Lack of Associations between Environmental Exposures and Environmental Enteric Dysfunction among 18-Month-Old Children in Rural Malawi
Abstract
:1. Introduction
2. Materials and Methods
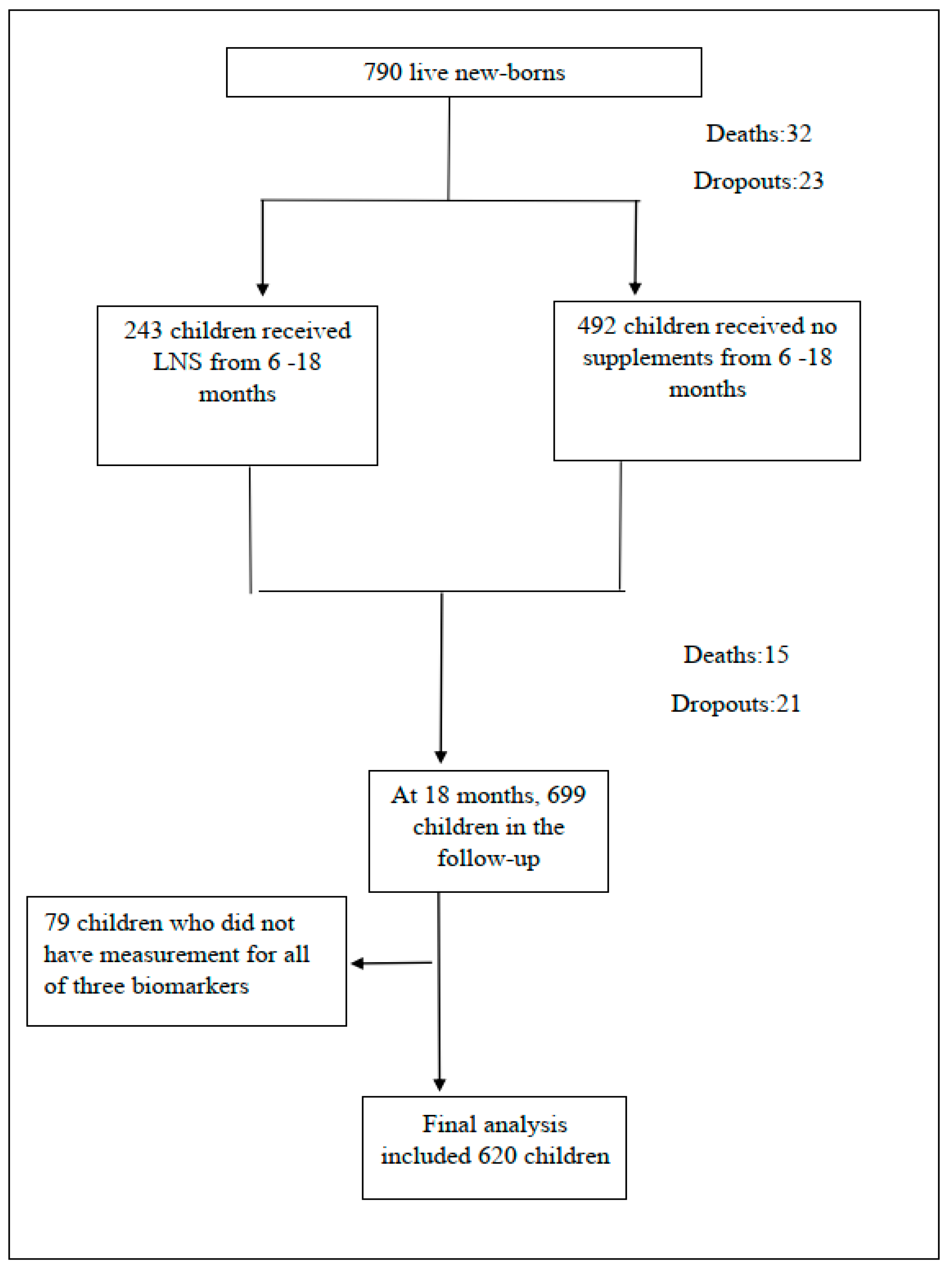
2.1. Study Participants
2.2. Environmental Exposures
2.3. Outcomes
2.4. Other Variables
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crane, R.J.; Jones, K.D.; Berkley, J.A. Environmental enteric dysfunction: An overview. Food Nutr. Bull. 2015, 36, S76–S87. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.; Tembo, M.; Kelly, P. Intestinal Biopsies for the Evaluation of Environmental Enteropathy and Environmental Enteric Dysfunction. J. Infect. Dis. 2021, 224, S856–S863. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.B. Environmental Enteric Dysfunction: Reeemergence of an Old Disease. J. Infect. Dis. 2021, 224, S873–S875. [Google Scholar] [CrossRef]
- Myatt, M.; Khara, T.; Schoenbuchner, S.; Pietzsch, S.; Dolan, C.; Lelijveld, N.; Briend, A. Children who are both wasted and stunted are also underweight and have a high risk of death: A descriptive epidemiology of multiple anthropometric deficits using data from 51 countries. Arch. Public Health 2018, 76, 28. [Google Scholar] [CrossRef]
- Morais, M.B.; Silva, G.A.P.D. Environmental enteric dysfunction and growth. J. Pediatr. 2019, 95, 85–94. [Google Scholar] [CrossRef]
- George, C.M.; Oldja, L.; Biswas, S.K.; Perin, J.; Lee, G.O.; Ahmed, S.; Haque, R.; Sack, R.B.; Parvin, T.; Azmi, I.J.; et al. Fecal Markers of Environmental Enteropathy are Associated with Animal Exposure and Caregiver Hygiene in Bangladesh. Am. J. Trop. Med. Hyg. 2015, 93, 269–275. [Google Scholar] [CrossRef]
- Campbell, R.K.; Schulze, K.J.; Shaikh, S.; Mehra, S.; Ali, H.; Wu, L.; Raqib, R.; Baker, S.; Labrique, A.; West, K.P., Jr.; et al. Biomarkers of Environmental Enteric Dysfunction Among Children in Rural Bangladesh. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 40–46. [Google Scholar] [CrossRef]
- McCormick, B.J.J.; Lee, G.O.; Seidman, J.C.; Haque, R.; Mondal, D.; Quetz, J.; Lima, A.A.M.; Babji, S.; Kang, G.; Shrestha, S.K.; et al. Dynamics and Trends in Fecal Biomarkers of Gut Function in Children from 1–24 Months in the MAL-ED Study. Am. J. Trop. Med. Hyg. 2017, 96, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Exum, N.G.; Lee, G.O.; Olórtegui, M.P.; Yori, P.P.; Salas, M.S.; Trigoso, D.R.; Colston, J.M.; Schwab, K.J.; McCormick, B.J.J.; Kosek, M.N. A Longitudinal Study of Household Water, Sanitation, and Hygiene Characteristics and Environmental Enteropathy Markers in Children Less than 24 Months in Iquitos, Peru. Am. J. Trop. Med. Hyg. 2018, 98, 995–1004. [Google Scholar] [CrossRef]
- Lin, A.; Arnold, B.F.; Afreen, S.; Goto, R.; Huda, T.M.N.; Haque, R.; Raqib, R.; Unicomb, L.; Ahmed, T.; Colford, J.M.; et al. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. Am. J. Trop. Med. Hyg. 2013, 89, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Crane, R.J.; Parker, E.; Fleming, S.; Gwela, A.; Gumbi, W.; Ngoi, J.M.; de Laurent, Z.R.; Nyatichi, E.; Ngari, M.; Wambua, J.; et al. Cessation of exclusive breastfeeding and seasonality, but not small intestinal bacterial overgrowth, are associated with environmental enteric dysfunction: A birth cohort study amongst infants in rural Kenya. EClinicalMedicine 2022, 47, 101403. [Google Scholar] [CrossRef] [PubMed]
- Gough, E.K.; Moulton, L.H.; Mutasa, K.; Ntozini, R.; Stoltzfus, R.J.; Majo, F.D.; Smith, L.E.; Panic, G.; Giallourou, N.; Jamell, M.; et al. Effects of improved water, sanitation, and hygiene and improved complementary feeding on environmental enteric dysfunction in children in rural Zimbabwe: A cluster-randomized controlled trial. PLoS Negl. Trop. Dis. 2020, 14, e0007963. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, P.A.; Fernández, M.S.; Turjanski, P.; Pérez, A.; Rivero, M.R.; De Angelo, C.; Salomón, O.D.; Cueto, G. Substantial reduction in child stunting is differentially associated to geographical and socioeconomic disparities in Misiones Province, Argentina. Trop. Med. Int. Health 2020, 25, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.N.; Stephenson, K.B.; Trehan, I.; Shulman, R.J.; Thakwalakwa, C.; Murray, E.; Maleta, K.; Manary, M.J. Zinc or albendazole attenuates the progression of environmental enteropathy: A randomized controlled trial. Clin. Gastroenterol. Hepatol. 2014, 12, 1507–1513. [Google Scholar] [CrossRef]
- Belayneh, M.; Loha, E.; Lindtjørn, B. Seasonal Variation of Household Food Insecurity and Household Dietary Diversity on Wasting and Stunting among Young Children in A Drought Prone Area in South Ethiopia: A Cohort Study. Ecol. Food Nutr. 2021, 60, 44–69. [Google Scholar] [CrossRef] [PubMed]
- Uwiringiyimana, V.; Osei, F.; Amer, S.; Veldkamp, A. Bayesian geostatistical modelling of stunting in Rwanda: Risk factors and spatially explicit residual stunting burden. BMC Public Health 2022, 22, 159. [Google Scholar] [CrossRef]
- Tusting, L.S.; Gething, P.W.; Gibson, H.S.; Greenwood, B.; Knudsen, J.; Lindsay, S.W.; Bhatt, S. Housing and child health in sub-Saharan Africa: A cross-sectional analysis. PLoS Med. 2020, 17, e1003055. [Google Scholar] [CrossRef]
- Kortekangas, E.; Fan, Y.M.; Chaima, D.; Lehto, K.M.; Malamba-Banda, C.; Matchado, A.; Chingwanda, C.; Liu, Z.; Ashorn, U.; Cheung, Y.B.; et al. Associations between Gut Microbiota and Intestinal Inflammation, Permeability and Damage in Young Malawian Children. J. Trop. Pediatr. 2022, 68, fmac012. [Google Scholar] [CrossRef]
- Kortekangas, E.; Kamng’ona, A.W.; Fan, Y.M.; Cheung, Y.B.; Ashorn, U.; Matchado, A.; Poelman, B.; Maleta, K.; Dewey, K.G.; Ashorn, P. Environmental exposures and child and maternal gut microbiota in rural Malawi. Paediatr. Perinat. Epidemiol. 2020, 34, 161–170. [Google Scholar] [CrossRef]
- Kim, H.; Sitarik, A.R.; Woodcroft, K.; Johnson, C.C.; Zoratti, E. Birth Mode, Breastfeeding, Pet Exposure, and Antibiotic Use: Associations with the Gut Microbiome and Sensitization in Children. Curr. Allergy Asthma Rep. 2019, 19, 22. [Google Scholar] [CrossRef]
- McDonnell, L.; Gilkes, A.; Ashworth, M.; Rowland, V.; Harries, T.H.; Armstrong, D.; White, P. Association between antibiotics and gut microbiome dysbiosis in children: Systematic review and meta-analysis. Gut Microbes 2021, 13, 1870402. [Google Scholar] [CrossRef] [PubMed]
- Kamng’ona, A.W.; Young, R.; Arnold, C.D.; Patson, N.; Jorgensen, J.M.; Kortekangas, E.; Chaima, D.; Malamba, C.; Ashorn, U.; Cheung, Y.B.; et al. Provision of Lipid-Based Nutrient Supplements to Mothers During Pregnancy and 6 Months Postpartum and to Their Infants from 6 to 18 Months Promotes Infant Gut Microbiota Diversity at 18 Months of Age but Not Microbiota Maturation in a Rural Malawian Setting: Secondary Outcomes of a Randomized Trial. J. Nutr. 2020, 150, 918–928. [Google Scholar]
- Kolho, K.L.; Alfthan, H. Concentration of fecal calprotectin in 11,255 children aged 0–18 years. Scand. J. Gastroenterol. 2020, 55, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Mumolo, M.G.; Bellini, M.; Romano, M.R.; Ceccarelli, L.; Arpe, P.; Sterpi, C.; Marchi, S.; Maltinti, G. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig. Liver Dis. 2003, 35, 642–647. [Google Scholar] [CrossRef]
- Guerrant, R.L.; Leite, A.M.; Pinkerton, R.; Medeiros, P.H.; Cavalcante, P.A.; DeBoer, M.; Kosek, M.; Duggan, C.; Gewirtz, A.; Kagan, J.C.; et al. Biomarkers of Environmental Enteropathy, Inflammation, Stunting, and Impaired Growth in Children in Northeast Brazil. PLoS ONE 2016, 11, e0158772. [Google Scholar] [CrossRef]
- Kosek, M.; Haque, R.; Lima, A.; Babji, S.; Shrestha, S.; Qureshi, S.; Amidou, S.; Mduma, E.; Lee, G.; Yori, P.P.; et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am. J. Trop. Med. Hyg. 2013, 88, 390–396. [Google Scholar] [CrossRef]
- Ashorn, P.; Alho, L.; Ashorn, U.; Cheung, Y.B.; Dewey, K.G.; Harjunmaa, U.; Lartey, A.; Nkhoma, M.; Phiri, N.; Phuka, J.; et al. The impact of lipid-based nutrient supplement provision to pregnant women on newborn size in rural Malawi: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 387–397. [Google Scholar] [CrossRef]
- Ashorn, P.; Alho, L.; Ashorn, U.; Cheung, Y.B.; Dewey, K.G.; Gondwe, A.; Harjunmaa, U.; Lartey, A.; Phiri, N.; Phiri, T.E.; et al. Supplementation of Maternal Diets during Pregnancy and for 6 Months Postpartum and Infant Diets Thereafter with Small-Quantity Lipid-Based Nutrient Supplements Does Not Promote Child Growth by 18 Months of Age in Rural Malawi: A Randomized Controlled Trial. J. Nutr. 2015, 145, 1345–1353. [Google Scholar] [CrossRef]
- Penakalapati, G.; Swarthout, J.; Delahoy, M.J.; McAliley, L.; Wodnik, B.; Levy, K.; Freeman, M.C. Exposure to Animal Feces and Human Health: A Systematic Review and Proposed Research Priorities. Environ. Sci. Technol. 2017, 51, 11537–11552. [Google Scholar] [CrossRef]
- Headey, D.; Nguyen, P.; Kim, S.; Rawat, R.; Ruel, M.; Menon, P. Is Exposure to Animal Feces Harmful to Child Nutrition and Health Outcomes? A Multicountry Observational Analysis. Am. J. Trop. Med. Hyg. 2017, 96, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.; Swindale, A.; Bilinsky, P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide V3; FHI 360/FANTA: Washington, DC, USA, 2007. [Google Scholar]
- Deitchler, M.; Ballard, T.; Swindale, A.; Coates, J. Introducing a Simple Measure of Household Hunger for Cross-Cultural Use; FANTA2: Washington, DC, USA, 2011. [Google Scholar]
- Lin, A.; Ali, S.; Arnold, B.F.; Rahman, M.Z.; Alauddin, M.; Grembi, J.; Mertens, A.N.; Famida, S.L.; Akther, S.; Hossen, M.S.; et al. Effects of Water, Sanitation, Handwashing, and Nutritional Interventions on Environmental Enteric Dysfunction in Young Children: A Cluster-randomized, Controlled Trial in Rural Bangladesh. Clin. Infect. Dis. 2020, 70, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Naylor, C.; Lu, M.; Haque, R.; Mondal, D.; Buonomo, E.; Nayak, U.; Mychaleckyj, J.C.; Kirkpatrick, B.; Colgate, R.; Carmolli, M.; et al. Environmental Enteropathy, Oral Vaccine Failure and Growth Faltering in Infants in Bangladesh. EBioMedicine 2015, 2, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Asgarshirazi, M.; Shariat, M.; Nayeri, F.; Dalili, H.; Abdollahi, A. Comparison of Fecal Calprotectin in Exclusively Breastfed and Formula or Mixed Fed Infants in the First Six Months of Life. Acta Med. Iran. 2017, 55, 53–58. [Google Scholar] [PubMed]
- Peterson, K.M.; Buss, J.; Easley, R.; Yang, Z.; Korpe, P.S.; Niu, F.; Ma, J.Z.; Olortegui, M.P.; Haque, R.; Kosek, M.N.; et al. REG1B as a predictor of childhood stunting in Bangladesh and Peru. Am. J. Clin. Nutr. 2013, 97, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Castagno, E.; Calabrese, R.; Viola, S.; Oggero, R.; Miniero, R. High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology 2010, 97, 299–304. [Google Scholar] [CrossRef]
- Lankelma, J.M.; Cranendonk, D.R.; Belzer, C.; de Vos, A.F.; de Vos, W.M.; van der Poll, T.; Wiersinga, W.J. Antibiotic-induced gut microbiota disruption during human endotoxemia: A randomised controlled study. Gut 2017, 66, 1623–1630. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- European Food Safety Authority. Management of left-censored data in dietary exposure assessment of chemical substances. EFSA J. 2010, 8, 1557. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Church, J.A.; Parker, E.P.; Kosek, M.N.; Kang, G.; Grassly, N.C.; Kelly, P.; Prendergast, A.J. Exploring the relationship between environmental enteric dysfunction and oral vaccine responses. Future Microbiol. 2018, 13, 1055–1070. [Google Scholar] [CrossRef]
- Miyake, H.; Lee, C.; Chusilp, S.; Bhalla, M.; Li, B.; Pitino, M.; Seo, S.; O’Connor, D.L.; Pierro, A. Human breast milk exosomes attenuate intestinal damage. Pediatr. Surg. Int. 2020, 36, 155–163. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Bandsma, R.H.J.; Sadiq, K.; Bhutta, Z.A. Persistent diarrhoea: Current knowledge and novel concepts. Paediatr. Int. Child Health 2019, 39, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Nickles, M.A.; Hasan, A.; Shakhbazova, A.; Wright, S.; Chambers, C.J.; Sivamani, R.K. Alternative Treatment Approaches to Small Intestinal Bacterial Overgrowth: A Systematic Review. J. Altern. Complement. Med. 2021, 27, 108–119. [Google Scholar] [CrossRef]
- Candido, F.G.; Valente, F.X.; Grześkowiak, Ł.M.; Moreira, A.P.B.; Rocha, D.M.U.P.; Alfenas, R.C.G. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef]
- Woodruff, C.; Fabacher, D.; Latham, C. Fecal alpha 1-antitrypsin and infant feeding. J. Pediatr. 1985, 106, 228–232. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chen, E.Z.; Baldassano, R.N.; Otley, A.R.; Griffiths, A.M.; Lee, D.; Bittinger, K.; Bailey, A.; Friedman, E.S.; Hoffmann, C.; et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe 2017, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Marill, K.A. Advanced statistics: Linear regression, part II: Multiple linear regression. Acad. Emerg. Med. 2004, 11, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Fahim, S.M.; Das, S.; Gazi, M.A.; Alam, M.A.; Hasan, M.M.; Hossain, M.S.; Mahfuz, M.; Rahman, M.M.; Haque, R.; Sarker, S.A.; et al. Helicobacter pylori infection is associated with fecal biomarkers of environmental enteric dysfunction but not with the nutritional status of children living in Bangladesh. PLoS Negl. Trop. Dis. 2020, 14, e0008243. [Google Scholar] [CrossRef]
- Sterne, J.A.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Yaya, S.; Hudani, A.; Udenigwe, O.; Shah, V.; Ekholuenetale, M.; Bishwajit, G. Improving Water, Sanitation and Hygiene Practices, and Housing Quality to Prevent Diarrhea among Under-Five Children in Nigeria. Trop. Med. Infect. Dis. 2018, 3, 41. [Google Scholar] [CrossRef]
- Jenkins, M.W.; Cumming, O.; Cairncross, S. Pit latrine emptying behavior and demand for sanitation services in Dar Es Salaam, Tanzania. Int. J. Environ. Res. Public Health 2015, 12, 2588–2611. [Google Scholar] [CrossRef] [PubMed]
- Holm, R.; Tembo, J.M.; Thole, B. A comparative study of fecal sludge management in Malawi and Zambia: Status, challenges and opportunities in pit latrine emptying. Afr. J. Environ. Sci. Tech. 2015, 9, 783–792. [Google Scholar] [CrossRef]
- Blackett, C.I.; Hawkins, P.; Heymans, C. The Missing Link in Sanitation Service Delivery: A Review of Fecal Sludge Management in 12 Cities; World Bank Group: Washington, DC, USA, 2014. [Google Scholar] [CrossRef]
- Morita, T.; Perin, J.; Oldja, L.; Biswas, S.; Sack, R.B.; Ahmed, S.; Haque, R.; Bhuiyan, N.A.; Parvin, T.; Bhuyian, S.I.; et al. Mouthing of Soil Contaminated Objects is Associated with Environmental Enteropathy in Young Children. Trop. Med. Int. Health 2017, 22, 670–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristic | Included N = 620 | Excluded N = 170 | p-Value ‡ |
|---|---|---|---|
| Maternal age, years | 25 (6) | 24 (6) | 0.077 |
| Mother, HIV infected a | 77 (12%) | 17 (10%) | 0.588 |
| Educational achievement, years | 4 (3) | 5 (4) | <0.001 ** |
| Child sex, boys | 299 (48%) | 73 (46%) | 0.595 |
| WLZ at 18 months | −0.2 (1.0) | −0.1 (0.9) | 0.606 |
| WLZ at 18 months < −2, wasting | 22 (4%) | 4 (0%) | 0.250 |
| LAZ at 18 months | −1.7 (1.1) | −1.6 (1.1) | 0.600 |
| LAZ at 18months < −2, stunting | 226 (37%) | 18 (29%) | 0.267 |
| Continued breastfeeding by 18 months | 537 (93%) | 40 (83%) | 0.045 * |
| Number of days of antibiotic use between birth and 18 months | 8 (18) | 13 (20) | 0.135 |
| Environmental Exposures | Calprotectin | § REG1B | Alpha-1-antitrypsin | |||
|---|---|---|---|---|---|---|
| Coef. (95%CI) | p-Value | Coef. (95%CI) | p-Value | Coef. (95%CI) | p-Value | |
| Well, lake and river as drinking water source, 543/620 (vs. piped water and borehole) | −0.25 (−0.54, 0.05) | 0.100 | −0.03 (−0.48, 0.42) | 0.899 | 0.03 (−0.20, 0.27) | 0.785 |
| Regular pit latrine and none, 563/620 (vs. improved latrine) | −0.32 (−0.64, −0.01) | 0.045 * | −0.11 (−0.59, 0.38) | 0.665 | −0.16 (−0.42, 0.09) | 0.205 |
| Exposure to chickens, 303/620 (vs. not exposure) | −0.00 (−0.19, 0.18) | 0.977 | 0.04 (−0.24, 0.32) | 0.793 | 0.04 (−0.11, 0.19) | 0.604 |
| Exposure to goats, 147/620 (vs. not exposure) | −0.01 (−0.23, 0.20) | 0.916 | −0.20 (−0.53, 0.13) | 0.242 | 0.00 (−0.17, 0.18) | 0.974 |
| Exposure to cows, 16/620 (vs. not exposure) | 0.31 (−0.27, 0.88) | 0.295 | 0.56 (−0.32, 1.45) | 0.212 | 0.06 (−0.40, 0.52) | 0.802 |
| Poor quality wall of the main house, 391/620 (vs. burnt brick) | −0.19 (−0.38, 0.00) | 0.051 | 0.11(−0.18, 0.40) | 0.455 | −0.14 (−0.29, 0.01) | 0.069 |
| Poor quality roofing material of the main house, 498/620 (vs. iron sheets or tiles) | −0.03 (−0.26, 0.20) | 0.818 | −0.08 (−0.44, 0.27) | 0.649 | −0.13 (−0.31, 0.06) | 0.171 |
| Season (vs. Cold-dry) | ||||||
| Rainy, 216/620 | 0.45 (0.23, 0.67) | <0.001 ** | −0.06 (−0.41, 0.29) | 0.750 | 0.20 (0.01, 0.38) | 0.034 * |
| Hot-dry, 218/620 | 0.43 (0.21, 0.66) | <0.001 ** | −0.02 (−0.37, 0.33) | 0.923 | 0.13 (−0.05, 0.31) | 0.154 |
| Residential area (vs. Lungwena) | ||||||
| Malindi, 117/620 | 0.23 (−0.01, 0.47) | 0.066 | −0.05 (−0.43, 0.32) | 0.789 | 0.10 (−0.09, 0.30) | 0.297 |
| Mangochi 171/620 | 0.09 (−0.12, 0.31) | 0.386 | −0.28 (−0.60, 0.05) | 0.100 | 0.13 (−0.04, 0.30) | 0.144 |
| Food insecurity (vs. food secure) | ||||||
| Mildly food insecure, 76/613 | −0.09 (−0.44, 0.26) | 0.618 | 0.16 (−0.38, 0.69) | 0.562 | −0.06 (−0.33, 0.22) | 0.684 |
| Moderately food insecure, 196/613 | −0.07 (−0.36, 0.21) | 0.606 | 0.05 (−0.38, 0.48) | 0.813 | −0.14 (−0.36, 0.09) | 0.229 |
| Severely food insecure, 243/613 | −0.04 (−0.32, 0.23) | 0.750 | −0.18 (−0.60, 0.24) | 0.403 | −0.02 (−0.23, 0.20) | 0.862 |
| Environmental Exposures | Calprotectin | § REG1B | Alpha-1-antitrypsin | |||
|---|---|---|---|---|---|---|
| Coef. (95%CI) | p-Value | Coef. (95%CI) | p-Value | Coef. (95%CI) | p-Value | |
| N = 558 | N = 558 | N = 558 | ||||
| Well, lake and river as drinking water source, 543/620 (vs. piped water and borehole) | −0.14 (−0.45, 0.17) | 0.379 | −0.06 (−0.54, 0.41) | 0.793 | 0.10 (−0.15, 0.34) | 0.431 |
| Regular pit latrine and none, 563/620 (vs. improved latrine) | −0.22 (−0.56, 0.13) | 0.216 | −0.10 (−0.64, 0.43) | 0.708 | −0.05 (−0.32, 0.23) | 0.730 |
| Exposure to chicken, 303/620 (vs. not exposure) | −0.02 (−0.22, 0.18) | 0.858 | 0.06 (−0.25, 0.37) | 0.703 | 0.02 (−0.14, 0.18) | 0.806 |
| Exposure to goats, 147/620 (vs. not exposure) | −0.00 (−0.23, 0.23) | 0.996 | −0.22 (−0.58, 0.14) | 0.228 | 0.02 (−0.17, 0.20) | 0.847 |
| Exposure to cows, 16/620 (vs. not exposure) | 0.24 (−0.35, 0.82) | 0.425 | 0.64 (−0.27, 1.54) | 0.168 | −0.03 (−0.49, 0.43) | 0.904 |
| Poor quality wall material of the main house, 391/620 (vs. burnt brick) | −0.10 (−0.31, 0.11) | 0.367 | 0.22 (−0.11, 0.55) | 0.195 | −0.05 (−0.22, 0.11) | 0.525 |
| Season (vs. Cold-dry) | ||||||
| Rainy, 216/620 | 0.39 (0.13, 0.65) | 0.003 ** | −0.07 (−0.47, 0.32) | 0.715 | 0.16 (−0.05, 0.36) | 0.133 |
| Hot-dry, 218/620 | 0.37 (0.12, 0.62) | 0.004 ** | 0.04 (−0.35, 0.43) | 0.846 | 0.10 (−0.10, 0.30) | 0.315 |
| Residential area (vs. Lungwena) | ||||||
| Malindi, 117/620 | 0.02 (−0.26, 0.31) | 0.868 | −0.10 (−0.54, 0.35) | 0.670 | 0.04 (−0.18, 0.27) | 0.698 |
| Mangochi, 171/620 | −0.08 (−0.34, 0.18) | 0.547 | −0.45 (−0.86, −0.04) | 0.031 * | 0.02 (−0.19, 0.23) | 0.873 |
| Food insecurity (vs. food secure) | ||||||
| Mildly food insecure, 76/613 | 0.02 (−0.37, 0.42) | 0.910 | 0.27 (−0.34, 0.88) | 0.388 | −0.01 (−0.32, 0.30) | 0.940 |
| Moderately food insecure, 196/613 | −0.01 (−0.33, 0.32) | 0.970 | 0.05 (−0.45, 0.56) | 0.834 | −0.08 (−0.34, 0.18) | 0.548 |
| Severely food insecure, 243/613 | 0.08 (−0.24, 0.40) | 0.612 | −0.13 (−0.63, 0.36) | 0.595 | 0.10 (−0.15, 0.35) | 0.430 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Fan, Y.-M.; Ashorn, P.; Chingwanda, C.; Maleta, K.; Hallamaa, L.; Hyöty, H.; Chaima, D.; Ashorn, U. Lack of Associations between Environmental Exposures and Environmental Enteric Dysfunction among 18-Month-Old Children in Rural Malawi. Int. J. Environ. Res. Public Health 2022, 19, 10891. https://doi.org/10.3390/ijerph191710891
Liu Z, Fan Y-M, Ashorn P, Chingwanda C, Maleta K, Hallamaa L, Hyöty H, Chaima D, Ashorn U. Lack of Associations between Environmental Exposures and Environmental Enteric Dysfunction among 18-Month-Old Children in Rural Malawi. International Journal of Environmental Research and Public Health. 2022; 19(17):10891. https://doi.org/10.3390/ijerph191710891
Chicago/Turabian StyleLiu, Zhifei, Yue-Mei Fan, Per Ashorn, Chilungamo Chingwanda, Kenneth Maleta, Lotta Hallamaa, Heikki Hyöty, David Chaima, and Ulla Ashorn. 2022. "Lack of Associations between Environmental Exposures and Environmental Enteric Dysfunction among 18-Month-Old Children in Rural Malawi" International Journal of Environmental Research and Public Health 19, no. 17: 10891. https://doi.org/10.3390/ijerph191710891
APA StyleLiu, Z., Fan, Y.-M., Ashorn, P., Chingwanda, C., Maleta, K., Hallamaa, L., Hyöty, H., Chaima, D., & Ashorn, U. (2022). Lack of Associations between Environmental Exposures and Environmental Enteric Dysfunction among 18-Month-Old Children in Rural Malawi. International Journal of Environmental Research and Public Health, 19(17), 10891. https://doi.org/10.3390/ijerph191710891






