Study on the Changes in Immobilized Petroleum–Degrading Bacteria Beads in a Continuous Bioreactor Related to Physicochemical Performance, Degradation Ability, and Microbial Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Immobilized Bacteria
2.2. Bioreactor Set-Up and Experimental Design
2.3. Analysis Method
2.4. Statistical Analysis
3. Results and Discussion
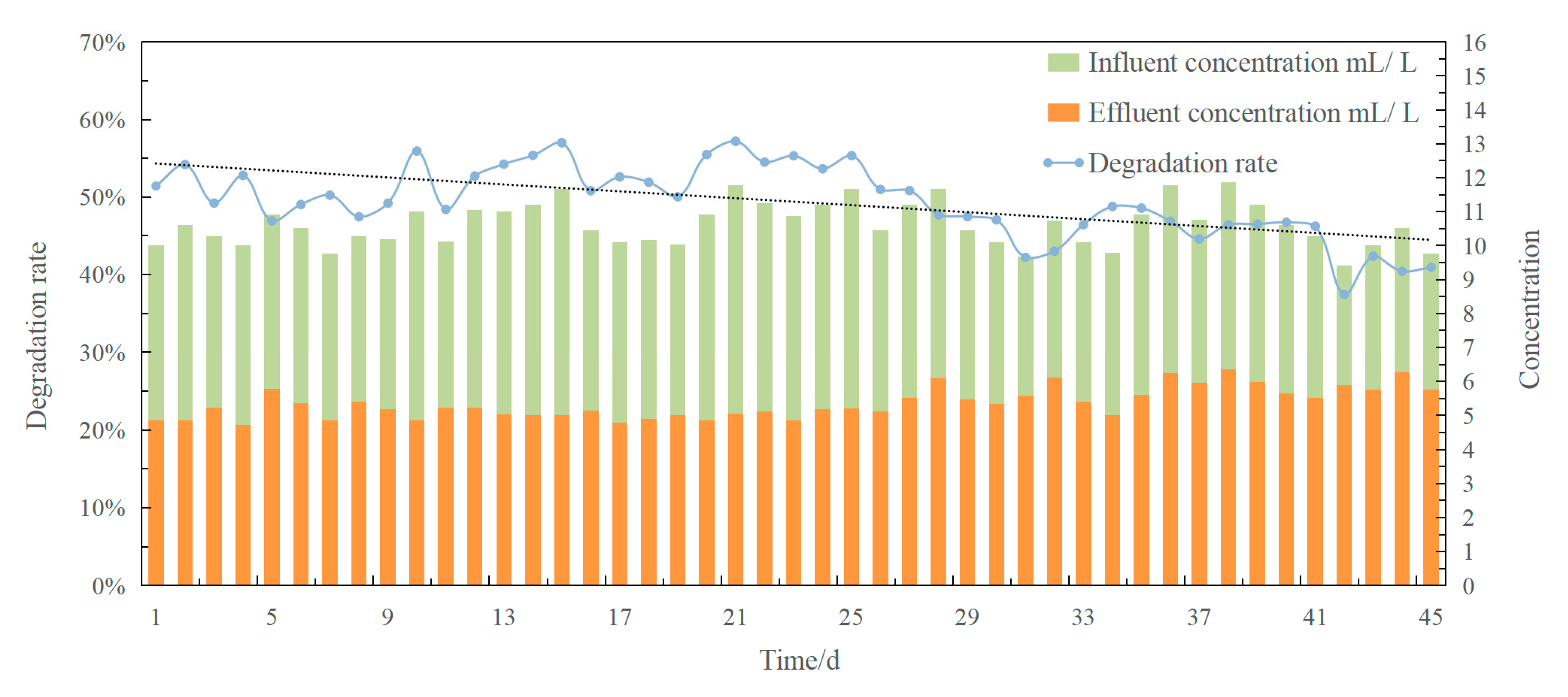
3.1. Diesel Degradation Rate in a Bioreactor under Continuous Operation
3.2. Physicochemical Analysis of Immobilized Petroleum–Degrading Bacteria Beads under Continuous Operation
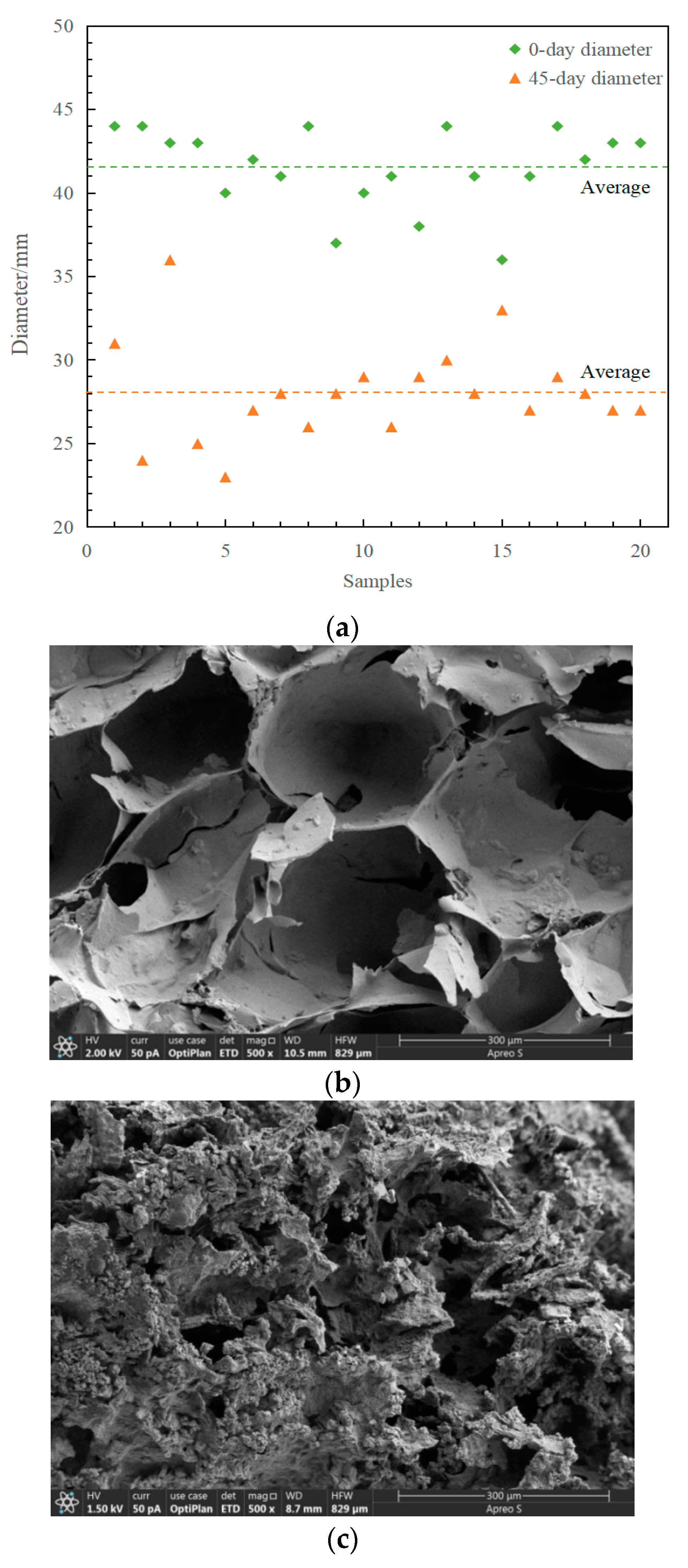
3.2.1. Morphological Analysis of Immobilized Petroleum–Degrading Bacteria Beads
3.2.2. Group Types and Number of Immobilized Petroleum–Degrading Bacteria Beads
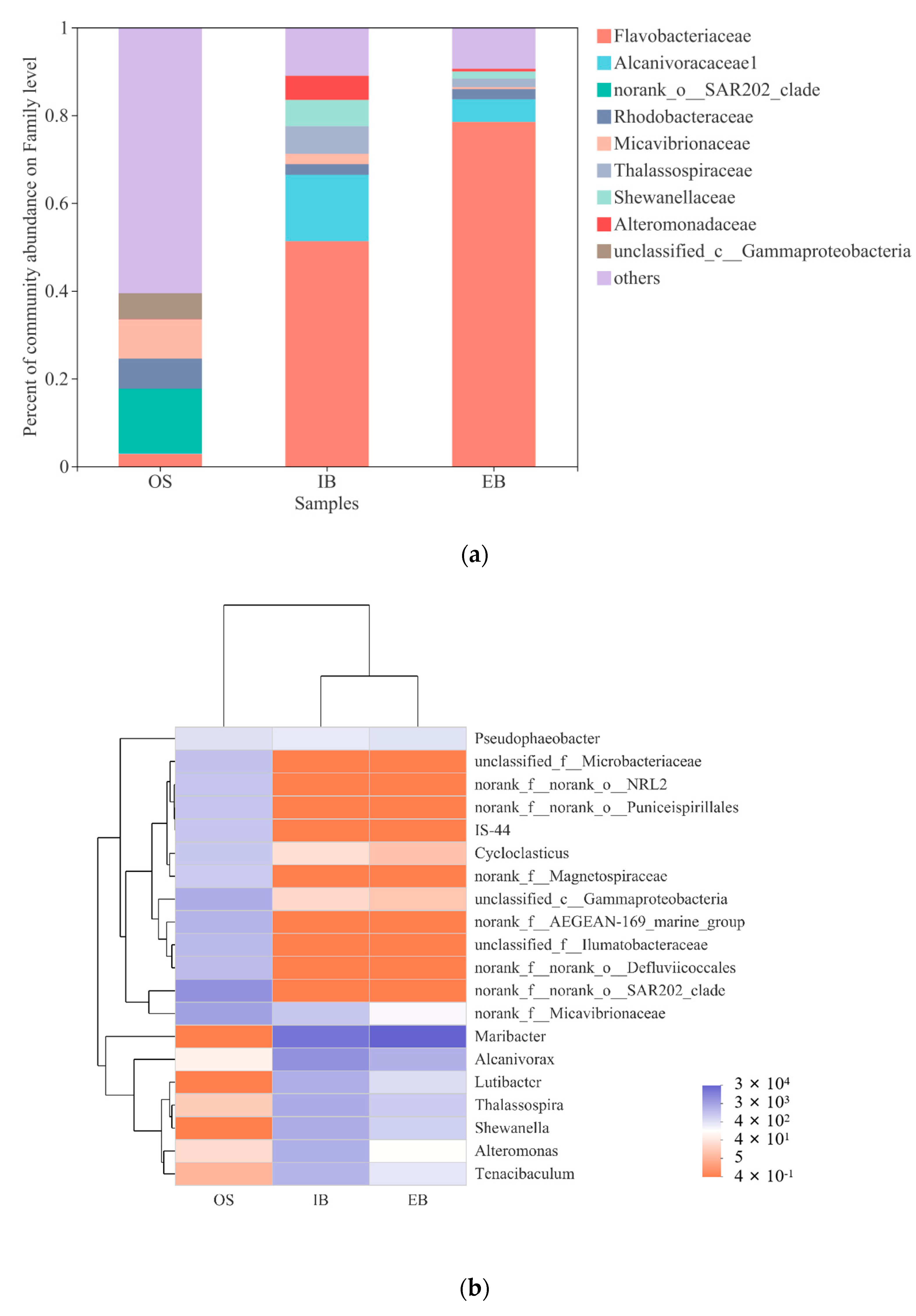
3.3. Community Structure of Immobilized Petroleum–Degrading Bacteria Beads in the Bioreactor
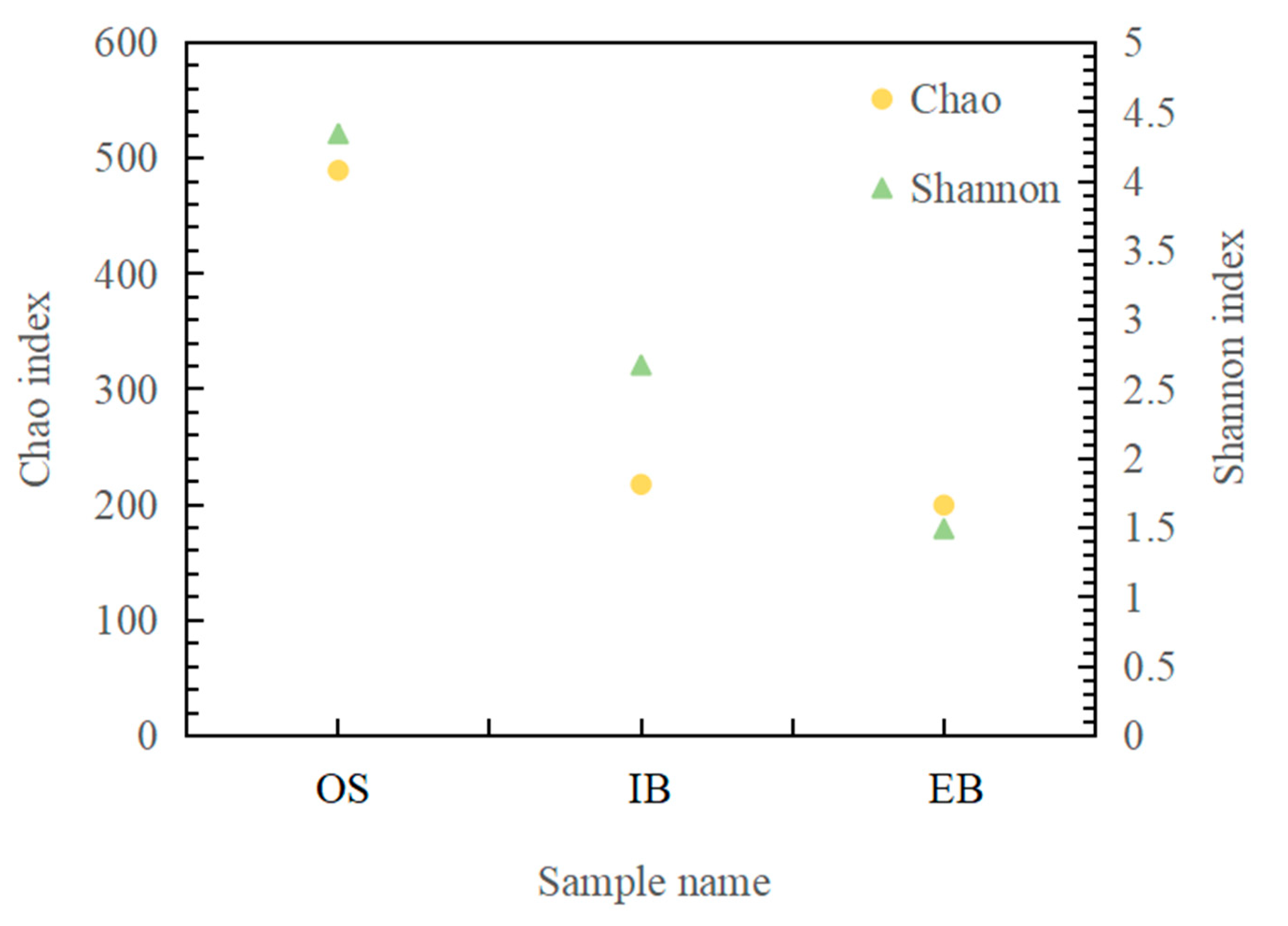
3.3.1. Biological Diversity
3.3.2. Community Structure
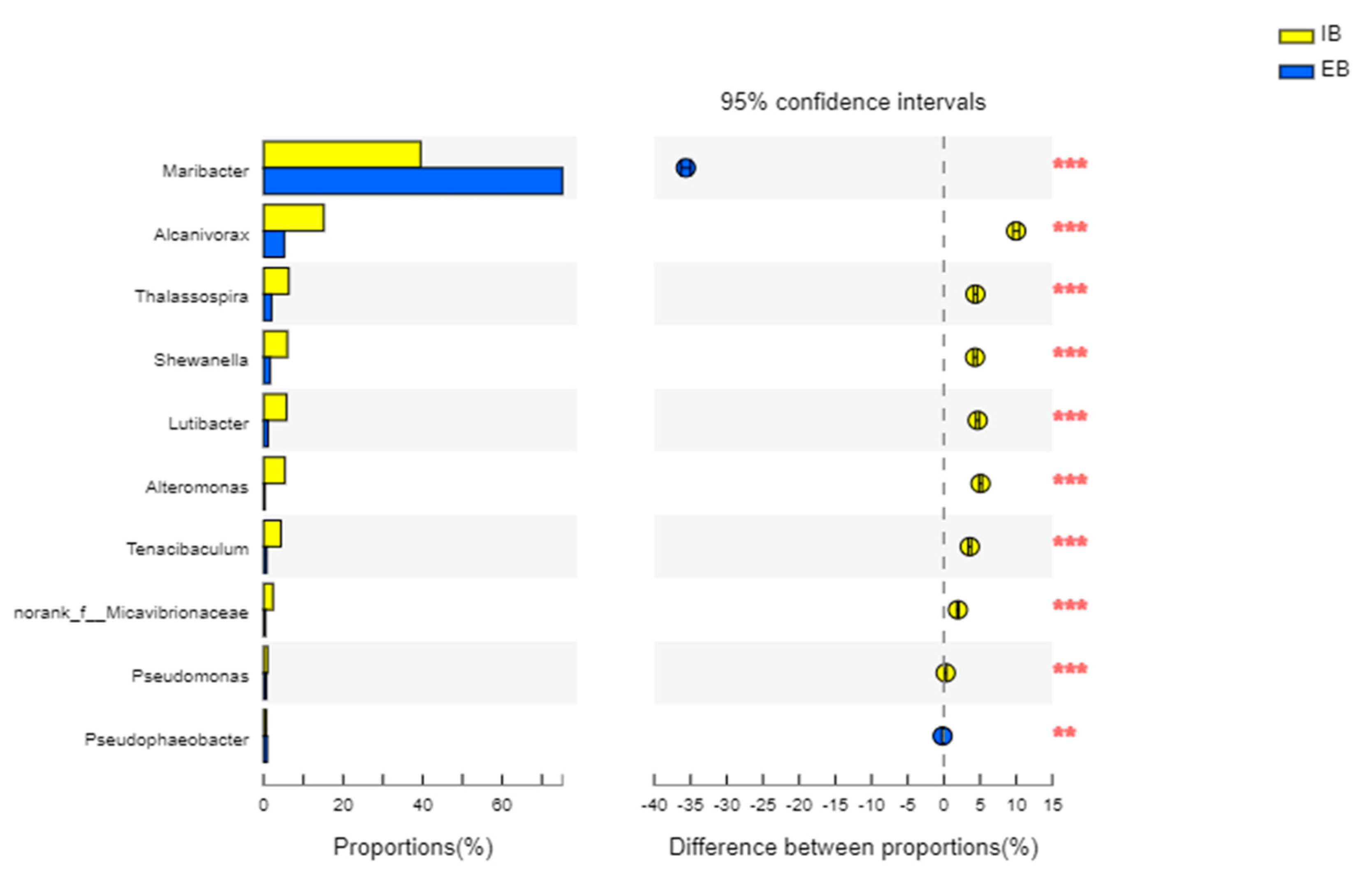
3.3.3. The Main Difference in the Bacteria between Immobilized Petroleum–Degrading Bacteria Beads
3.3.4. COG Functional Classification Statistics
3.4. Analysis of the Change in the Immobilized Petroleum–Degrading Bacteria in the Bioreactor
4. Conclusions
- (1)
- The bioreactor could maintain a degradation rate above 50% during earlier weeks, indicating that renewal of the immobilized petroleum bacteria beads over time would be beneficial for bioreactor performance;
- (2)
- Bacterial migration led to more kinds of petroleum–degrading bacteria, such as Alcanivorax, Shewanella, and Thalassospira, and so on, becoming part of the community structure in the immobilized petroleum–degrading bacteria beads; however, the total abundance of petroleum–degrading bacteria decreased significantly;
- (3)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, S.M.C.; Bautista, M.A.; Cramm, M.A.; Hubert, C.R.J. Diesel and Crude Oil Biodegradation by Cold-Adapted Microbial Communities in the Labrador Sea. Appl. Environ. Microbiol. 2021, 87, e0080021. [Google Scholar] [CrossRef] [PubMed]
- Scoma, A.; Yakimov, M.M.; Daffonchio, D.; Boon, N. Self-healing capacity of deep-sea ecosystems affected by petroleum hydrocarbons: Understanding microbial oil degradation at hydrocarbon seeps is key to sustainable bioremediation protocols. EMBO Rep. 2017, 18, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Galitskaya, P.; Biktasheva, L.; Blagodatsky, S.; Selivanovskaya, S. Response of bacterial and fungal communities to high petroleum pollution in different soils. Sci. Rep. 2021, 11, 164. [Google Scholar] [CrossRef]
- Hazaimeh, M.D.; Ahmed, E.S. Bioremediation perspectives and progress in petroleum pollution in the marine environment: A review. Environ. Sci. Pollut. Res. 2021, 28, 54238–54259. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, S.; Paul, J.H.; Joye, S.B. Using dispersants after oil spills: Impacts on the composition and activity of microbial communities. Nat. Rev. Genet. 2015, 13, 388–396. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, W.; Wang, Y.; Guo, J.; Zhang, H.; Hu, X. Nutrient depletion is the main limiting factor in the crude oil bioaugmentation process. J. Environ. Sci. 2021, 100, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, H.; Bai, Y.; Xue, J.; Gao, Y.; Hu, S.; Wu, T.; Sun, J. Systematic degradation mechanism and pathways analysis of the immobilized bacteria: Permeability and biodegradation, kinetic and molecular simulation. Environ. Sci. Ecotechnol. 2020, 2, 100028. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, M.; Qian, L. Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J. Soils Sediments 2012, 12, 1350–1359. [Google Scholar] [CrossRef]
- Guo, S.; Liu, X.; Tang, J. Enhanced degradation of petroleum hydrocarbons by immobilizing multiple bacteria on wheat bran biochar and its effect on greenhouse gas emission in saline-alkali soil. Chemosphere 2022, 286 Pt 2, 131663. [Google Scholar] [CrossRef]
- Li, Y.; Gong, H.; Cheng, H.; Wang, L.; Bao, M. Individually immobilized and surface-modified hydrocarbon-degrading bacteria for oil emulsification and biodegradation. Mar. Pollut. Bull. 2017, 125, 433–439. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, M. Bioremediation of crude oil-contaminated soil: Comparison of different biostimulation and bioaugmentation treatments. J. Hazard. Mater. 2010, 183, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Roy, A.; Pal, S.; Mohapatra, B.; Kazy, S.K.; Maiti, M.K.; Sar, P. Enrichment and characterization of hydrocarbon-degrading bacteria from petroleum refinery waste as potent bioaugmentation agent for in situ bioremediation. Bioresour. Technol. 2017, 242, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wu, T.; Li, H.; Xue, J.; Sun, J.; Li, L.; Qiao, Y.; Li, C. Study on the preparation conditions and degradation performance of an efficient immobilized microbial agent for marine oil pollution. Environ. Technol. 2021, 43, 2352–2358. [Google Scholar] [CrossRef]
- Bayat, Z.; Hassanshahian, M.; Cappello, S. Immobilization of Microbes for Bioremediation of Crude Oil Polluted Environments: A Mini Review. Open Microbiol. J. 2015, 9, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Shen, X.; Luo, Q.; He, Y.; Wang, Q.; Liu, Q. Enhancement of the diesel oil degradation ability of a marine bacterial strain by immobilization on a novel compound carrier material. Mar. Pollut. Bull. 2013, 67, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, J.; Wang, L.; Liu, J.; Gurav, R.G.; Sun, K. A novel bioremediation strategy for petroleum hydrocarbon pollutants using salt tolerant Corynebacterium variabile HRJ4 and biochar. J. Environ. Sci. 2016, 47, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, Y.; Shi, K.; Qiao, Y.; Cheng, D.; Bai, Y.; Shen, C.; Jiang, Q. Responses of seawater bacteria in the bioremediation process of petroleum contamination by immobilized bacteria. J. Environ. Chem. Eng. 2022, 10, 107133. [Google Scholar] [CrossRef]
- Bordenave, S.; Goñi-Urriza, M.S.; Caumette, P.; Duran, R. Effects of Heavy Fuel Oil on the Bacterial Community Structure of a Pristine Microbial Mat. Appl. Environ. Microbiol. 2007, 73, 6089–6097. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, M.; Zhang, R.; Zhu, W.; Mao, S. Comparative studies of the composition of bacterial microbiota associated with the ruminal content, ruminal epithelium and in the faeces of lactating dairy cows. Microb. Biotechnol. 2016, 9, 257–268. [Google Scholar] [CrossRef]
- Sirichoat, A.; Lulitanond, V.; Faksri, K. Analysis of bacterial and fungal communities in fermented fish (pla-ra) from Northeast Thailand. Arch. Microbiol. 2022, 204, 302. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Fanesi, A.; Wagner, H.; Birarda, G.; Vaccari, L.; Wilhelm, C. Quantitative macromolecular patterns in phytoplankton communities resolved at the taxonomical level by single-cell Synchrotron FTIR-spectroscopy. BMC Plant Biol. 2019, 19, 142. [Google Scholar] [CrossRef] [PubMed]
- Fanesi, A.; Wagner, H.; Wilhelm, C. Phytoplankton growth rate modelling: Can spectroscopic cell chemotyping be superior to physiological predictors? Proc. R. Soc. B Biol. Sci. 2017, 284, 20161956. [Google Scholar] [CrossRef]
- Filip, Z.; Hermann, S. An attempt to differentiate Pseudomonas spp. and other soil bacteria by FT-IR spectroscopy. Eur. J. Soil Biol. 2001, 37, 137–143. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Hou, L.; Wu, P. Exploring the hydrogen-bond structures in sodium alginate through two-dimensional correlation infrared spectroscopy. Carbohydr. Polym. 2019, 205, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shao, Z. The long-chain alkane metabolism network of Alcanivorax dieselolei. Nat. Commun. 2014, 5, 5755. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, D.; Vega-Alvarado, L.; Taboada, B.; Estradas-Romero, A.; Soto, L.; Juárez, K. Bacterial diversity in surface sediments from the continental shelf and slope of the North West gulf of Mexico and the presence of hydrocarbon degrading bacteria. Mar. Pollut. Bull. 2020, 150, 110590. [Google Scholar] [CrossRef] [PubMed]
- Bôto, M.L.; Magalhães, C.; Perdigão, R.; Alexandrino, D.A.M.; Fernandes, J.P.; Bernabeu, A.M.; Ramos, S.; Carvalho, M.F.; Semedo, M.; LaRoche, J.; et al. Harnessing the Potential of Native Microbial Communities for Bioremediation of Oil Spills in the Iberian Peninsula NW Coast. Front. Microbiol. 2021, 12, 633659. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, J.; Wu, Y.; Li, M.; Sun, X.; Cui, H.; Cheng, L.; Gao, Y.; Xiao, X. Preliminary Analysis of Diesel-Degrading Bacteria Immobilized on Organic Carriers in Seawater. China Pet. Process. Petrochem. Technol. 2017, 19, 33–39. [Google Scholar]
- Li, J.; Liu, G.; Yin, L.; Xue, J.; Qi, H.; Li, Y. Distribution characteristics of polycyclic aromatic hydrocarbons in sediments and biota from the Zha Long Wetland, China. Environ. Monit. Assess. 2013, 185, 3163–3171. [Google Scholar] [CrossRef]
- Reyes-Sosa, M.B.; Apodaca-Hernández, J.E.; Arena-Ortiz, M.L. Bioprospecting for microbes with potential hydrocarbon remediation activity on the northwest coast of the Yucatan Peninsula, Mexico, using DNA sequencing. Sci. Total Environ. 2018, 642, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhong, R.; Shan, D.; Shao, Z. Indigenous oil-degrading bacteria in crude oil-contaminated seawater of the Yellow sea, China. Appl. Microbiol. Biotechnol. 2014, 98, 7253–7269. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yin, X.; Mi, T.; Zhang, Y.; Lin, F.; Han, B.; Zhao, X.; Luan, X.; Cui, Z.; Zheng, L. Microbial diversity and ecotoxicity of sediments 3 years after the Jiaozhou Bay oil spill. AMB Express 2018, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.J. The family Flavobacteriaceae. In The Prokaryotes: Other Major Lineages of Bacteria and the Archaea, 4th ed.; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg Germany, 2014; pp. 643–676. [Google Scholar] [CrossRef]
- Xie, X.; He, Z.; Hu, X.; Yin, H.; Liu, X.; Yang, Y. Large-scale seaweed cultivation diverges water and sediment microbial communities in the coast of Nan’ao Island, South China Sea. Sci. Total Environ. 2017, 598, 97–108. [Google Scholar] [CrossRef] [PubMed]

| Sample | Name | Details |
|---|---|---|
| Original seawater | OS | Microbial sample of original seawater |
| Influent immobilized beads | IB | Microbial sample of 45-day immobilized petroleum–degrading bacteria beads in the influent site of the bioreactor |
| Effluent immobilized beads | EB | Microbial sample of 45-day immobilized petroleum–degrading bacteria beads in the effluent site of the bioreactor |
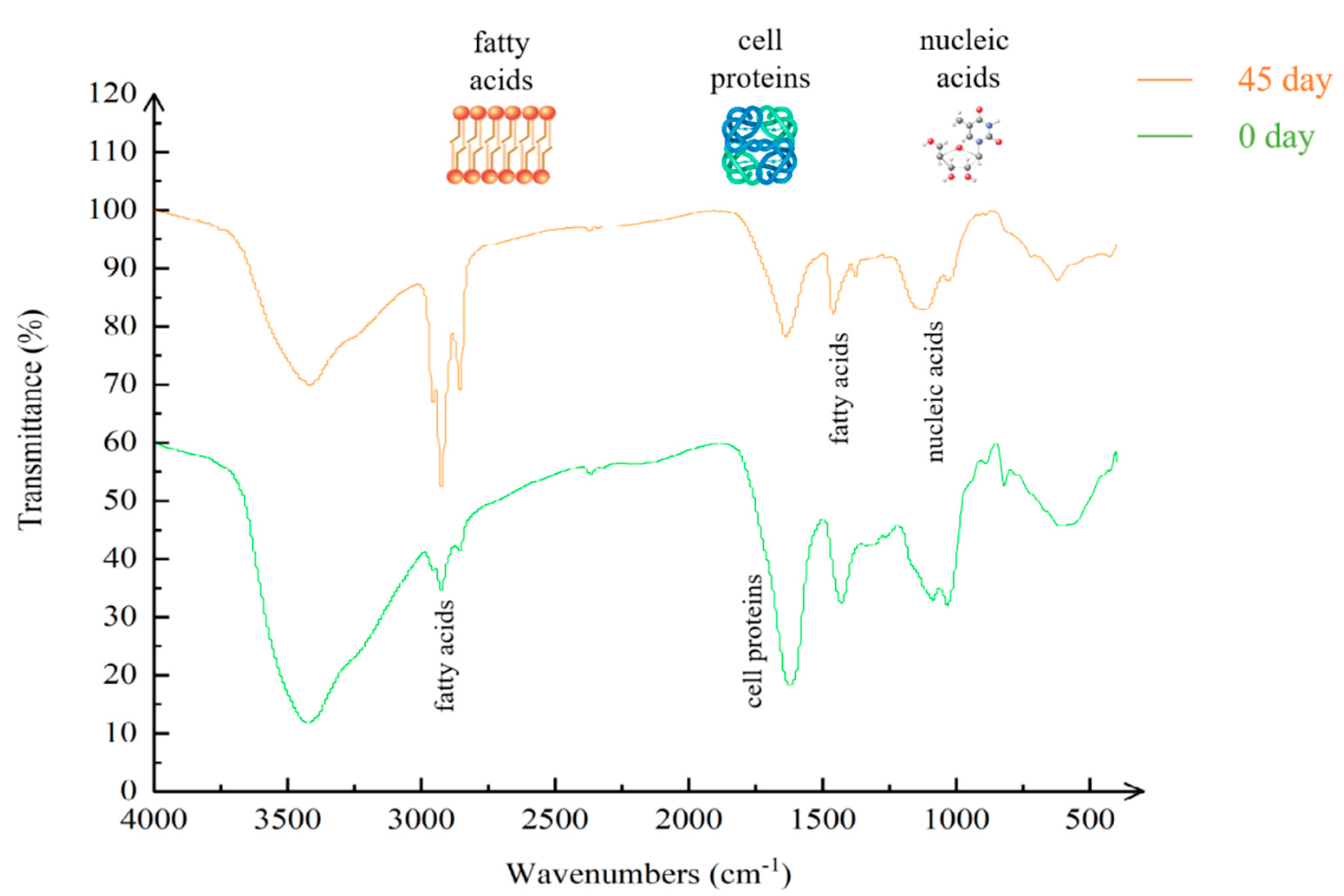
| Bond (cm−1) | Related Structures |
|---|---|
| 2800–3000 | Cell membrane fatty acids |
| 1500–1800 | Cell proteins |
| 1400–1500 | Fatty acids |
| 900–1200 | Glycopeptides and phosphate groups of nucleic acid constituents |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, W.; Qiao, Y.; Yu, F.; Wang, B.; Xue, J.; Wang, M.; Jiang, Q.; Zhou, Z. Study on the Changes in Immobilized Petroleum–Degrading Bacteria Beads in a Continuous Bioreactor Related to Physicochemical Performance, Degradation Ability, and Microbial Community. Int. J. Environ. Res. Public Health 2022, 19, 11348. https://doi.org/10.3390/ijerph191811348
Liu Y, Li W, Qiao Y, Yu F, Wang B, Xue J, Wang M, Jiang Q, Zhou Z. Study on the Changes in Immobilized Petroleum–Degrading Bacteria Beads in a Continuous Bioreactor Related to Physicochemical Performance, Degradation Ability, and Microbial Community. International Journal of Environmental Research and Public Health. 2022; 19(18):11348. https://doi.org/10.3390/ijerph191811348
Chicago/Turabian StyleLiu, Yixuan, Weisi Li, Yanlu Qiao, Fangying Yu, Bowen Wang, Jianliang Xue, Mianmian Wang, Qing Jiang, and Zhibin Zhou. 2022. "Study on the Changes in Immobilized Petroleum–Degrading Bacteria Beads in a Continuous Bioreactor Related to Physicochemical Performance, Degradation Ability, and Microbial Community" International Journal of Environmental Research and Public Health 19, no. 18: 11348. https://doi.org/10.3390/ijerph191811348
APA StyleLiu, Y., Li, W., Qiao, Y., Yu, F., Wang, B., Xue, J., Wang, M., Jiang, Q., & Zhou, Z. (2022). Study on the Changes in Immobilized Petroleum–Degrading Bacteria Beads in a Continuous Bioreactor Related to Physicochemical Performance, Degradation Ability, and Microbial Community. International Journal of Environmental Research and Public Health, 19(18), 11348. https://doi.org/10.3390/ijerph191811348






