Human Evidence of Perfluorooctanoic Acid (PFOA) Exposure on Hepatic Disease: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specifying the Research Question
- Population: Any population and life stage (occupational or general population, including children and other sensitive populations). The controlled exposure, cohort, or cross-sectional studies were used.
- Exposure: Studies providing quantitative estimates of PFOA exposure based on administered dose or concentration, biomonitoring data (e.g., urine, blood, or other specimens), and environmental or occupational setting measures.
- Comparator: A comparison or reference population exposed to a lower level (0 or no exposure/exposure below detection levels) or for a shorter period.
- Outcome: All hepatic disease types.
2.2. Literature Search and Screening
- Literature Selection Criteria: We selected studies associated with all hepatic disease types in which human exposure to PFOA was measured or estimated.
- Literature Exclusion Criteria: We excluded studies if they did not contain original data or were epidemiology studies (i.e., prospective cohort studies, nested case-control studies, and case-cohort studies), if study subjects were not humans, if the study subject’s exposure to PFOA was not measured or estimated, or if they did not provide outcome/exposure of interest.
- Study Selection Process: We excluded duplicates that were found in two or more databases. Abstract screening was performed by using Rayyan, open-source, online software, by two investigators independently [12]. Two independent researchers examined the abstracts and titles of all citations using the defined criteria. Studies that were not excluded based on the title and abstract were screened through a full text review. After the initial screening, when there was a discrepancy between the researchers, they discussed each discrepancy and brought in the third researcher, if necessary, to discuss and decide whether to include or exclude each discrepancy.
2.3. Data Extraction and Rating the Risk of Bias (RoB)
2.4. Quantitative Synthesis and Meta-Analysis
3. Results
3.1. Flow Diagram for Search and Selection
3.2. Characteristics of Included Studies
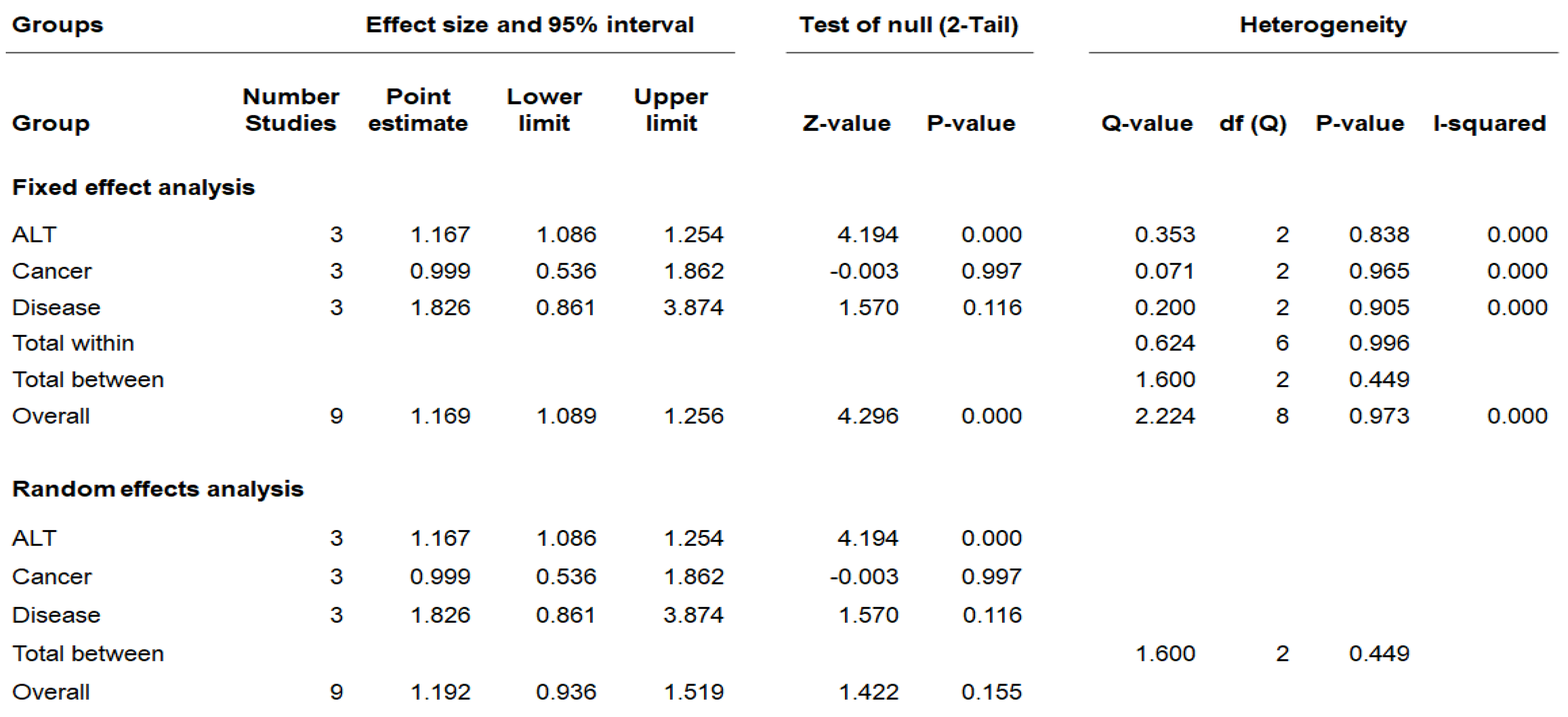
3.3. Meta-Analysis of PFOA Exposure on Hepatic Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, M.J.; Park, J.; Luo, L.; Min, J.; Kim, J.H.; Yang, H.D.; Kho, Y.; Kang, G.J.; Chung, M.S.; Shin, S.; et al. Effect of washing, soaking, and cooking methods on perfluorinated compounds in mackerel (Scomber japonicus). Food Sci. Nutr. 2020, 8, 4399–4408. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.C.; Bech, B.H.; Brix, N.; Nohr, E.A.; Bonde, J.P.; Henriksen, T.B. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: A systematic review. Crit. Rev. Toxicol. 2015, 45, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Jeddy, Z.; Hartman, T.J.; Taylor, E.V.; Poteete, C.; Kordas, K. Prenatal concentrations of Perfluoroalkyl substances and early communication development in British girls. Early Hum. Dev. 2017, 109, 15–20. [Google Scholar] [CrossRef]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Hundley, S.G.; Sarrif, A.M.; Kennedy, G.L. Absorption, distribution, and excretion of ammonium perfluorooctanoate (APFO) after oral administration to various species. Drug Chem. Toxicol. 2006, 29, 137–145. [Google Scholar] [CrossRef]
- Shane, H.L.; Baur, R.; Lukomska, E.; Weatherly, L.; Anderson, S.E. Immunotoxicity and allergenic potential induced by topical application of perfluorooctanoic acid (PFOA) in a murine model. Food Chem. Toxicol. 2020, 136, 111114. [Google Scholar] [CrossRef]
- Jin, R.; McConnell, R.; Catherine, C.; Xu, S.; Walker, D.I.; Stratakis, N.; Jones, D.P.; Miller, G.W.; Peng, C.; Conti, D.V.; et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in Children: An untargeted metabolomics approach. Environ. Int. 2020, 134, 105220. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Zhang, Y.; Guan, S.; Gong, X.; Wang, X. Maternal exposure to perfluorooctanoic acid (PFOA) causes liver toxicity through PPAR-alpha pathway and lowered histone acetylation in female offspring mice. Environ. Sci. Pollut. Res. Int. 2019, 26, 18866–18875. [Google Scholar] [CrossRef]
- Naturvårdsverket, N. Swedish National Implementation Plan for the Stockholm Convention on Persistent Organic Pollutants: Update 2020 to Include Substances Listed 2017 and 2019. 2020. Available online: https://www.naturvardsverket.se/om-oss/publikationer/6900/swedish-national-implementation-plan-for-the-stockholm-convention-on-persistent-organic-pollutants/ (accessed on 26 August 2022).
- Fiedler, H.; Sadia, M. Regional occurrence of perfluoroalkane substances in human milk for the global monitoring plan under the Stockholm Convention on Persistent Organic Pollutants during 2016–2019. Chemosphere 2021, 277, 130287. [Google Scholar] [CrossRef]
- EPA. Systematic Review Protocol for the PFBA, PFHxA, PFHxS, PFNA, and PFDA IRIS Assessments; Environmental Protection Agency: Washington, DC, USA, 2019. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A.; Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). The Cochrane Collaboration. Available online: https://training.cochrane.org/handbook/current (accessed on 29 September 2021).
- Harrison, H.; Griffin, S.J.; Kuhn, I.; Usher-Smith, J.A. Software tools to support title and abstract screening for systematic reviews in healthcare: An evaluation. BMC Med. Res. Methodol. 2020, 20, 7. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Salihovic, S.; Stubleski, J.; Kärrman, A.; Larsson, A.; Fall, T.; Lind, L.; Lind, P.M. Changes in markers of liver function in relation to changes in perfluoroalkyl substances-a longitudinal study. Environ. Int. 2018, 117, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Groth, A.C.; Winquist, A.; Shin, H.-M.; Bartell, S.M.; Steenland, K. Modeled perfluorooctanoic acid (PFOA) exposure and liver function in a mid-Ohio valley community. Environ. Health Perspect. 2016, 124, 1227–1233. [Google Scholar] [CrossRef]
- Steenland, K.; Zhao, L.; Winquist, A. A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA). Occup. Environ. Med. 2015, 72, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.M.; Hoffman, K.; Shin, H.-M.; Weinberg, J.M.; Webster, T.F.; Fletcher, T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: A geographic analysis. Environ. Health Perspect. 2013, 121, 318–323. [Google Scholar] [CrossRef]
- Eriksen, K.T.; Sørensen, M.; McLaughlin, J.K.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J. Natl. Cancer Inst. 2009, 101, 605–609. [Google Scholar] [CrossRef]
- Steenland, K.; Barry, V.; Savitz, D. Serum perfluorooctanoic acid and birthweight: An updated meta-analysis with bias analysis. Epidemiology 2018, 29, 765–776. [Google Scholar] [CrossRef]
- Forns, J.; Verner, M.-A.; Iszatt, N.; Nowack, N.; Bach, C.C.; Vrijheid, M.; Costa, O.; Andiarena, A.; Sovcikova, E.; Høyer, B.B. Early life exposure to perfluoroalkyl substances (PFAS) and ADHD: A meta-analysis of nine European population-based studies. Environ. Health Perspect. 2020, 128, 057002. [Google Scholar] [CrossRef]
- Liu, P.; Yang, F.; Wang, Y.; Yuan, Z. Perfluorooctanoic acid (PFOA) exposure in early life increases risk of childhood adiposity: A meta-analysis of prospective cohort studies. Int. J. Environ. Res. Public Health 2018, 15, 2070. [Google Scholar] [CrossRef]
- Luo, Y.; Deji, Z.; Huang, Z. Exposure to perfluoroalkyl substances and allergic outcomes in children: A systematic review and meta-analysis. Environ. Res. 2020, 191, 110145. [Google Scholar] [CrossRef]
- Cao, T.; Qu, A.; Li, Z.; Wang, W.; Liu, R.; Wang, X.; Nie, Y.; Sun, S.; Zhang, X.; Liu, X. The relationship between maternal perfluoroalkylated substances exposure and low birth weight of offspring: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2021, 28, 67053–67065. [Google Scholar] [CrossRef] [PubMed]
- Qu, A.; Cao, T.; Li, Z.; Wang, W.; Liu, R.; Wang, X.; Nie, Y.; Sun, S.; Liu, X.; Zhang, X. The association between maternal perfluoroalkyl substances exposure and early attention deficit hyperactivity disorder in children: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2021, 28, 67066–67081. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.I.; Sutton, P.; Atchley, D.S.; Koustas, E.; Lam, J.; Sen, S.; Robinson, K.A.; Axelrad, D.A.; Woodruff, T.J. The Navigation Guide—evidence-based medicine meets environmental health: Systematic review of human evidence for PFOA effects on fetal growth. Environ. Health Perspect. 2014, 122, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Soheimi, S.S.; Abdul Rahman, A.; Abd Latip, N.; Ibrahim, E.; Sheikh Abdul Kadir, S.H. Understanding the Impact of Perfluorinated Compounds on Cardiovascular Diseases and Their Risk Factors: A Meta-Analysis Study. Int. J. Environ. Res. Public Health 2021, 18, 8345. [Google Scholar] [CrossRef]
- Maina, I.; Rule, J.A.; Wians, F.H.; Poirier, M.; Grant, L.; Lee, W.M. α-Glutathione S-Transferase: A New Biomarker for Liver Injury? J. Appl. Lab. Med. 2016, 1, 119–128. [Google Scholar] [CrossRef]
- Senior, J.R. Alanine aminotransferase: A clinical and regulatory tool for detecting liver injury-past, present, and future. Clin. Pharmacol. Ther. 2012, 92, 332–339. [Google Scholar] [CrossRef]
- Liu, H.; Sun, W.; Zhou, Y.; Griffin, N.; Faulkner, S.; Wang, L. iTRAQ-based quantitative proteomics analysis of Sprague-Dawley rats liver reveals perfluorooctanoic acid-induced lipid metabolism and urea cycle dysfunction. Toxicol. Lett. 2022, 357, 20–32. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, R.; Guo, X.; Hu, J.; Dai, J. Low dose perfluorooctanoate exposure promotes cell proliferation in a human non-tumor liver cell line. J. Hazard. Mater. 2016, 313, 18–28. [Google Scholar] [CrossRef]
- Jain, R.B. Concentration of selected liver enzymes across the stages of glomerular function: The associations with PFOA and PFOS. Heliyon 2019, 5, e02168. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Schwerdtle, T.; et al. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J. 2018, 16, e05194. [Google Scholar] [CrossRef]
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Hungerbühler, K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 2013, 60, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Clewell, H.; Cox, T.; Dourson, M.; Ethridge, S.; Forsberg, N.; Gadagbui, B.; Hamade, A.; Naidu, R.; Pechacek, N.; et al. The Conundrum of the PFOA human half-life, an international collaboration. Regul. Toxicol. Pharmacol. 2022, 132, 105185. [Google Scholar] [CrossRef] [PubMed]
- Schlezinger, J.; Hyötyläinen, T.; Sinioja, T.; Boston, C.; Puckett, H.; Oliver, J.; Heiger-Bernays, W.; Webster, T. Perfluorooctanoic acid induces liver and serum dyslipidemia in humanized pparα mice fed an american diet. Toxicol. Appl. Pharmacol. 2021, 426, 115644. [Google Scholar] [CrossRef] [PubMed]


| Outcome Categories | Author, Year | Study Design | Location (e.g., Country) | Study Period | Population | Exposure | Sample matrix | Outcome | Mean Exposure (IQR) | Effect Estimate | Study Evaluation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant | N Enrolled | Mean (SD) Age, yr | Exposure | Outcome | Selection | Confounding | Analysis | Reporting | Sensitivity | Overall confidenc | ||||||||||
| Hepatic | Salihovic et al., 2018 [15] | Longitudinal study | Sweden | 2001–2014 | General population, elderly (age 70: 2001–2004) (age 75: 2006–2009) (age 80: 2011–2014) | 1002 (age 70), 817 (age 75), 603 (age 80) | 70.0 ± 0.2 | PFOA | Plasma | Bilirubin, μmol/L PFOA −1.39 (−1.78, −1.01) | PFOA (median (IQR)) Age 70:3.31 (2.52, 4.39); Age 75:3.81 (2.71, 5.41); Age 80:2.53 (1.82, 3.61) | Coefficients (β) (CI) | A | A | A | A | A | A | A | Medium |
| ALT, μkat/L PFOA 0.04 (0.03, 0.06) | ||||||||||||||||||||
| ALP, μkat/L PFOA 0.11 (0.06, 0.15) | ||||||||||||||||||||
| GGT, μkat/L PFOA 0.07 (0.01, 0.12) | ||||||||||||||||||||
| Hepatic | Darrow et al., 2016 [16] | Cohort study (retrospective) | USA | 2005-2011 | C8 Health Project Survey Participants August 2005–August 2006 and Original DuPont Cohort | Liver biomarkers n = 30,723 (including 1892 workers), combined cohort for liver disease n = 32,254 | 46 (for liver disease) | PFOA | Serum | OR (95% CI) for above normal ALT 1.04 (1.01,1.07) Q1: reference; Q2: 1.12 (1.00, 1.27); Q3: 1.14 (1.01, 1.29); Q4: 1.20 (1.06, 1.35); Q5: 1.16 (1.02, 1.33) (p for trend: 0.0078) | Q1: 50.3–191.2; Q2: 191.2–311.3; Q3: 311.3–794.1; Q4: 794.1–3997.6; Q5: 3997.6–20,5667.3 | OR (CI) | A | G | A | A | A | A | A | Medium |
| Hepatic | Steenland et al., 2015 [17] | Cohort study (retrospective) | USA | 2008–2011 | The C8 Health Project (C8HP), workers employed between 1948 and 2002 at a DuPont plant in West Virginia | 3713 | Mean birth year: 1951 (SD 14) | PFOA | Serum | Non-hepatitis liver disease (10-years lag) Q1 1.00, Q2 1.46 (0.42, 5.04), Q3 2.13 (0.59, 7.71), Q4 2.02 (0.50, 8.10) | Mean measured exposure: 325 (SD 920) Mean predicted exposure: 218 (SD 358) | RR (CI) | G | A | G | A | A | A | A | Medium |
| Hepatic | Vieira et al., 2013 [18] | Cohort study (retrospective) | USA | 1996–2005 | OH Cancer Incidence Surveillance System (OCISS), WV Cancer Registry (WVCR) | 25,107 (7869 OH cases and 17,238 WV cases) | Median 67 years | PFOA | Serum | Liver cancer 0.9 (0.3, 2.5) | Medium = 12.9–30.7µg/L (very high = 110–655 µg/L; high = 30.8–109 µg/L; medium = 12.9–30.7 µg/L; low = 3.7–12.8 µg/L) | AOR (CI) | G | A | A | A | A | A | A | Medium |
| Hepatic | Eriksen et al., 2009 [19] | Cohort study (prospective) | Denmark | 1993–2006 | Diet, Cancer and Health (DCH) cohort, Patients with prostate, bladder, pancreatic, liver cancer | 1240 (prostate cancer n = 713, liver cancer n = 67) | Median 59 years (5–95th percentiles: 51–65) | PFOA | Plasma | PFOA (quartile trend) Liver cancer 0.95 (0.86, 1.06) | Liver cancer PFOA median 5.4 (5–95th percentiles: 2.5–13.7) | RR (CI) | A | G | G | A | A | A | A | Medium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Kim, J.-Y.; Lee, H.-J. Human Evidence of Perfluorooctanoic Acid (PFOA) Exposure on Hepatic Disease: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 11318. https://doi.org/10.3390/ijerph191811318
Choi J, Kim J-Y, Lee H-J. Human Evidence of Perfluorooctanoic Acid (PFOA) Exposure on Hepatic Disease: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(18):11318. https://doi.org/10.3390/ijerph191811318
Chicago/Turabian StyleChoi, Jihee, Jong-Yeon Kim, and Hae-Jeung Lee. 2022. "Human Evidence of Perfluorooctanoic Acid (PFOA) Exposure on Hepatic Disease: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 18: 11318. https://doi.org/10.3390/ijerph191811318
APA StyleChoi, J., Kim, J.-Y., & Lee, H.-J. (2022). Human Evidence of Perfluorooctanoic Acid (PFOA) Exposure on Hepatic Disease: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(18), 11318. https://doi.org/10.3390/ijerph191811318







