Abstract
Limited previous work has identified a relationship between exposure to ambient air pollution and aggressive somatic lung tumor mutations. More work is needed to confirm this relationship, especially using spatially resolved air pollution. We aimed to quantify the association between different air pollution metrics and aggressive tumor biology. Among patients treated at City of Hope Comprehensive Cancer Center in Duarte, CA (2013–2018), three non-small cell lung cancer somatic tumor mutations, TP53, KRAS, and KRAS G12C/V, were documented. PM2.5 exposure was assessed using state-of-the art ensemble models five and ten years before lung cancer diagnosis. We also explored the role of NO2 using inverse-distance-weighting approaches. We fitted logistic regression models to estimate odds ratio (OR) and their 95% confidence intervals (CIs). Among 435 participants (median age: 67, female: 51%), an IQR increase in NO2 exposure (3.5 μg/m3) five years before cancer diagnosis was associated with an increased risk in TP53 mutation (OR, 95% CI: 1.30, 0.99–1.71). We found an association between highly-exposed participants to PM2.5 (>12 μg/m3) five and ten years before cancer diagnosis and TP53 mutation (OR, 95% CI: 1.61, 0.95–2.73; 1.57, 0.93–2.64, respectively). Future studies are needed to confirm this association and better understand how air pollution impacts somatic profiles and the molecular mechanisms through which they operate.
1. Introduction
An estimated 131,880 Americans will die from lung cancer in 2021, accounting for 22% of all cancer deaths and making it the leading cause of cancer death in the United States (US) []. An important factor associated with lung cancer mortality is tumor biology and the presence of somatic mutations. Some mutations in certain genes can aid in the selection of targeted therapies and lead to improvements in survival outcomes, such as EGFR mutations and tyrosine kinase inhibitor treatments []. However, mutations in other genes such as KRAS and TP53 are associated with drug resistance [,], disease recurrence [,], and decreased survival [,]. Especially important are KRAS G12C and G12V mutations, which are associated with a uniquely elevated risk of disease recurrence and decreased overall survival [,,]. While cigarette smoking is a known primary risk factor for KRAS and TP53 mutations [,], exposure to environmental pollutants may also be related to the etiology of these mutations []. It is important to hone our understanding of the dose–response relationship between environmental pollutants and these deadly lung cancer somatic mutations.
Exposure to ambient air pollution has been linked to both lung cancer risk and mortality, even after accounting for smoking [,,,]. Fine particulate matter (PM2.5) and gaseous pollutants such as nitrogen dioxide (NO2) have specifically been implicated in this relationship [,,]. PM2.5 is a common urban pollutant, measuring the concentration of ambient particles with an aerodynamic diameter of less than 2.5 µm. PM2.5 is a mixture of pollutants originating from a variety of sources, including but not limited to transportation, power generation, and wildfires []. NO2 is a byproduct of fossil fuel combustion and is frequently used as a proxy for exposure to traffic-related air pollution [].
Previous research has identified potential biologic processes that can explain the link between ambient air pollution and lung cancer. In vitro work found that exposure to PM2.5 at similar concentrations to urban background levels leads to significantly modified cell cycles and altered cell organelles, leading to DNA damage that could ultimately lead to the development of lung cancer []. Additionally, PM2.5 can induce epigenetic modifications, including DNA methylation linked to the function of bronchial epithelial cells []. By contrast, NO2 may not be directly carcinogenic [], but its impact on lung cancer may be due to its high degree of correlation with known traffic-related carcinogens [,]. While some previous work has identified a relationship between exposure to ambient air pollution and aggressive somatic lung tumor mutations, more work is needed to confirm this relationship, especially using relevant air pollution assessment.
Measurement of PM2.5 relies heavily on the use of the Environmental Protection Agency (EPA) or state-sponsored sensor networks. While highly reliable/accurate and temporally extensive, these networks have course spatial resolution and uneven distribution between urban and rural areas. Models of PM2.5 relying solely on course sensor network inputs tend to assume smooth and linear change between the large distances of each node in the network. Yet PM2.5 concentrations are subject to hyper-localized variation due to the volatile nature of their sources. Considerable advances have been made in more spatially precise PM2.5 models using ensemble models that combine various machine learning algorithms and a large set of predictors including land-use or meteorological data or remote sensing products including aerosol optical depth, for example. It is unclear how a previously estimated dose–response stands when using more refined air pollution models.
In our previous study among patients who were treated at City of Hope Comprehensive Cancer Center (COH) in California (CA), patients living in areas with higher PM2.5 exposure had 1.66 (95% CI: 1.02–2.72) increased odds of TP53-mutated non-small cell lung cancer (NSCLC) []. That study measured air pollution (PM2.5 and ozone) with the EPA’s Environmental Justice Screening and Mapping Tool (EJScreen). The EJScreen tool is available at the census tract level and is not available at any period of time, thus limiting the ability to assign air pollution exposures based on the incidence of the disease. It is important to note that in this previous study, PM2.5 exposure was assigned in the year or two prior to diagnosis, and neither the role of NO2 nor the effect of air pollution on KRAS mutations were investigated.
In this present study, we aim to improve on previous research by relying on spatially- and temporally-resolved PM2.5 exposure. We used a state-of-the-art ensemble model for PM2.5 that we recently developed for California (combining multiple machine learning algorithms). We also explored the role of NO2 using traditional approaches based on inverse-distance-weighting. We assessed PM2.5 and NO2 exposures 5 and 10 years prior to cancer diagnosis.
2. Materials and Methods
2.1. Study Design and Participants
We reviewed all patients with a primary NSCLC diagnosis who were treated at City of Hope Comprehensive Cancer Center (COH) in Duarte, CA, USA, from 2013 through to 2018. We included patients in this analysis if they had received somatic TP53 or KRAS sequencing documented in the electronic medical record (EMR) and had a valid home address. Patients with non-US addresses or PO boxes were not included. We excluded patients with (i) diagnosis of small cell lung cancer, carcinoid tumors, or sarcomas; (ii) in situ lung cancer; (iii) <18 years of age; and/or (iv) multiple primary NSCLCs with different somatic phenotypes.
Patients included in the study provided written consent, and the study was approved by the COH Institutional Review Board and conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects.
2.2. Air Pollution Exposure Assessment
Data on concentrations of fine particulate matter < 2.5 µm (PM2.5) and nitrogen dioxide (NO2) in µg/m3 were routinely collected by US Environmental Protection Agency through ambient air pollutant monitoring stations. For PM2.5 exposure, we relied on an ensemble model we recently developed for California []. Briefly, we estimated daily levels of PM2.5 at the ZIP code level using a validated ensemble model combining multiple machine learning algorithms (e.g., random forest, gradient boosting) and multiple predictors (e.g., meteorological factors such as temperature, precipitation or wind patterns, satellite-derived aerosol optical depth, or land-use variables). For NO2, we relied on a traditional inverse-distance-weighting (IDW) method to estimate daily NO2 concentrations at the ZIP code population-weighted centroid. We used PM2.5 and NO2 annual concentrations five and ten years before lung cancer diagnosis based on participants’ home addresses.
2.3. Covariates
Data on patient demographics and clinical characteristics were obtained from the COH hospital-based cancer registry. Sociodemographic characteristics included age (continuous), sex (female or male), race/ethnicity (Asian, Black, Hispanic, or Non-Hispanic White), educational attainment (<HS grad, HS grad, college degree, or graduate degree), insurance status (Medicaid or not Medicaid), and cigarette smoking (current, former, or never). Clinical characteristics included cancer stage (I, II, III, or IV), cancer histology (adenocarcinoma, squamous, or other) and year of lung cancer diagnosis (from 2013 to 2018). We also assigned patients an estimated exposure to neighborhood-level socioeconomic status based on their home address using the Area Deprivation Index. Briefly, this measure ranks a census block group’s disadvantage within a given state, as measured by a composite of the area’s income, education, employment, and housing quality [].
2.4. Outcomes
The main outcomes of interest are the following 3 NSCLC somatic tumor mutations: TP53, all KRAS mutations, and KRAS G12C and G12V mutations (KRAS G12C/V). Somatic genomic tests were ordered as part of usual clinical care and sequencing results were obtained from the COH EMR, which contains test results from both internal and external laboratories. Results were typically generated from either the COH Clinical Molecular Diagnostics Laboratory, Foundation Medicine, Inc. (San Diego, CA, USA), or Guardant Health, Inc. (Redwood City, CA, USA). For patients who received multiple tests but had discrepant results, study staff prioritized findings from tissue over blood-based assays.
2.5. Statistical Analysis
To assess association between air pollution and lung cancer tumor mutations, we fit logistic regression models to estimate odds ratio (OR) and their 95% confidence intervals (CIs) per interquartile range (IQR) increase in PM2.5 and NO2 concentrations. Separate models were considered for each combination of air pollution estimates and tumor mutations for five and ten years prior to diagnosis. First, the crude association between air pollution exposure and cancer tumor mutations was investigated. Then, all models were adjusted for previously identified cofounders: age (in continuous), sex, race/ethnicity, educational level, insurance status, area deprivation level, smoking status, cancer stage, cancer histology, and year of diagnosis.
In supplementary analyses, we categorized the air pollution estimates (PM2.5 and NO2) in tertiles. Then, we used absolute cutoffs to define high exposure to PM2.5 according to the US EPA guidelines []. When the annual PM2.5 concentration was higher than 12 μg/m3, participants were classified as high exposed.
Missing data were observed on a few of the variables we assessed (Table S1). In sensitivity analyses, missing data for exposures and covariates were handled using multiple imputations by chained equations (MICE) package in R with 10 imputed datasets [].
All analyses were performed using R, version 3.6.0.
3. Results
3.1. Characteristics of the Study Sample
Among the 694 participants included at baseline, we restricted the population to participants with TP53 or KRAS data and with air pollution exposure data (PM2.5 or NO2). The sample selection is explained in Figure S1. The characteristics of the 435 participants included in this study are described in Table 1. The average age was 67 years (SD, 12), 51% were female and 42% had at least some college education. The majority of participants were non-Hispanic White (56%), followed by Asian participants (31%), Hispanic White (8.5%), and Black (4.6%). Few participants in the study population (7%) reported receiving Medicaid insurance. The year of lung cancer diagnosis was from 2013 to 2018. Sixteen percent of the participants were current smokers, 46% were former smokers, and 38% had never smoked. Most participants had stage IV lung cancer (70%) and a lung adenocarcinoma diagnosis (86%).

Table 1.
Characteristics distribution of selected participants from the CCPS data (n = 435).
3.2. Air Pollution Level 5 and 10 Y Prior to Diagnosis
The average PM2.5 level for all participants was 13.9 μg/m3 (IQR, 12.2–15.6) five years before lung cancer diagnosis, and 13.9 μg/m3 (IQR, 12.2–15.5) ten years before lung cancer diagnosis. The average NO2 level was 17 μg/m3 (IQR, 14.8–18.3) five years before diagnosis, and 18.9 μg/m3 (IQR, 16.3–20.5) ten years before diagnosis.
The average air pollution level according to each mutation status is shown in Table 2. Among 409 participants with complete data for TP53 mutation and PM2.5 exposure, 238 were positive for the TP53 mutation with an average PM2.5 level five years prior to diagnosis at 14.1 μg/m3 (IQR, 12.7–15.7). The average PM2.5 level five years prior to diagnosis was at 13.9 μg/m3 (IQR, 11.8–15.6) for participants without TP53 mutation (n = 171).

Table 2.
Distribution of PM2.5 and NO2 exposure according to mutation status.
3.3. Association between Air Pollution and Lung Cancer Tumor Mutations
Table 3 shows crude and adjusted odds ratios and 95% confidence intervals for TP53, KRAS, and KRAS G12C/V mutation status for every IQR (3.3 μg/m3) increase in PM2.5 exposure five or ten years prior to cancer diagnosis. In crude and adjusted models, an IQR increase in PM2.5 exposure five or ten years before diagnosis was not significantly associated with overall mutation status. However, the odds ratio was higher for TP53 mutation status with adjusted ORs (95% CI) of 1.24 (0.93–1.67) for five years prior to diagnosis and of 1.25 (0.93–1.67) for ten years prior to diagnosis.

Table 3.
Association between PM2.5 and NO2 concentrations 5 and 10 years before cancer diagnosis and lung cancer tumor mutations.
Crude and adjusted ORs and 95% confidence intervals for overall mutation status and NO2 exposure five and ten years prior to cancer diagnosis for an IQR increase (3.5 μg/m3 for five years and 4.2 μg/m3 for ten years prior to diagnosis) are shown in Table 3. An increase of 3.5 μg/m3 in NO2 exposure five years before cancer diagnosis was associated with TP53 tumor mutation (OR: 1.30, 95% CI: 0.99, 1.71). No associations were highlighted for the two KRAS mutations five and ten years before cancer diagnosis.
Using the lowest tertile as the reference, the adjusted OR (95% CI) evaluating the association between PM2.5 exposure five years before diagnosis and TP53 tumor mutation was 1.63 (0.98, 2.75) for the highest tertile, corresponding to an exposure higher than 15 μg/m3 (Figure 1). No association was observed with the other mutation status and for NO2 exposure in tertile.
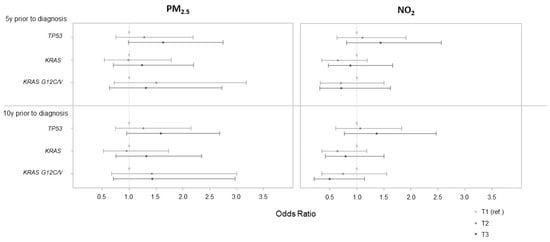
Figure 1.
Associations between PM2.5 and NO2 categorized in tertile (assessed 5 and 10 years prior to cancer diagnosis) and lung cancer tumor mutations. Based on tertile distribution, PM2.5 5 y prior to diagnosis was classified as [6.4;13.1], (13.1;15.0], and (15.0;19.6]; PM2.5 10 y prior to diagnosis as [6.3;13.0], (13.0;15.0], and (15.0;19.6]; NO2 5 y prior to diagnosis as [2.9;16.1], (16.1;17.8], and (17.8;21.5]; and NO2 10 y prior to diagnosis as [3.4;17.5], (17.5;19.9], and (19.9;24.6].
For the association between highly exposed participants to PM2.5 (i.e., with an exposure higher than 12 μg/m3, US EPA guidelines) five and ten years before cancer diagnosis and TP53 mutation, the adjusted ORs (95% CI) were 1.61 (0.95, 2.73) and 1.57 (0.93, 2.64), respectively (Figure 2).
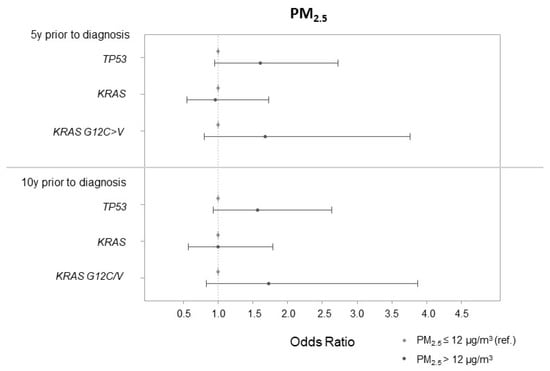
Figure 2.
Associations between PM2.5 in binary according to US EPA guidelines (PM2.5 > 12 μg/m3 5 and 10 years prior to cancer diagnosis) and lung cancer tumor mutations.
When we performed multiple imputation to handle missing data for exposures and covariates, the ORs were weaker and more imprecise (Table S2).
4. Discussion
In this study, we investigated the associations between air pollution concentration level and somatic non-small cell lung cancer mutations: TP53, KRAS, and KRAS G12C/V mutations. Among approximately 400 participants, the associations appeared to be higher between air pollution level (PM2.5 and NO2 exposure) five years before cancer diagnosis and TP53-mutated NSCLC. In contrast, no association was found between air pollution estimates and KRAS and KRAS G12C/V mutations.
The association between air pollution and lung cancer incidence is well established [,,,]. Due to sufficient evidence for a causal association between particulate matter and an increased risk of lung cancer, air pollution was classified as a carcinogen by the World Health Organization International Agency for Research on Cancer (IARC) in 2013 []. The mixture of carcinogenic and mutagenic substances present in PM, such as benzo(a)pyrene (BaP) and polycyclic aromatic hydrocarbons (PAHs), can be metabolized in the body and cause DNA damage, genomic instability, and promote malignant neoplasms [,]. The NSCLC mutations are induced by DNA adducts that are formed by the release of reactive intermediates when BaP and other PAHs are metabolized. Inhalation of PM2.5 particles may attract lymphocytes to tissues, resulting in angiogenesis and inflammation that could promote tumor growth [,].
Limited previous work has identified a relationship between exposure to ambient air pollution and aggressive somatic lung tumor mutations. In our study, we found that environmental pollutants (PM2.5 and NO2) five years before cancer diagnosis could be related to the etiology of the TP53 mutation. However, we did not find any association with other NSCLC somatic tumor mutations (KRAS and KRAS G12C/V), thus requiring further investigations. The biological mechanism of if and how exposure to air pollution impacts NSCLC biology is not clear. An association between air pollution and TP53 mutations has been observed in both mouse models and human cell lines [,,]. TP53 mutations have been observed in mouse cell lines that were experimentally exposed to different environmental toxins, such as BaP and 3-nitrobenzanthrone []. In a previous in vitro study, human cell lines exposed to 3-nitrobenzanthrone (a component of diesel exhaust) presented numerous mutations in TP53 [].
To the best of our knowledge, only two epidemiological studies have studied the link between outdoor air pollution and specific NSCLC mutations. In a cohort of patients living in China, an association between highly-polluted regions and specific somatic NSCLC mutations was reported []. Patients who lived in highly-polluted regions had three times higher mutated genes, including TP53, as those in control, lower-pollution regions. Our previous work found that TP53-mutated NSCLC was linked to areas with higher PM2.5 exposure []. However, this work only focused on TP53-mutated NSCLC and on two pollutants (PM2.5 and ozone), which were assessed through EPA’s EJScreen in the year or two prior to cancer diagnosis. We hope to now expand that work by including two other NSCLC somatic tumor mutations (KRAS and KRAS G12C/V), and by overcoming several limitations of previous studies using more a precise exposure assessment and by limiting the exposure misclassification. We used improved estimation methods to assess exposure to PM2.5 []. Moreover, because health effects can be caused by both short-term exposure and long-term exposure to pollutants, we looked at PM2.5 and NO2 concentration levels up to ten years prior to cancer diagnosis.
Our study has some limitations. First, due to the relatively small number of participants (~400), we are insufficiently powered to conduct analyses stratified by stage of diagnosis or cancer histology (adenocarcinoma, squamous, other). Moreover, we focused on a small number of genes, TP53 and KRAS. We do not have the historic residential patients’ addresses prior to diagnosis, so we were unable to assess patients’ previous exposure to carcinogens. We do not consider potential co-exposure, other than smoking, while other environmental risk factors, such as exposure to radon, household pollutants, and occupational exposure, could be important to take into account as a person may be exposed to several and often synergistic exposures []. Finally, in our study a third of the patients were non-smoking Asians and only a few Hispanic Americans and African Americans were included. This may impact the generalizability of these findings to other populations with different racial/ethnic compositions. Our study also has some strengths including the study sample with almost 400 participants with tumor sequencing results and smoking data, which is a relatively large sample that contains information on somatic oncogenic molecular abnormalities []. Moreover, we adjusted for potential cofounders that have previously been omitted, including smoking status and area deprivation level.
5. Conclusions
Even if the associations were at the limit of significance, our results suggest that the concentration of environmental pollutants (PM2.5 and NO2) five years before cancer diagnosis is associated with TP53-mutated NSCLC, using improved estimation methods to assess air pollution. In contrast, we did not find any association with KRAS and KRAS G12C/V mutations. Future studies are needed to confirm this association and better understand how air pollution affects somatic profiles and through which molecular mechanisms. This improved understanding could help better identify individuals who may be at high risk of developing aggressive disease, implement accurate screening of high-risk patients, and improve the use of targeted therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph191711027/s1, Figure S1. Sample selection; Table S1. Distribution of missing data; Table S2. Association between PM2.5 and NO2 concentrations 5 and 10 years before cancer diagnosis and lung cancer tumor mutations after multiple imputation.
Author Contributions
Conceptualization, S.E.W., L.E. and M.M.J.; data curation, N.L. and S.E.W.; formal analysis, N.L.; methodology, N.L, T.B. and M.M.J.; writing—original draft preparation, N.L. and S.E.W.; writing—review and editing, J.-A.Y., T.B., S.W.G., L.E. and M.M.J.; supervision, T.B., L.E. and M.M.J.; funding acquisition, T.B., L.E. and M.M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by The City of Hope Paul Calabresi Career Development Award for Clinical Oncology (K12 CA001727) and the National Cancer Institute (R01CA228147).
Institutional Review Board Statement
The study was approved by the COH Institutional Review Board and conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects (# 18257, 7 October 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used for analyses during the current study are not publicly available due to ethical restrictions and participant confidentiality but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cancer of the Lung and Bronchus—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 24 June 2021).
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar] [PubMed]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2016, 8, 8921–8946. [Google Scholar] [CrossRef]
- Pao, W.; Wang, T.Y.; Riely, G.J.; Miller, V.A.; Pan, Q.; Ladanyi, M.; Zakowski, M.F.; Heelan, R.T.; Kris, M.G.; Varmus, H.E. KRAS Mutations and Primary Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib. PLoS Med. 2005, 2, e17. [Google Scholar] [CrossRef]
- Mak, R.H.; Hermann, G.; Lewis, J.H.; Aerts, H.J.W.L.; Baldini, E.H.; Chen, A.B.; Colson, Y.L.; Hacker, F.H.; Kozono, D.; Wee, J.O.; et al. Outcomes by Tumor Histology and KRAS Mutation Status After Lung Stereotactic Body Radiation Therapy for Early-Stage Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2015, 16, 24–32. [Google Scholar] [CrossRef]
- Qin, K.; Hou, H.; Liang, Y.; Zhang, X. Prognostic value of TP53 concurrent mutations for EGFR- TKIs and ALK-TKIs based targeted therapy in advanced non-small cell lung cancer: A meta-analysis. BMC Cancer 2020, 20, 328. [Google Scholar] [CrossRef]
- Johnson, M.L.; Sima, C.S.; Chaft, J.; Paik, P.K.; Pao, W.; Kris, M.G.; Ladanyi, M.; Riely, G.J. Association of KRAS and EGFR Mutations with Survival in Patients with Advanced Lung Adenocarcinomas. Cancer 2013, 119, 356–362. [Google Scholar] [CrossRef]
- Renaud, S.; Falcoz, P.-E.; Schaëffer, M.; Guenot, D.; Romain, B.; Olland, A.; Reeb, J.; Santelmo, N.; Chenard, M.-P.; Legrain, M.; et al. Prognostic value of the KRAS G12V mutation in 841 surgically resected Caucasian lung adenocarcinoma cases. Br. J. Cancer 2015, 113, 1206–1215. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, T.; Li, X.; Zhao, C.; Zhang, L.; Zhao, S.; Liu, X.; Qiao, M.; Luo, J.; Shi, J.; et al. Characterization of distinct types of KRAS mutation and its impact on first-line platinum-based chemotherapy in Chinese patients with advanced non-small cell lung cancer. Oncol. Lett. 2017, 14, 6525–6532. [Google Scholar] [CrossRef]
- Nadal, E.; Chen, G.; Prensner, J.R.; Shiratsuchi, H.; Sam, C.; Zhao, L.; Kalemkerian, G.P.; Brenner, D.; Lin, J.; Reddy, R.M.; et al. KRAS-G12C Mutation Is Associated with Poor Outcome in Surgically Resected Lung Adenocarcinoma. J. Thorac. Oncol. 2014, 9, 1513–1522. [Google Scholar] [CrossRef]
- Gibbons, D.L.; Byers, L.A.; Kurie, J.M. Smoking, p53 Mutation, and Lung Cancer. Mol. Cancer Res. 2014, 12, 3–13. [Google Scholar]
- Varghese, A.M.; Sima, C.S.; Chaft, J.E.; Johnson, M.L.; Riely, G.J.; Ladanyi, M.; Kris, M.G. Lungs Don’t Forget: Comparison of the KRAS and EGFR Mutation Profile and Survival of Collegiate Smokers and Never Smokers with Advanced Lung Cancers. J. Thorac. Oncol. 2013, 8, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Bumroongkit, K.; Rannala, B.; Traisathit, P.; Srikummool, M.; Wongchai, Y.; Kangwanpong, D. TP53 gene mutations of lung cancer patients in upper northern Thailand and environmental risk factors. Cancer Genet. Cytogenet. 2008, 185, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-B.; Shim, J.-Y.; Park, B.; Lee, Y.-J. Long-Term Exposure to Air Pollutants and Cancer Mortality: A Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public. Health 2018, 15, 2608. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Huang, Y.; Han, J.; Song, F.; Chen, K. Ambient particulate matter and lung cancer incidence and mortality: A meta-analysis of prospective studies. Eur. J. Public Health 2015, 25, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Turner, M.C.; Krewski, D.; Pope, C.A.; Chen, Y.; Gapstur, S.M.; Thun, M.J. Long-term Ambient Fine Particulate Matter Air Pollution and Lung Cancer in a Large Cohort of Never-Smokers. Am. J. Respir. Crit. Care Med. 2011, 184, 1374–1381. [Google Scholar] [CrossRef]
- Pope, C.A., III. Lung Cancer, Cardiopulmonary Mortality, and Long-term Exposure to Fine Particulate Air Pollution. JAMA 2002, 287, 1132. [Google Scholar] [CrossRef]
- Hamra, G.B.; Laden, F.; Cohen, A.J.; Raaschou-Nielsen, O.; Brauer, M.; Loomis, D. Lung Cancer and Exposure to Nitrogen Dioxide and Traffic: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2015, 123, 1107–1112. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Particulate Matter (PM) Basics. Available online: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics (accessed on 24 June 2021).
- Brook, J.R.; Burnett, R.T.; Dann, T.F.; Cakmak, S.; Goldberg, M.S.; Fan, X.; Wheeler, A.J. Further interpretation of the acute effect of nitrogen dioxide observed in Canadian time-series studies. J. Expo. Sci. Environ. Epidemiol. 2007, 17, S36–S44. [Google Scholar] [CrossRef]
- Longhin, E.; Holme, J.A.; Gutzkow, K.B.; Arlt, V.M.; Kucab, J.E.; Camatini, M.; Gualtieri, M. Cell cycle alterations induced by urban PM2.5 in bronchial epithelial cells: Characterization of the process and possible mechanisms involved. Part. Fibre Toxicol. 2013, 10, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zhao, T.; Yang, X.; Sun, B.; Li, Y.; Duan, J.; Sun, Z. PM2.5-induced alteration of DNA methylation and RNA-transcription are associated with inflammatory response and lung injury. Sci. Total Environ. 2019, 650, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Curren, K.C.; Dann, T.F.; Wang, D.K. Ambient air 1,3-butadiene concentrations in Canada (1995–2003): Seasonal, day of week variations, trends, and source influences. Atmos. Environ. 2006, 40, 170–181. [Google Scholar] [CrossRef]
- Erhunmwunsee, L.; Wing, S.E.; Shen, J.; Hu, H.; Sosa, E.; Lopez, L.N.; Raquel, C.; Sur, M.; Ibarra-Noriega, P.; Currey, M.; et al. The Association between Polluted Neighborhoods and TP53-Mutated Non–Small Cell Lung Cancer. Cancer Epidemiol. Prev. Biomark. 2021, 30, 1498–1505. [Google Scholar] [CrossRef]
- Aguilera, R.; Luo, N.; Basu, R.; Wu, J.; Gershunov, A.; Benmarhnia, T. Using machine learning to estimate wildfire PM2.5 at California ZIP codes (2006–2020). ChemRxiv 2021. [Google Scholar] [CrossRef]
- Kind, A.J.H.; Buckingham, W.R. Making Neighborhood-Disadvantage Metrics Accessible—The Neighborhood Atlas. N. Engl. J. Med. 2018, 378, 2456–2458. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. National Ambient Air Quality Standards (NAAQS) for PM. Available online: https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm (accessed on 24 June 2021).
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- IARC: Outdoor Air Pollution a Leading Environmental Cause of Cancer Deaths—IARC. Available online: https://www.iarc.who.int/news-events/iarc-outdoor-air-pollution-a-leading-environmental-cause-of-cancer-deaths/ (accessed on 13 October 2021).
- Li, J.; Li, W.X.; Bai, C.; Song, Y. Particulate matter-induced epigenetic changes and lung cancer. Clin. Respir. J. 2017, 11, 539–546. [Google Scholar] [CrossRef]
- Li, R.; Zhou, R.; Zhang, J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol. Lett. 2018, 15, 7506–7514. [Google Scholar] [CrossRef]
- Evans, J.; van Donkelaar, A.; Martin, R.V.; Burnett, R.; Rainham, D.G.; Birkett, N.J.; Krewski, D. Estimates of global mortality attributable to particulate air pollution using satellite imagery. Environ. Res. 2013, 120, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.; Verdin, A.; Escande, F.; Saint-Georges, F.; Cazier, F.; Mulliez, P.; Courcot, D.; Shirali, P.; Gosset, P.; Garçon, G. In vitro short-term exposure to air pollution PM2.5-0.3 induced cell cycle alterations and genetic instability in a human lung cell coculture model. Environ. Res. 2016, 147, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Kucab, J.E.; Phillips, D.H.; Arlt, V.M. Linking environmental carcinogen exposure to TP53 mutations in human tumours using the human TP53 knock-in (Hupki) mouse model. FEBS J. 2010, 277, 2567–2583. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tian, D.; He, J.; Wang, Y.; Zhang, L.; Cui, L.; Jia, L.; Zhang, L.; Li, L.; Shu, Y.; et al. Repeated PM2.5 exposure inhibits BEAS-2B cell P53 expression through ROS-Akt-DNMT3B pathway-mediated promoter hypermethylation. Oncotarget 2016, 7, 20691–20703. [Google Scholar] [CrossRef] [PubMed]
- vom Brocke, J.; Krais, A.; Whibley, C.; Hollstein, M.C.; Schmeiser, H.H. The carcinogenic air pollutant 3-nitrobenzanthrone induces GC to TA transversion mutations in human p53 sequences. Mutagenesis 2009, 24, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-J.; Yang, M.-J.; Zhou, B.; Wang, G.-Z.; Huang, Y.-C.; Wu, L.-C.; Cheng, X.; Wen, Z.-S.; Huang, J.-Y.; Zhang, Y.-D.; et al. Characterization of Somatic Mutations in Air Pollution-Related Lung Cancer. eBioMedicine 2015, 2, 583–590. [Google Scholar] [CrossRef]
- Gibelin, C.; Couraud, S. Somatic alterations in lung cancer: Do environmental factors matter? Lung Cancer Amst. Neth. 2016, 100, 45–52. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).