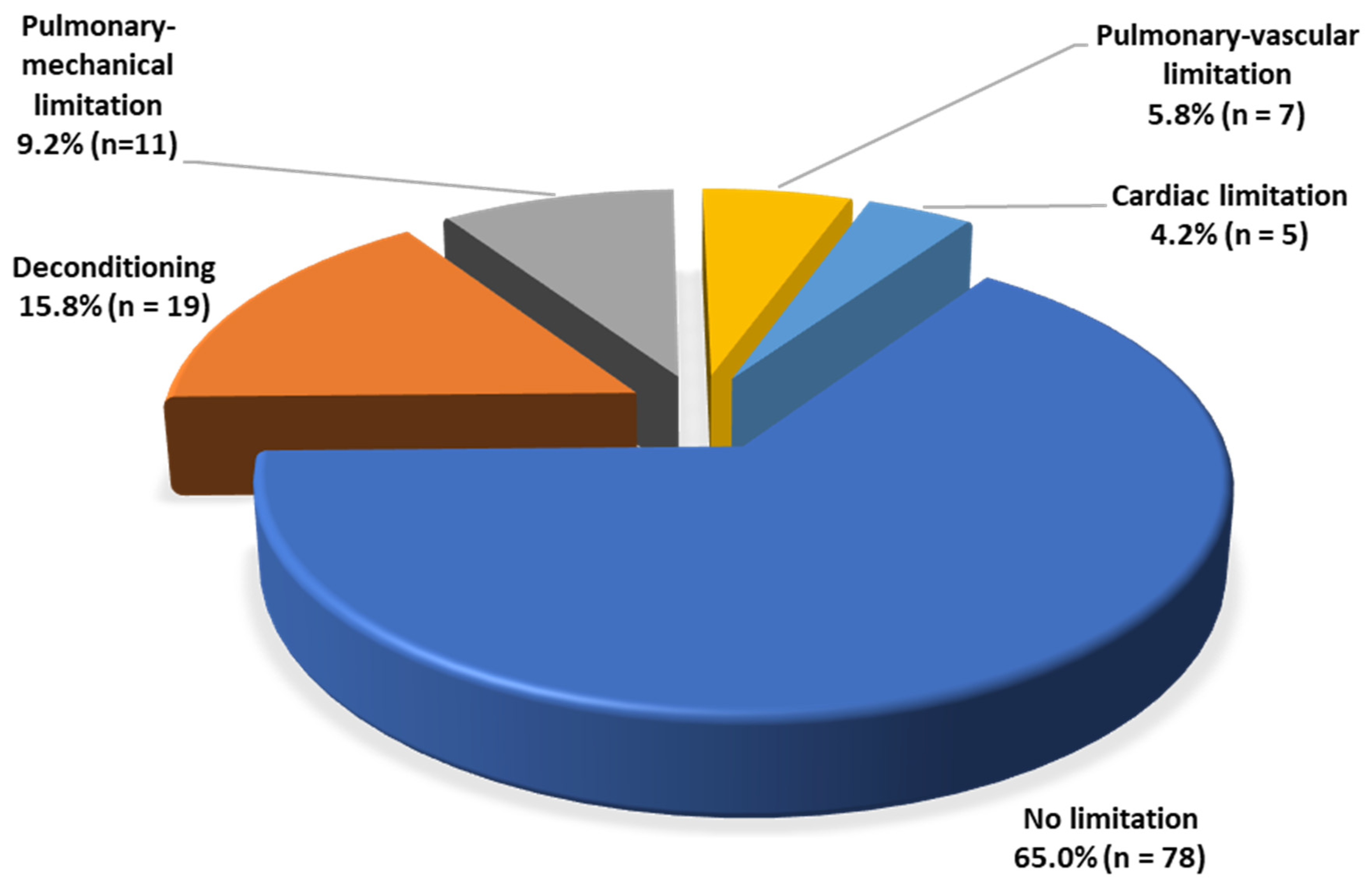
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Proal, A.D.; Van Elzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Kersten, J.; Baumhardt, M.; Hartveg, P.; Hoyo, L.; Hüll, E.; Imhof, A.; Kropf-Sanchen, C.; Nita, N.; Mörike, J.; Rattka, M.; et al. Long COVID: Distinction between Organ Damage and Deconditioning. J. Clin. Med. 2021, 10, 3782. [Google Scholar] [CrossRef]
- Arena, R.; Sietsema, K.E. Cardiopulmonary Exercise Testing in the Clinical Evaluation of Patients With Heart and Lung Disease. Circulation 2011, 123, 668–680. [Google Scholar] [CrossRef]
- Cassar, M.P.; Tunnicliffe, E.M.; Petousi, N.; Lewandowski, A.J.; Xie, C.; Mahmod, M.; Samat, A.H.A.; Evans, R.A.; Brightling, C.E.; Ho, L.-P.; et al. Symptom Persistence Despite Improvement in Cardiopulmonary Health—Insights from longitudinal CMR, CPET and lung function testing post-COVID-19. eClinicalMedicine 2021, 41, 101159. [Google Scholar] [CrossRef]
- Alba, G.A.; Ziehr, D.R.; Rouvina, J.N.; Hariri, L.P.; Knipe, R.S.; Medoff, B.D.; Hibbert, K.A.; Kowal, A.; Hoenstine, C.; Ginns, L.C.; et al. Exercise performance in patients with post-acute sequelae of SARS-CoV-2 infection compared to patients with unexplained dyspnea. eClinicalMedicine 2021, 39, 101066. [Google Scholar] [CrossRef]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to Cardiopulmonary Exercise Testing in Adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [Green Version]
- American Thoracic Society. ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Solberg, G.; Robstad, B.; Skjønsberg, O.H.; Borchsenius, F. Respiratory gas exchange indices for estimating the anaerobic threshold. J. Sports Sci. Med. 2005, 4, 29–36. [Google Scholar] [PubMed]
- Dickstein, K.; Barvik, S.; Aarsland, T.; Snapinn, S.; Millerhagen, J. Validation of a computerized technique for detection of the gas exchange anaerobic threshold in cardiac disease. Am. J. Cardiol. 1990, 66, 1363–1367. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Aupetit, J.F.; Gueret, P.; Kolsky, H.; Zannad, F. Can anaerobic threshold be used as an end-point for therapeutic trials in heart failure? Lessons from a multicentre randomized placebo-controlled trial. Eur. Heart J. 1994, 15, 236–241. [Google Scholar] [CrossRef]
- Yeh, M.P.; Gardner, R.M.; Adams, T.D.; Yanowitz, F.G.; Crapo, R.O. “Anaerobic threshold”: Problems of determination and validation. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 1178–1186. [Google Scholar] [CrossRef]
- Glaab, T.; Schmidt, O.; Fritsch, J. Spiroergometrie kompakt—Physiologie, Durchführung und Auswertung. Pneumologie 2020, 74, 88–102. [Google Scholar] [CrossRef]
- Datta, D.; Normandin, E.; ZuWallack, R. Cardiopulmonary exercise testing in the assessment of exertional dyspnea. Ann. Thorac. Med. 2015, 10, 77–86. [Google Scholar] [CrossRef]
- Mancini, D.M.; Brunjes, D.L.; Lala, A.; Trivieri, M.G.; Contreras, J.P.; Natelson, B.H. Use of Cardiopulmonary Stress Testing for Patients with Unexplained Dyspnea Post-Coronavirus Disease. JACC Heart Fail. 2021, 9, 927–937. [Google Scholar] [CrossRef]
- Ionescu, M.F.; Mani-Babu, S.; Degani-Costa, L.H.; Johnson, M.; Paramasivan, C.; Sylvester, K.; Fuld, J. Cardiopulmonary Exercise Testing in the Assessment of Dysfunctional Breathing. Front. Physiol. 2021, 11, 620955. [Google Scholar] [CrossRef]
- Kersten, J.; Wolf, A.; Hoyo, L.; Hüll, E.; Tadic, M.; Andreß, S.; D’Almeida, S.; Scharnbeck, D.; Roder, E.; Beschoner, P.; et al. Symptom burden correlates to impairment of diffusion capacity and exercise intolerance in long COVID patients. Sci. Rep. 2022, 12, 8801. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ortuno, R.; Jennings, G.; Xue, F.; Duggan, E.; Gormley, J.; Monaghan, A. Predictors of Submaximal Exercise Test Attainment in Adults Reporting Long COVID Symptoms. J. Clin. Med. 2022, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, R.F.; Mondoni, M.; Parazzini, E.M.; Pitari, F.; Brambilla, E.; Luraschi, S.; Balbi, M.; Papa, G.F.S.; Sotgiu, G.; Guazzi, M.; et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur. Respir. J. 2021, 58, 2100870. [Google Scholar] [CrossRef]
- Zaccagni, L.; Toselli, S.; Barbieri, D. Physical Activity during COVID-19 Lockdown in Italy: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6416. [Google Scholar] [CrossRef]
- Agergaard, J.; Leth, S.; Pedersen, T.H.; Harbo, T.; Blicher, J.U.; Karlsson, P.; Østergaard, L.; Andersen, H.; Tankisi, H. Myopathic changes in patients with long-term fatigue after COVID-19. Clin. Neurophysiol. 2021, 132, 1974–1981. [Google Scholar] [CrossRef]
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Rudroff, T.; Workman, C.D.; Ponto, L.L.B. 18F-FDG-PET Imaging for Post-COVID-19 Brain and Skeletal Muscle Alterations. Viruses 2021, 13, 2283. [Google Scholar] [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Pediatr. 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Shan, Z.Y.; Barnden, L.R.; Kwiatek, R.A.; Bhuta, S.; Hermens, D.F.; Lagopoulos, J. Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A systematic review. J. Transl. Med. 2020, 18, 335. [Google Scholar] [CrossRef]
- Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K.; Harmon, K.G.; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar] [CrossRef] [PubMed]
- Cattadori, G.; Di Marco, S.; Baravelli, M.; Picozzi, A.; Ambrosio, G. Exercise Training in Post-COVID-19 Patients: The Need for a Multifactorial Protocol for a Multifactorial Pathophysiology. J. Clin. Med. 2022, 11, 2228. [Google Scholar] [CrossRef] [PubMed]
- Kortianou, E.A.; Mavronasou, A.S.; Sapouna, V. Practicalities for Exercise Prescription in Long-COVID-19 Rehabilitation. A Narrative Review. Med Res. Arch. 2022, 10. [Google Scholar] [CrossRef]
- Everaerts, S.; Heyns, A.; Langer, D.; Beyens, H.; Hermans, G.; Troosters, T.; Gosselink, R.; Lorent, N.; Janssens, W. COVID-19 recovery: Benefits of multidisciplinary respiratory rehabilitation. BMJ Open Respir. Res. 2021, 8, e000837. [Google Scholar] [CrossRef] [PubMed]
- White, P.D.; Goldsmith, K.A.; Johnson, A.L.; Potts, L.; Walwyn, R.; DeCesare, J.C.; Baber, H.L.; Burgess, M.; Clark, L.V.; Cox, D.L.; et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomised trial. Lancet 2011, 377, 823–836. [Google Scholar] [CrossRef]
- Larun, L.; Brurberg, K.G.; Odgaard-Jensen, J.; Price, J.R. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst. Rev. 2019, 10, CD003200. [Google Scholar] [CrossRef]
- Thomas, M.; McKinley, R.; Freeman, E.; Foy, C.; Prodger, P.; Price, D. Breathing retraining for dysfunctional breathing in asthma: A randomised controlled trial. Thorax 2003, 58, 110–115. [Google Scholar] [CrossRef]
- Buonsenso, D.; Di Giuda, D.; Sigfrid, L.; Pizzuto, D.A.; Di Sante, G.; De Rose, C.; Lazzareschi, I.; Sali, M.; Baldi, F.; Chieffo, D.P.R.; et al. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child Adolesc. Health 2021, 5, 677–680. [Google Scholar] [CrossRef]

| Characteristic | Value |
|---|---|
| Age (years), mean ± SD | 49.7 ± 15.2 |
| Women, n (%) | 74 (61.7) |
| Body mass index (kg/m2), mean ± SD | 25.4 ± 4.3 |
| Patient history, n (%) | |
| Cardiac diseases | 10 (8.3) |
| Pulmonary diseases | 15 (12.5) (asthma bronchial, 14 (11.7)) |
| Malignant diseases | 2 (1.7) |
| COVID-19 history, n (%) | |
| Oligosymptomatic/asymptomatic course | 10 (8.3) |
| Hospitalization | 19 (15.8) |
| Invasive ventilation | 6 (5.0) |
| Therapy with corticosteroids | 12 (10.0) |
| Therapy with antibiotics | 14 (11.7) |
| Cardiovascular risk profile, n (%) | |
| Arterial hypertension | 30 (25.0) |
| Diabetes mellitus type I | 2 (1.7) |
| Diabetes mellitus type II | 6 (5.0) |
| Dyslipidemia | 68 (56.7) |
| Current/past smoking | 29 (24.2) |
| Post-COVID-19 symptoms, n (%) | |
| Thoracic pain/pressure | 32 (26.7) |
| Dyspnea | 82 (68.3) |
| Anosmia/ageusia | 10 (8.3) |
| Headaches | 10 (8.3) |
| Sleep disorders | 16 (13.4) |
| Exhaustion/fatigue | 71 (59.2) |
| Memory and concentration disorders | 41 (34.5) |
| Measurement | Value | Predicted/Norm * |
|---|---|---|
| Workload (W) | 144 ± 50 | 132 ± 46 |
| Workload per bodyweight (W/kg) | 1.9 ± 0.6 | 1.8 ± 0.6 |
| Heart rate (min−1) | 147 ± 25 | 170 ± 15 |
| Expiratory volume (L/min) | 77.0 ± 22.7 | 91.5 ± 16.7 |
| Peak VO2 (mL/min/kg) | 24.6 ± 7.1 | 26.2 ± 7.5 |
| VO2AT (mL/min/kg) | 17.3 ± 5.7 | 10.5 ± 3.0 |
| VE/VCO2 | 33.1 ± 6.2 | <35 |
| VO2 pulse (mL/beat) | 12.8 ± 3.7 | ≥9 |
| VO2 work rate (mL/W) | 13.1 ± 1.5 | ≥10 |
| pO2 baseline (mmHg) | 77.2 ± 7.8 | |
| pO2 maximum workload (mmHg) | 81.9 ± 10.4 | |
| pCO2 baseline (mmHg) | 36.0 ± 3.5 | |
| pCO2 maximum workload (mmHg) | 33.8 ± 3.9 | |
| AaDO2 baseline (mmHg) | 16.3 ± 8.1 | |
| AaDO2 maximum workload (mmHg) | 24.1 ± 10.0 | |
| Maximum lactate (mmol/L) | 7.3 ± 2.6 |
| Characteristic/Measurement | Hospitalized (n = 19) | Non-Hospitalized (n = 91) | Oligo-/Asymptomatic Course (n = 10) | p-Value |
|---|---|---|---|---|
| Workload (W) | 144 ± 36 | 141 ± 47 | 178 ± 78 | 0.074 |
| Workload per bodyweight (W/kg) | 1.7 ± 0.5 | 1.9 ± 0.6 | 2.2 ± 0.8 | 0.104 |
| Heart rate (min−1) | 134 ± 28 | 150 ± 25 | 149 ± 20 | 0.053 |
| Expiratory volume (L/min) | 80.4 ± 21.1 | 75.7 ± 23.1 | 82.2 ± 23.1 | 0.538 |
| Peak VO2 (mL/min/kg) | 20.8 ± 5.4 | 25.0 ± 6.9 | 27.8 ± 9.4 | 0.022 |
| VO2AT (mL/min/kg) | 16.3 ± 3.6 | 17.1 ± 5.8 | 20.8 ± 7.2 | 0.109 |
| VE/VCO2 | 33.8 ± 8.7 | 33.1 ± 5.7 | 31.1 ± 5.2 | 0.511 |
| VO2 pulse (mL/beat) | 13.9 ± 3.4 | 12.3 ± 3.4 | 15.0 ± 5.4 | 0.034 |
| VO2 work rate (mL/W) | 12.7 ± 1.2 | 13.2 ± 1.5 | 12.8 ± 1.2 | 0.297 |
| pO2 baseline (mmHg) | 72.4 ± 8.3 | 78.1 ± 7.7 | 76.6 ± 6.7 | 0.057 |
| pO2 maximum workload (mmHg) | 74.2 ± 12.6 | 84.0 ± 9.1 | 77.3 ± 9.0 | <0.001 |
| pCO2 baseline (mmHg) | 36.2 ± 3.6 | 35.8 ± 3.6 | 37.1 ± 2.9 | 0.529 |
| pCO2 maximum workload (mmHg) | 35.8 ± 5.1 | 33.2 ± 3.4 | 35.7 ± 4.2 | 0.007 |
| AaDO2 baseline (mmHg) | 20.6 ± 7.8 | 15.4 ± 7.9 | 15.6 ± 8.1 | 0.037 |
| AaDO2 maximum workload (mmHg) | 30.1 ± 13.3 | 22.6 ± 9.0 | 26.1 ± 7.3 | 0.008 |
| Maximum lactate (mmol/L) | 7.6 ± 2.9 | 7.3 ± 2.6 | 7.1 ± 1.9 | 0.861 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kersten, J.; Hoyo, L.; Wolf, A.; Hüll, E.; Nunn, S.; Tadic, M.; Scharnbeck, D.; Rottbauer, W.; Buckert, D. Cardiopulmonary Exercise Testing Distinguishes between Post-COVID-19 as a Dysfunctional Syndrome and Organ Pathologies. Int. J. Environ. Res. Public Health 2022, 19, 11421. https://doi.org/10.3390/ijerph191811421
Kersten J, Hoyo L, Wolf A, Hüll E, Nunn S, Tadic M, Scharnbeck D, Rottbauer W, Buckert D. Cardiopulmonary Exercise Testing Distinguishes between Post-COVID-19 as a Dysfunctional Syndrome and Organ Pathologies. International Journal of Environmental Research and Public Health. 2022; 19(18):11421. https://doi.org/10.3390/ijerph191811421
Chicago/Turabian StyleKersten, Johannes, Luis Hoyo, Alexander Wolf, Elina Hüll, Samuel Nunn, Marijana Tadic, Dominik Scharnbeck, Wolfgang Rottbauer, and Dominik Buckert. 2022. "Cardiopulmonary Exercise Testing Distinguishes between Post-COVID-19 as a Dysfunctional Syndrome and Organ Pathologies" International Journal of Environmental Research and Public Health 19, no. 18: 11421. https://doi.org/10.3390/ijerph191811421
APA StyleKersten, J., Hoyo, L., Wolf, A., Hüll, E., Nunn, S., Tadic, M., Scharnbeck, D., Rottbauer, W., & Buckert, D. (2022). Cardiopulmonary Exercise Testing Distinguishes between Post-COVID-19 as a Dysfunctional Syndrome and Organ Pathologies. International Journal of Environmental Research and Public Health, 19(18), 11421. https://doi.org/10.3390/ijerph191811421







