Validation of the Norma Latina Neuropsychological Assessment Battery in Patients with Alzheimer’s Disease in Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.3. Procedure
2.4. Data Analyses
3. Results
4. Discussion
4.1. Implications
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samadi, M.; Moradi, S.; Moradinazar, M.; Mostafai, R.; Pasdar, Y. Dietary Pattern in Relation to the Risk of Alzheimer’s Disease: A Systematic Review. Neurol. Sci. 2019, 40, 2031–2043. [Google Scholar] [CrossRef]
- World Health Organization. World Failing to Address Dementia Challenge. Available online: https://www.who.int/es/news/item/02-09-2021-world-failing-to-address-dementia-challenge (accessed on 22 February 2022).
- World Health Organization. The Epidemiology and Impact of Dementia: Current Stage and Future Trends; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global Prevalence of Dementia: A Delphi Consensus Study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2019 Alzheimer’s Disease Facts and Figures. Alzheimer’s Amp Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Ibáñez, A.; Parra, M.A.; Butler, C. The Latin America and the Caribbean Consortium on Dementia (LAC-CD): From Networking to Research to Implementation Science. J. Alzheimer’s Dis. 2021, 82, S379–S394. [Google Scholar] [CrossRef]
- Custodio, N.; Wheelock, A.; Thumala, D.; Slachevsky, A. Dementia in Latin America: Epidemiological Evidence and Implications for Public Policy. Front. Aging Neurosci. 2017, 9, 221. [Google Scholar] [CrossRef]
- Ibáñez, A.; Sedeño, L.; García, A.M.; Deacon, R.M.J.; Cogram, P. Human and Animal Models for Translational Research on Neurodegeneration: Challenges and Opportunities From South America. Front. Aging Neurosci. 2018, 10, 95. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Herrera, R.F.G. Demencias y enfermedad de Alzheimer en América Latina y el Caribe. Rev. Cubana Salud Pública 2014, 40, 378–387. [Google Scholar]
- Wortmann, M. Dementia: A Global Health Priority—Highlights from an ADI and World Health Organization Report. Alzheimer’s Res. Therapy 2012, 4, 40. [Google Scholar] [CrossRef]
- Silva, M.V.F.; Loures, C.D.M.G.; Alves, L.C.V.; De Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s Disease: Risk Factors and Potentially Protective Measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef]
- Guarino, A.; Favieri, F.; Boncompagni, I.; Agostini, F.; Cantone, M.; Casagrande, M. Executive Functions in Alzheimer Disease: A Systematic Review. Front. Aging Neurosci. 2019, 10, 437. [Google Scholar] [CrossRef]
- Wadley, V.G.; Bull, T.P.; Zhang, Y.; Barba, C.; Bryan, R.N.; Crowe, M.; Desiderio, L.; Deutsch, G.; Erus, G.; Geldmacher, D.S.; et al. Cognitive Processing Speed Is Strongly Related to Driving Skills, Financial Abilities, and Other Instrumental Activities of Daily Living in Persons with Mild Cognitive Impairment and Mild Dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Merino, E.N.; Sendin, M.A.C.; Osorio, J.A.V. Enfermedad de Alzheimer. Medicine 2015, 11, 4306–4315. [Google Scholar] [CrossRef]
- Akram, M.; Nawaz, A. Effects of Medicinal Plants on Alzheimer’s Disease and Memory Deficits. Neural. Regen. Res. 2017, 12, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Schonhaut, D.R.; Schöll, M.; Lockhart, S.N.; Ayakta, N.; Baker, S.L.; O’Neil, J.P.; Janabi, M.; Lazaris, A.; Cantwell, A.; et al. Tau PET Patterns Mirror Clinical and Neuroanatomical Variability in Alzheimer’s Disease. Brain 2016, 139, 1551–1567. [Google Scholar] [CrossRef] [PubMed]
- Cova, I.; Nicotra, A.; Maestri, G.; Canevelli, M.; Pantoni, L.; Pomati, S. Translations and Cultural Adaptations of the Montreal Cognitive Assessment: A Systematic and Qualitative Review. Neurol. Sci. 2022, 43, 113–124. [Google Scholar] [CrossRef]
- De Roeck, E.E.; Engelborghs, S.; Dierckx, E. Next Generation Brain Health Depends on Early Alzheimer Disease Diagnosis: From a Timely Diagnosis to Future Population Screening. J. Am. Med. Dir. Assoc. 2016, 17, 452–453. [Google Scholar] [CrossRef]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): I. Clinical and Neuropsychological Assessment of Alzheimer’s Disease. Neurology 1989, 39, 1159–1165. [Google Scholar] [CrossRef]
- Fillenbaum, G.G.; van Belle, G.; Morris, J.C.; Mohs, R.C.; Mirra, S.S.; Davis, P.C.; Tariot, P.N.; Silverman, J.M.; Clark, C.M.; Welsh-Bohmer, K.A.; et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): The First Twenty Years. Alzheimer’s Dement. 2008, 4, 96–109. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Clinical Validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef]
- Johnson, S.C.; Koscik, R.L.; Jonaitis, E.M.; Clark, L.R.; Mueller, K.D.; Berman, S.E.; Bendlin, B.B.; Engelman, C.D.; Okonkwo, O.C.; Hogan, K.J.; et al. The Wisconsin Registry for Alzheimer’s Prevention: A Review of Findings and Current Directions. Alzheimer’s Dement. 2018, 10, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Mattis, S. Dementia Rating Scale: Professional Manual; Psychological Assessment Resources, Incorporated: Odessa, FL, USA, 1988. [Google Scholar]
- Ball, S.; Holland, T.; Huppert, F.; Treppner, P.; Dodd, K. CAMDEX-DS. The Cambridge Examination for Mental Disorders of Older People with Down’s Syndrome and Others with Intellectual Disabilitties; TEA Ediciones: Cambridge, UK, 2013. [Google Scholar]
- Green, A.; Garrick, T.; Sheedy, D.; Blake, H.; Shores, A.; Harper, C. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Australian Normative Data. Aust. J. Psychol. 2008, 60, 72–79. [Google Scholar] [CrossRef]
- Arnold, B.R.; Cuellar, I.; Guzman, N. Statistical and Clinical Evaluation of the Mattis Dementia Rating Scale-Spanish Adaptation: An Initial Investigation. J. Gerontol. B Psychol. Sci. Soc. Sci. 1998, 53B, P364–P369. [Google Scholar] [CrossRef]
- Hall, J.R.; Balldin, V.H.; Gamboa, A.; Edwards, M.L.; Johnson, L.A.; O’Bryant, S.E. Texas Mexican American Adult Normative Studies: Normative Data for the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Dev. Neuropsychol. 2018, 43, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Heyman, A.; Fillenbaum, G.G. Overview: Clinical Sites, Case Material, and Special Studies. Neurology 1997, 49, S2–S6. [Google Scholar] [CrossRef]
- Strutt, A.M.; Ayanegui, I.G.; Scott, B.M.; Mahoney, M.L.; York, M.K.; San Miguel Montes, L.E. Influence of Socio-Demographic Characteristics on DRS-2 Performance in Spanish-Speaking Older Adults. Arch. Clin. Neuropsychol. 2012, 27, 545–556. [Google Scholar] [CrossRef]
- Tsatali, M.; Fotiadou, F.; Giaglis, G.; Tsolaki, M. The Repeatable Battery for the Assessment of the Neuropsychological Status (RBANS): A Diagnostic Validity Study in Greek Elderly. Aging Clin. Exp. Res. 2019, 31, 1305–1312. [Google Scholar] [CrossRef]
- Blvshtein, M. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Russian Language Adaptation. Ph.D. Thesis, Capella University, Minneapolis, MN, USA, 2004. [Google Scholar]
- Lee, J.H.; Lee, K.U.; Lee, D.Y.; Kim, K.W.; Jhoo, J.H.; Kim, J.H.; Lee, K.H.; Kim, S.Y.; Han, S.H.; Woo, J.I. Development of the Korean Version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): Clinical and Neuropsychological Assessment Batteries. J. Gerontol. B Psychol. Sci. Soc. Sci. 2002, 57, P47–P53. [Google Scholar] [CrossRef]
- Safaz, I.; Kurt, M.; Cakir, G.; Yasar, E.; Alaca, R. Test-Retest Reliability and Practice Effects of the Turkish Version of Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in Healthy Persons. Klin. Psikofarmakol. Bul. 2015, 25, 243–247. [Google Scholar] [CrossRef]
- Demers, P.; Robillard, A.; Laflèche, G.; Nash, F.; Heyman, A.; Fillenbaum, G. Translation of Clinical and Neuropsychological Instruments into French: The CERAD Experience. Age Ageing 1994, 23, 449–451. [Google Scholar] [CrossRef]
- De la Torre, G.G.; Suárez-Llorens, A.; Caballero, F.J.; Ramallo, M.A.; Randolph, C.; Lleó, A.; Sala, I.; Sánchez, B. Norms and Reliability for the Spanish Version of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) Form A. J. Clin. Exp. Neuropsychol. 2014, 36, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Jurica, P.; Leitten, C.L.; Mattis, S. DRS-2: Dementia Rating Scale-2.; PAR, Psychological Assessment Resources: Rocklin, CA, USA, 2001. [Google Scholar]
- Aguirre-Acevedo, D.C.; Gómez, R.D.; Moreno, S.; Henao-Arboleda, E.; Motta, M.; Muñoz, C.; Arana, A.; Pineda, D.A.; Lopera, F. Validez y fiabilidad de la batería neuropsicológica CERAD-Col. RevNeurol 2007, 45, 655–660. [Google Scholar] [CrossRef]
- Henao-Arboleda, E.; Muñoz, C.; Aguirre-Acevedo, D.C.; Lara, E.; Pineda, D.A.; Lopera, F. Datos Normativos de Pruebas Neuropsicológicas En Adultos Mayores En Una Población Colombiana. Rev. Chil. Neuropsicol. 2010, 5, 214–226. [Google Scholar]
- Torres, V.L.; Vila-Castelar, C.; Bocanegra, Y.; Baena, A.; Guzmán-Vélez, E.; Aguirre-Acevedo, D.C.; Tirado, V.; Munoz, C.; Henao, E.; Moreno, S.; et al. Normative Data Stratified by Age and Education for a Spanish Neuropsychological Test Battery: Results from the Colombian Alzheimer’s Prevention Initiative Registry. Appl. Neuropsychol. Adult 2021, 28, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Guàrdia-Olmos, J.; Peró-Cebollero, M.; Rivera, D.; Arango-Lasprilla, J.C. Methodology for the Development of Normative Data for Ten Spanish-Language Neuropsychological Tests in Eleven Latin American Countries. NeuroRehabilitation 2015, 37, 493–499. [Google Scholar] [CrossRef]
- Porto, C.S.; Fichman, H.C.; Caramelli, P.; Bahia, V.S.; Nitrini, R. Brazilian Version of the Mattis Dementia Rating Scale: Diagnosis of Mild Dementia in Alzheimer’s Disease. Arq. Neuro-Psiquiatr. 2003, 61, 339–345. [Google Scholar] [CrossRef]
- Grandi, F.; Martínez-Pernía, D.; Parra, M.A.; Olavarría, L.; Huepe, D.; Alegría, P.; Aliaga, Á.; Lillo, P.; Delgado, C.; Tenorio, M.; et al. Standardization and Diagnostic Utility of the Frontal Assessment Battery for Healthy People and Patients with Dementia in the Chilean Population. Dement. Neuropsycho. 2022, 16, 69–78. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Lyoo, C.H.; Jester-Broms, J.; Sudre, C.H.; Cho, H.; Ryu, Y.H.; Choi, J.Y.; Smith, R.; Strandberg, O.; Palmqvist, S.; et al. Assessment of Demographic, Genetic, and Imaging Variables Associated With Brain Resilience and Cognitive Resilience to Pathological Tau in Patients With Alzheimer Disease. JAMA Neurol. 2020, 77, 632–642. [Google Scholar] [CrossRef]
- Dehghani, N.; Bras, J.; Guerreiro, R. How Understudied Populations Have Contributed to Our Understanding of Alzheimer’s Disease Genetics. Brain 2021, 144, 1067–1081. [Google Scholar] [CrossRef]
- Arango-Lasprilla, J.C.; Stevens, L.; Morlett Paredes, A.; Ardila, A.; Rivera, D. Profession of Neuropsychology in Latin America. Appl. Neuropsychol. Adult 2017, 24, 318–330. [Google Scholar] [CrossRef]
- Fonseca-Aguilar, P.; Olabarrieta Landa, L.; Rivera, D.; Arelis, A.A.; Jiménez, X.A.O.; Barajas, B.V.R.; Agudelo, Y.R.; Álvarez, E.; Arango-Lasprilla, J.C. Situación actual de la práctica profesional de la neuropsicología en México. Psicol. Desde Caribe 2015, 32, 343–364. [Google Scholar] [CrossRef]
- Parra, M.A. Overcoming Barriers in Cognitive Assessment of Alzheimer’s Disease. Dement. Neuropsychol. 2014, 8, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arango-Lasprilla, J.C. Commonly Used Neuropsychological Tests for Spanish Speakers: Normative Data from Latin America. NeuroRehabilitation 2015, 37, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Arango-Lasprilla, J.C. Methodology for the Development of Normative Data for Spanish-Speaking Pediatric Populations. NeuroRehabilitation 2017, 41, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lorenzana, A.; Benito-Sánchez, I.; Adana-Díaz, L.; Paz, C.P.; Yacelga-Ponce, T.; Rivera, D.; Arango-Lasprilla, J.C. Normative Data for Test of Verbal Fluency and Naming on Ecuadorian Adult Population. Front. Psychol. 2020, 11, 830. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzana, A.; Núñez-Fernández, S.; Adana-Díaz, L.; Mascialino, G.; Ponce, T.Y.; Rivera, D.; Arango-Lasprilla, J.C. Normative Data for Test of Learning and Memory in an Ecuadorian Adult Population. Clin. Neuropsychol. 2020, 34, 54–69. [Google Scholar] [CrossRef]
- Vicente, S.G.; Ramos-Usuga, D.; Barbosa, F.; Gaspar, N.; Dores, A.R.; Rivera, D.; Arango-Lasprilla, J.C. Regression-Based Norms for the Hopkins Verbal Learning Test-Revised and the Rey–Osterrieth Complex Figure in a Portuguese Adult Population. Arch. Clin. Neuropsychol. 2021, 36, 587–596. [Google Scholar] [CrossRef]
- Vicente, S.G.; Rivera, D.; Barbosa, F.; Gaspar, N.; Dores, A.R.; Mascialino, G.; Arango-Lasprilla, J.C. Normative Data for Tests of Attention and Executive Functions in a Sample of European Portuguese Adult Population. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2021, 28, 418–437. [Google Scholar] [CrossRef]
- McKhann, G.M.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group* under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State” A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Villaseñor-Cabrera, T.; Guàrdia-Olmos, J.; Jiménez-Maldonado, M.; Rizo-Curiel, G.; Peró-Cebollero, M. Sensitivity and Specificity of the Mini-Mental State Examination in the Mexican Population. Qual. Quant. 2010, 44, 1105–1112. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Rey, A. REY: Test de Copia y de Reproducción de Memoria de Figuras Geométricas Complejas; TEA Ediciones: Madrid, Spain, 2009. [Google Scholar]
- Tupler, L.A.; Welsh, K.A.; Asare-aboagye, Y.; Dawson, D.V. Reliability of the Rey-Osterrieth Complex Figure in Use with Memory-Impaired Patients. J. Clin. Exp. Neuropsychol. 1995, 17, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G.; Delgado, A.R.; Perea, M.V.; Ladera, V. Scoring Neuropsychological Tests Using the Rasch Model: An Illustrative Example With the Rey-Osterrieth Complex Figure. Clin. Neuropsychol. 2010, 24, 45–56. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Schretlen, D.; Groninger, L.; Brandt, J. Hopkins Verbal Learning Test—Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. Clin. Neuropsychol. 1998, 12, 43–55. [Google Scholar] [CrossRef]
- Brandt, J. The Hopkins Verbal Learning Test: Development of a New Memory Test with Six Equivalent Forms. Clin. Neuropsychol. 1991, 5, 125–142. [Google Scholar] [CrossRef]
- Schretlen, D. Modified Wisconsin Card Sorting Test Professional Manual.; Psychological Assessment Resources Inc.: Odessa, FL, USA, 2010. [Google Scholar]
- Bird, C.M.; Papadopoulou, K.; Ricciardelli, P.; Rossor, M.N.; Cipolotti, L. Monitoring Cognitive Changes: Psychometric Properties of Six Cognitive Tests. Br. J. Clin. Psychol. 2004, 43, 197–210. [Google Scholar] [CrossRef]
- Golden, C.J. Manual Del Test de Colores y Palabras, 5th ed.; TEA Ediciones: Madrid, Spain, 2010. [Google Scholar]
- Rodríguez-Barreto, L.C.; del Carmen Pulido, N.; Pineda-Roa, C.A. Propiedades psieométrieas del Stroop, test de colores y palabras en población colombiana no patológica. Univ. Psychol. 2016, 15, 255–272. [Google Scholar] [CrossRef]
- Olabarrieta-Landa, L.; Rivera, D.; Galarza-del-Angel, J.; Garza, M.; Saracho, C.; Rodríguez, W.; Chávez-Oliveros, M.; Rábago, B.; Leibach, G.; Schebela, S.; et al. Verbal Fluency Tests: Normative Data for the Latin American Spanish Speaking Adult Population. NeuroRehabilitation 2015, 37, 515–561. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; Kozak, J.; Rees, L. Normative Data Stratified by Age and Education for Two Measures of Verbal Fluency: FAS and Animal Naming. Arch. Clin. Neuropsychol. 1999, 14, 167–177. [Google Scholar] [PubMed]
- Delis, D.C.; Kaplan, E.; Kramer, J.H. Delis-Kaplan Executive Function System; Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar]
- Riva, D.; Nichelli, F.; Devoti, M. Developmental Aspects of Verbal Fluency and Confrontation Naming in Children. Brain Lang. 2000, 71, 267–284. [Google Scholar] [CrossRef]
- Buré-Reyes, A.; Hidalgo-Ruzzante, N.; Vilar-López, R.; Gontier, J.; Sánchez, L.; Pérez-García, M.; Puente, A.E. Neuropsychological Test Performance of Spanish Speakers: Is Performance Different across Different Spanish-Speaking Subgroups? J. Clin. Exp. Neuropsychol. 2013, 35, 404–412. [Google Scholar] [CrossRef]
- Kaplan, E.; Goodglass, H.; Barresi, B. Evaluación de La Afasia y de Trastornos Relacionados, 3rd ed.; Médica Panamericana: Madrid, Spain, 2005. [Google Scholar]
- del Toro, C.M.; Bislick, L.P.; Comer, M.; Velozo, C.; Romero, S.; Gonzalez Rothi, L.J.; Kendall, D.L. Development of a Short Form of the Boston Naming Test for Individuals With Aphasia. J. Speech Lang. Hear. Res. 2011, 54, 1089–1100. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Jackson, S.T. Test-Retest Reliability of Three Aphasia Tests: Performance of Non-Brain-Damaged Older Adults. J. Commun. Disord. 1997, 30, 33–43. [Google Scholar] [CrossRef]
- Wolinsky, F.D.; Vander Weg, M.W.; Howren, M.B.; Jones, M.P.; Dotson, M.M. A Randomized Controlled Trial of Cognitive Training Using a Visual Speed of Processing Intervention in Middle Aged and Older Adults. PLoS ONE 2013, 8, e61624. [Google Scholar] [CrossRef]
- Levine, A.J.; Miller, E.N.; Becker, J.T.; Selnes, O.A.; Cohen, B.A. Normative Data for Determining Significance of Test–Retest Differences on Eight Common Neuropsychological Instruments. Clin. Neuropsychol. 2004, 18, 373–384. [Google Scholar] [CrossRef]
- Smith, A. Manual de Test. de Símbolos y Dígitos SDMT; Publicaciones de Psicología Aplicada; TEA Ediciones: Madrid, Spain, 2002. [Google Scholar]
- Berrigan, L.I.; Fisk, J.D.; Walker, L.A.S.; Wojtowicz, M.; Rees, L.M.; Freedman, M.S.; Marrie, R.A. Reliability of Regression-Based Normative Data for the Oral Symbol Digit Modalities Test: An Evaluation of Demographic Influences, Construct Validity, and Impairment Classification Rates in Multiple Sclerosis Samples. Clin. Neuropsychol. 2014, 28, 281–299. [Google Scholar] [CrossRef]
- Lezak, M.D. Neuropsychological Assessment, 2nd ed.; Oxford University Press: New York, NY, USA, 1983. [Google Scholar]
- Lezak, M.; Howieson, D.B.; Loring, D.W. Neuropsychological Assessment, 4th ed.; Oxford University Press: New York, NY, USA, 2004; ISBN 978-0-19-511121-7. [Google Scholar]
- Reitan, R.M.; Wolfson, D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation; Neuropsychology Press: Tucson, AZ, USA, 1985. [Google Scholar]
- Drane, D.L.; Yuspeh, R.L.; Huthwaite, J.S.; Klingler, L.K. Demographic Characteristics and Normative Observations for Derived-Trail Making Test Indices. Neuropsychiatry Neuropsycho. Behav. Neurol. 2002, 15, 39–43. [Google Scholar]
- Allen, D.N.; Thaler, N.S.; Barchard, K.A.; Vertinski, M.; Mayfield, J. Factor Structure of the Comprehensive Trail Making Test in Children and Adolescents with Brain Dysfunction. Psychol. Assess. 2012, 24, 964–972. [Google Scholar] [CrossRef]
- Schretlen, D.; Bobholz, J.H.; Brandt, J. Development and Psychometric Properties of the Brief Test of Attention. Clin. Neuropsychol. 1996, 10, 80–89. [Google Scholar] [CrossRef]
- Arango-Lasprilla, J.C.; Rivera, D.; Garza, M.T.; Saracho, C.P.; Rodríguez, W.; Rodríguez-Agudelo, Y.; Aguayo, A.; Schebela, S.; Luna, M.; Longoni, M.; et al. Hopkins Verbal Learning Test– Revised: Normative Data for the Latin American Spanish Speaking Adult Population. NeuroRehabilitation 2015, 37, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Olabarrieta-Landa, L.; Brooks, B.L.; Ertl, M.M.; Benito-Sánchez, I.; Quijano, M.C.; Rodriguez-Irizarry, W.; Aguayo Arelis, A.; Rodríguez-Agudelo, Y.; Arango-Lasprilla, J.C. Multivariate Base Rates of Low Scores on Tests of Learning and Memory Among Latino Adult Populations. J. Int. Neuropsychol. Soc. 2019, 25, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lasprilla, J.C.; Rivera, D.; Longoni, M.; Saracho, C.P.; Garza, M.T.; Aliaga, A.; Rodríguez, W.; Rodríguez-Agudelo, Y.; Rábago, B.; Sutter, M.; et al. Modified Wisconsin Card Sorting Test (M-WCST): Normative Data for the Latin American Spanish Speaking Adult Population. NeuroRehabilitation 2015, 37, 563–590. [Google Scholar] [CrossRef]
- Arango-Lasprilla, J.C.; Rivera, D.; Rodríguez, G.; Garza, M.T.; Galarza-del-Angel, J.; Rodríguez, W.; Velázquez-Cardoso, J.; Aguayo, A.; Schebela, S.; Weil, C.; et al. Symbol Digit Modalities Test: Normative Data for the Latin American Spanish Speaking Adult Population. NeuroRehabilitation 2015, 37, 625–638. [Google Scholar] [CrossRef]
- Arango-Lasprilla, J.C.; Rivera, D.; Aguayo, A.; Rodríguez, W.; Garza, M.T.; Saracho, C.P.; Rodríguez-Agudelo, Y.; Aliaga, A.; Weiler, G.; Luna, M.; et al. Trail Making Test: Normative Data for the Latin American Spanish Speaking Adult Population. NeuroRehabilitation 2015, 37, 639–661. [Google Scholar] [CrossRef]
- Olabarrieta-Landa, L.; Rivera, D.; Morlett-Paredes, A.; Jaimes-Bautista, A.; Garza, M.T.; Galarza-del-Angel, J.; Rodríguez, W.; Rábago, B.; Schebela, S.; Perrin, P.B.; et al. Standard form of the Boston Naming Test: Normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation 2015, 37, 501–513. [Google Scholar] [CrossRef]
- Rivera, D.; Perrin, P.B.; Aliaga, A.; Garza, M.T.; Saracho, C.P.; Rodrŕguez, W.; Justo-Guillen, E.; Aguayo, A.; Schebela, S.; Gulin, S.; et al. Brief Test of Attention: Normative Data for the Latin American Spanish Speaking Adult Population. NeuroRehabilitation 2015, 37, 663–676. [Google Scholar] [CrossRef]
- Rivera, D.; Olabarrieta-Landa, L.; Van der Elst, W.; Gonzalez, I.; Rodríguez-Agudelo, Y.; Aguayo Arelis, A.; Rodriguez-Irizarry, W.; García de la Cadena, C.; Arango-Lasprilla, J.C. Normative Data for Verbal Fluency in Healthy Latin American Adults: Letter M, and Fruits and Occupations Categories. Neuropsychology 2019, 33, 287–300. [Google Scholar] [CrossRef]
- Rivera, D.; Perrin, P.B.; Morlett-Paredes, A.; Galarza-del-Angel, J.; Martínez, C.; Garza, M.T.; Saracho, C.P.; Rodríguez, W.; Rodríguez-Agudelo, Y.; Rábago, B.; et al. Rey–Osterrieth Complex Figure—Copy and Immediate Recall: Normative Data for the Latin American Spanish Speaking Adult Population. NeuroRehabilitation 2015, 37, 677–698. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Perrin, P.B.; Stevens, L.F.; Garza, M.T.; Weil, C.; Saracho, C.P.; Rodríguez, W.; Rodríguez-Agudelo, Y.; Rábago, B.; Weiler, G.; et al. Stroop Color-Word Interference Test: Normative Data for the Latin American Spanish Speaking Adult Population. NeuroRehabilitation 2015, 37, 591–624. [Google Scholar] [CrossRef] [PubMed]
- Olabarrieta-Landa, L.; Ramos-Usuga, D.; Rivera, D.; Leal, G.; Bailey, K.C.; Calderón Chagualá, A.; Rabago, B.; Esenarro, L.; Mascialino, G.; Arango-Lasprilla, J.C. Prevalence of Low Scores on Language Tests as a Potential Factor in Misdiagnosis of Cognitive Impairment in a Spanish-Speaking Adult Population. Appl. Neuropsychol. Adult 2019, 29, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Mascialino, G.; Brooks, B.L.; Olabarrieta-Landa, L.; Longoni, M.; Galarza-Del-Angel, J.; Arango-Lasprilla, J.C. Multivariate Base Rates of Low Scores on Tests of Executive Functions in a Multi-Country Latin American Sample. Dev. Neuropsychol. 2021, 46, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, M.C. Combining Probability from Independent Tests: The Weighted Z-Method Is Superior to Fisher’s Approach. J. Evol. Biol. 2005, 18, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.E.; Bachmann, L.M.; Jaeschke, R. A Readers’ Guide to the Interpretation of Diagnostic Test Properties: Clinical Example of Sepsis. Intensive Care Med. 2003, 29, 1043–1051. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The Inconsistency of “Optimal” Cutpoints Obtained Using Two Criteria Based on the Receiver Operating Characteristic Curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and Its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; Version 26.0; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Matallana, D.; de Santacruz, C.; Cano, C.; Reyes, P.; Samper-Ternent, R.; Markides, K.S.; Ottenbacher, K.J.; Reyes-Ortiz, C.A. The Relationship Between Education Level and Mini-Mental State Examination Domains Among Older Mexican Americans. J. Geriatr. Psychiatry Neurol. 2011, 24, 9–18. [Google Scholar] [CrossRef]
- Ostrosky-Solís, F.; López-Arango, G.; Ardila, A. Sensitivity and Specificity of the Mini-Mental State Examination in a Spanish-Speaking Population. Appl. Neuropsychol. 2000, 7, 25–31. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Haddad, G.G.; Mattar, G.P.; de Oliveira, M.C.; Simon, S.S.; Guilhoto, L.M.; Busatto, G.F.; Zaman, S.; Holland, A.J.; Hoexter, M.Q.; et al. The Validity and Reliability of the CAMDEX-DS for Assessing Dementia in Adults with Down Syndrome in Brazil. Braz. J. Psychiatry 2019, 41, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Custodio, N.; Duque, L.; Montesinos, R.; Alva-Diaz, C.; Mellado, M.; Slachevsky, A. Systematic Review of the Diagnostic Validity of Brief Cognitive Screenings for Early Dementia Detection in Spanish-Speaking Adults in Latin America. Front. Aging Neurosci. 2020, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Matías-Guiu, J.A.; Sánchez-Benavides, G.; Rivera-Àvila, N.; Cortés-Martínez, A.; Delgado-Alonso, C.; Delgado-Álvarez, A.; Montero, P.; Pytel, V.; Matías-Guiu, J.; Peña-Casanova, J. Validation of the Neuronorma Battery for Neuropsychological Assessment in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2020, 42, 102070. [Google Scholar] [CrossRef]
- Rivera, D.; Ramos-Usuga, D.; Fuentes-Mendoza, E.M.; Aguayo-Arelis, A.; Rabago Barajas, B.V.; Macías-Islas, M.Á.; Krch, D.; Lequerica, A.H.; Arango-Lasprilla, J.C. Validation of the Norma Latina Neuropsychological Assessment Battery in Individuals with Multiple Sclerosis in Mexico. Mult. Scler. Relat. Disord. 2022, 59, 103685. [Google Scholar] [CrossRef] [PubMed]
- Oltra-Cucarella, J.; Rivera, D.; Arango-Lasprilla, J. Principios básicos en estadística para neuropsicólogos clínicos e investigadores: Utilidad práctica e interpretación de análisis de variables continuas. Rev. Iberoam. Neuropsicol. 2020, 3, 29–40. [Google Scholar]
- Van der Elst, W.; Molenberghs, G.; Van Boxtel, M.P.J.; Jolles, J. Establishing Normative Data for Repeated Cognitive Assessment: A Comparison of Different Statistical Methods. Behav. Res. 2013, 45, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Waber, D.P.; Holmes, J.M. Assessing Children’s Copy Productions of the Rey-Osterrieth Complex Figure. J. Clin. Exp. Neuropsychol. 1985, 7, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.F.; Galindo, G.; Villa, M.; Salvador, J. La Figura Compleja de Rey: Propiedades Psicométricas. Salud Ment. 1996, 19, 42–48. [Google Scholar]
| HC (n = 117) | AD (n = 117) | Statistic | Sig. | Effect Size (r) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Min. | Max. | Median | Min. | Max. | |||||
| Age | 78 | 57 | 88 | 78 | 57 | 88 | U = 6433.5 | 0.427 | 0.056 | |
| Education | 6 | 1 | 20 | 6 | 0 | 20 | U = 6407.5 | 0.395 | 0.052 | |
| Sex | Female | 91 | 77.8% | 91 | 77.8% | X2 = 0.00 | 1.00 | 0.000 | ||
| Male | 26 | 22.2% | 26 | 22.2% | ||||||
| Test-Score | Group | Median | Min. | Max. | Mann–Whitney U | Sig. | r |
|---|---|---|---|---|---|---|---|
| ROCF Copy | AD | 7.0 | 0.0 | 36.0 | 1973.000 | <0.001 | 0.609 ††† |
| HC | 30.0 | 9.5 | 36.0 | ||||
| ROCF Recall | AD | 0.0 | 0.0 | 20.0 | 889.000 | <0.001 | 0.759 ††† |
| HC | 13.5 | 0.0 | 34.0 | ||||
| Stroop Word | AD | 50.5 | 7.0 | 100.0 | 3219.500 | <0.001 | 0.341 †† |
| HC | 70.0 | 21.0 | 110.0 | ||||
| Stroop Color | AD | 31.0 | 0.0 | 87.0 | 2609.500 | <0.001 | 0.415 †† |
| HC | 49.0 | 2.0 | 93.0 | ||||
| Stroop Word—Color | AD | 8.0 | 0.0 | 40.0 | 1192.000 | <0.001 | 0.630 ††† |
| HC | 26.0 | 0.0 | 52.0 | ||||
| Stroop Interference | AD | −7.5 | −31.0 | 26.0 | 3001.000 | <0.001 | 0.373 †† |
| HC | −1.9 | −16.0 | 17.9 | ||||
| M-WCST Categories | AD | 1.0 | 0.0 | 6.0 | 2948.000 | <0.001 | 0.391 †† |
| HC | 3.0 | 0.0 | 6.0 | ||||
| M-WCST Perseveration errors | AD | 11.0 | 1.0 | 44.0 | 4025.500 | 0.001 | 0.226 † |
| HC | 7.0 | 0.0 | 45.0 | ||||
| M-WCST Total errors | AD | 24.0 | 4.0 | 47.0 | 4951.500 | 0.172 | 0.089 |
| HC | 18.0 | 0.0 | 47.0 | ||||
| TMT-A | AD | 100.0 | 35.0 | 100.0 | 2664.000 | <0.001 | 0.438 †† |
| HC | 81.0 | 28.0 | 100.0 | ||||
| TMT-B | AD | 300.0 | 82.0 | 300.0 | 1167.500 | <0.001 | 0.596 ††† |
| HC | 172.0 | 34.0 | 300.0 | ||||
| BTA | AD | 5.0 | 0.0 | 19.0 | 2856.500 | <0.001 | 0.492 †† |
| HC | 12.0 | 0.0 | 20.0 | ||||
| VFT Letter F | AD | 4.0 | 0.0 | 14.0 | 2488.000 | <0.001 | 0.547 ††† |
| HC | 9.0 | 1.0 | 23.0 | ||||
| VFT Letter A | AD | 4.0 | 0.0 | 15.0 | 2975.500 | <0.001 | 0.486 †† |
| HC | 8.0 | 2.00 | 23.0 | ||||
| VFT Letter S | AD | 3.5 | 0.0 | 16.0 | 2843.000 | <0.001 | 0.503 ††† |
| HC | 8.0 | 2.0 | 21.0 | ||||
| VFT Letter M | AD | 4.0 | 0.0 | 17.0 | 2834.500 | <0.001 | 0.503 ††† |
| HC | 9.0 | 1.0 | 26.0 | ||||
| VFT Animals | AD | 7.0 | 0.0 | 21.0 | 1951.500 | <0.001 | 0.619 ††† |
| HC | 14.0 | 5.0 | 24.0 | ||||
| VFT Fruits | AD | 6.0 | 0.0 | 19.0 | 2078.500 | <0.001 | 0.603 ††† |
| HC | 12.0 | 4.0 | 21.0 | ||||
| VFT Occupations | AD | 4.0 | 0.0 | 14.0 | 1928.500 | <0.001 | 0.623 ††† |
| HC | 9.0 | 4.0 | 20.0 | ||||
| BNT | AD | 24.0 | 0.0 | 53.0 | 1855.000 | <0.001 | 0.631 ††† |
| HC | 45.0 | 0.0 | 60.0 | ||||
| SDMT | AD | 4.0 | 0.0 | 37.0 | 1411.500 | <0.001 | 0.678 ††† |
| HC | 22.0 | 3.0 | 50.0 | ||||
| HVLT-R Total learning | AD | 9.0 | 0.0 | 22.0 | 1098.500 | <0.001 | 0.726 ††† |
| HC | 17.0 | 6.0 | 29.0 | ||||
| HVLT-R Delayed recall | AD | 0.0 | 0.0 | 7.0 | 1235.500 | <0.001 | 0.745 ††† |
| HC | 5.0 | 0.0 | 11.0 | ||||
| HVLT-R Recognition | AD | 8.0 | 0.0 | 12.0 | 3669.000 | <0.001 | 0.406 †† |
| HC | 11.0 | 4.0 | 12.0 |
| Cut-Off | Group | Median | Min. | Max. | Mann–Whitney U | Sig. | r | AUC [CI95%] |
|---|---|---|---|---|---|---|---|---|
| <25th percentile | AD | 17 | 7 | 24 | 457.000 | <0.001 | 0.627 ††† | 0.936 [0.901, 0.970] |
| HC | 6 | 0 | 23 | |||||
| <16th percentile | AD | 15 | 5 | 24 | 395.000 | <0.001 | 0.640 ††† | 0.945 [0.912, 0.977] |
| HC | 4 | 0 | 22 | |||||
| <10th percentile | AD | 12 | 2 | 23 | 391.500 | <0.001 | 0.641 ††† | 0.945 [0.912, 0.977] |
| HC | 2 | 0 | 19 | |||||
| <5th percentile | AD | 9 | 1 | 21 | 376.000 | <0.001 | 0.649 ††† | 0.947 [0.917, 0.978] |
| HC | 1 | 0 | 14 | |||||
| <2nd percentile | AD | 5 | 0 | 20 | 427.500 | <0.001 | 0.653 ††† | 0.940 [0.905, 0.975] |
| HC | 0 | 0 | 7 |
| Cut Point | <5th Percentile | <10th Percentile | ||||||
|---|---|---|---|---|---|---|---|---|
| Se | Sp | J | UI | Se | Sp | J | UI | |
| ≥1 | 0.984 | 0.628 | 0.612 | 0.356 | 1.000 | 0.381 | 0.381 | 0.619 |
| ≥2 | 0.968 | 0.761 | 0.729 | 0.207 | 1.000 | 0.540 | 0.524 | 0.444 |
| ≥3 | 0.873 | 0.823 | 0.696 | 0.198 | 0.984 | 0.664 | 0.632 | 0.304 |
| ≥4 | 0.841 | 0.894 | 0.735 | 0.159 | 0.952 | 0.743 | 0.680 | 0.210 |
| ≥5 | 0.746 | 0.956 | 0.702 | 0.210 | 0.921 | 0.850 | 0.771 | 0.119 |
| ≥6 | 0.651 | 0.982 | 0.633 | 0.331 | 0.841 | 0.912 | 0.753 | 0.137 |
| ≥7 | 0.571 | 0.982 | 0.553 | 0.411 | 0.794 | 0.938 | 0.732 | 0.158 |
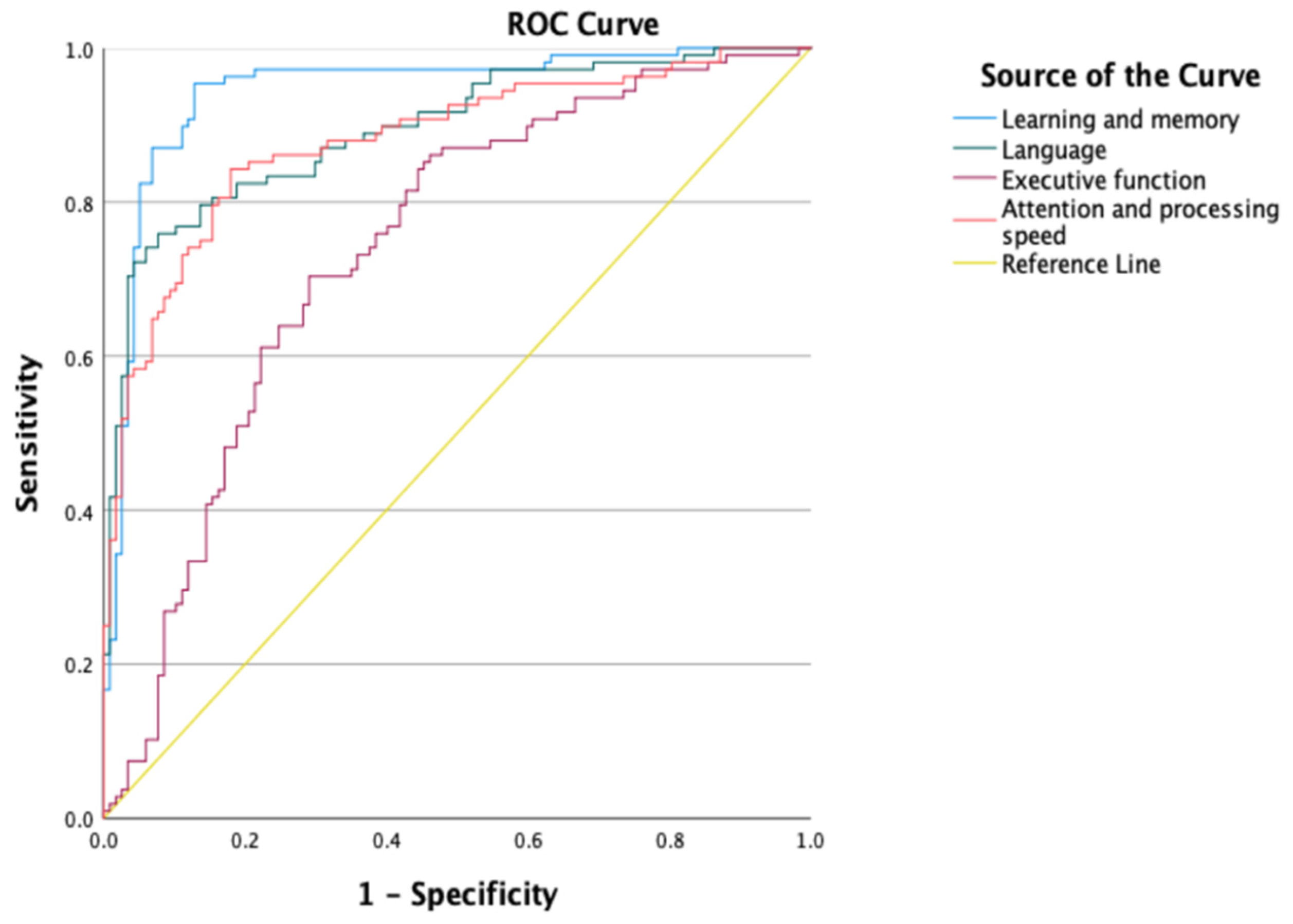
| Domains | Group | Median (Z-Score) | SD | Mann–Whitney U | Sig. | r | AUC [CI95%] |
|---|---|---|---|---|---|---|---|
| Executive Function | AD | −2.03 | 1.55 | 3314.000 | <0.001 | 0.403 †† | 0.785 [0.726, 0.845] |
| HC | −0.30 | 1.83 | |||||
| Attention and Processing Speed | AD | −3.64 | 1.68 | 1551.000 | <0.001 | 0.665 ††† | 0.892 [0.849, 0.936] |
| HC | −0.68 | 1.62 | |||||
| Language | AD | −4.72 | 2.48 | 1334.000 | <0.001 | 0.696 ††† | 0.892 [0.849, 0.935] |
| HC | −0.61 | 1.97 | |||||
| Learning and Memory | AD | −4.18 | 2.36 | 721.000 | <0.001 | 0.773 ††† | 0.944 [0.912, 0.976] |
| HC | 0.25 | 1.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Fernández, S.; Rivera, D.; Arroyo-Anlló, E.M.; Ortiz Jiménez, X.A.; Camino-Pontes, B.; Salinas Martínez, R.; Arango-Lasprilla, J.C. Validation of the Norma Latina Neuropsychological Assessment Battery in Patients with Alzheimer’s Disease in Mexico. Int. J. Environ. Res. Public Health 2022, 19, 11322. https://doi.org/10.3390/ijerph191811322
Núñez-Fernández S, Rivera D, Arroyo-Anlló EM, Ortiz Jiménez XA, Camino-Pontes B, Salinas Martínez R, Arango-Lasprilla JC. Validation of the Norma Latina Neuropsychological Assessment Battery in Patients with Alzheimer’s Disease in Mexico. International Journal of Environmental Research and Public Health. 2022; 19(18):11322. https://doi.org/10.3390/ijerph191811322
Chicago/Turabian StyleNúñez-Fernández, Silvia, Diego Rivera, Eva María Arroyo-Anlló, Xóchitl Angélica Ortiz Jiménez, Borja Camino-Pontes, Ricardo Salinas Martínez, and Juan Carlos Arango-Lasprilla. 2022. "Validation of the Norma Latina Neuropsychological Assessment Battery in Patients with Alzheimer’s Disease in Mexico" International Journal of Environmental Research and Public Health 19, no. 18: 11322. https://doi.org/10.3390/ijerph191811322
APA StyleNúñez-Fernández, S., Rivera, D., Arroyo-Anlló, E. M., Ortiz Jiménez, X. A., Camino-Pontes, B., Salinas Martínez, R., & Arango-Lasprilla, J. C. (2022). Validation of the Norma Latina Neuropsychological Assessment Battery in Patients with Alzheimer’s Disease in Mexico. International Journal of Environmental Research and Public Health, 19(18), 11322. https://doi.org/10.3390/ijerph191811322








